Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6636
Review Article(ISSN: 2637-6636) 
Immunosenescence in Autoimmune Related to Periodontal Disease : A Review Article Volume 5 - Issue 5
Nanda Rachmad Putra Gofur1, Aisyah Rachmadani Putri Gofur2, Soesilaningtyas3, Rizki Nur Rachman Putra Gofur4, Mega Kahdina4 and Hernalia Martadila Putri4
- 1Department of Health, Faculty of Vocational Studies, Universitas Airlangga, Indonesia
- 2Faculty of Dental Medicine, Universitas Airlangga, Indonesia
- 3Department of Dental Nursing, Poltekkes Kemenkes, Indonesia
- 4Faculty of Medicine, Universitas Airlangga, Indonesia
Received:February 19, 2021 Published: March 04, 2021
*Corresponding author: Nanda Rachmad Putra Gofur, Department of Health, Faculty of Vocational Studies, Universitas Airlangga, Surabaya, Indonesia
DOI: 10.32474/IPDOAJ.2021.05.000222
Abstract
Introduction: Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder characterized by the development of autoantibodies and immune complexes associated with a variety of clinical manifestations and tissue damage. SLE is the production of reactive antibodies against the body’s own cells. SLE is multifactorial and most likely involves complex interactions between genetic, environmental, and hormonal factors. There is an aging process in immune cells due to changes in the innate and adaptive immune system compartments. This phenomenon is known as Immunosenescence, also occurs on autoimmune. One of the characteristics of elderly people is their inability to respond to vaccines and infections properly.
Discussion This situation also occurs in patients with systemic lupus erythematosus (SLE). In SLES patients, the aging of the immune system is the concept of inflammation; a state where there is a chronic pro-inflammatory status, characterized by increased levels of pro-inflammatory cytokines such as TNF, or IL-6, thereby stimulating a decrease in IL-2 and IFNγ and an increase in IL-10. Clotting factor and acute phase reactants under constant conditions. These biomarkers correlate with the incidence of various age-related diseases, such as heart disease, cognitive decline, cancer, and other physical disabilities. Immunosenescence and inflammation are the result of disruption in the cellular immunity properties of the innate and adaptive immune.
Conclusion: Defect production of T cells and B cells in autoimmune disease could results immune aging. This phenomenon causes neutrophil activation, forming antibody DNA consisting of immune complexes resulting in severe inflammation and tissue damage, as periodontal disease in oral cavity.
Introduction
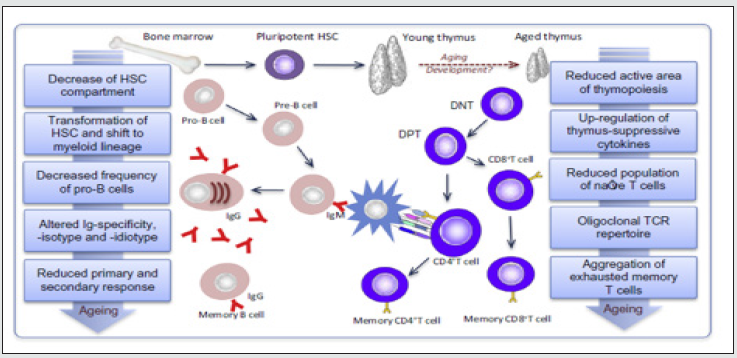
As we age, there is an aging process in immune cells due to changes in the innate and adaptive immune system compartments. This phenomenon is known as Immunosenescence. One of the characteristics of elderly people is their inability to respond to vaccines and infections properly. This condition can be the result of low immune system efficiency and a thymus involution in which the thymus loses the ability to produce and replace naïve T cells in the periphery. As a result, thymus dysfunction results in decreased cellmediated responses to foreign antigens, self-tolerance, and naive T cell populations. These changes lead not only to modifications in the lymphocyte subsets but also to functional changes in the cell population subsets [1]. This situation also occurs in patients with systemic lupus erythematosus (SLE). In SLES patients, the aging of the immune system is the concept of inflammaging; a state where there is a chronic pro-inflammatory status, characterized by increased levels of pro-inflammatory cytokines such as TNF, or IL-6, thereby stimulating a decrease in IL-2 and IFNγ and an increase in IL-10. Clotting factor and acute phase reactants under constant conditions. These biomarkers correlate with the incidence of various age-related diseases, such as heart disease, cognitive decline, cancer, and other physical disabilities. Immunosenescence and inflammaging are the result of disruption in the cellular immunity properties of the innate and adaptive immune [2]. Proinflammatory cytokines in systemic inflammation cause an inflammatory process, which is a loss of balance between the inflammatory response and the efficiency of anti-inflammatory control in the elderly. Furthermore, in the natural aging process, this control fails to neutralize the inflammatory process so that the elderly will experience a higher risk of being more susceptible to infection, malignancy and the process of atherosclerosis, as periodontal disease. This article aims to review immunosenescence in Autoimmune related to periodontal Disease [1].
Immunosenescence in autoimmune disease
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder characterized by the development of autoantibodies and immune complexes associated with a variety of clinical manifestations and tissue damage. SLE is the production of reactive antibodies against the body’s own cells. SLE is multifactorial and most likely involves complex interactions between genetic, environmental, and hormonal factors. The clinical manifestation of SLE patients is inflammation of various organ systems, including skin and mucous membranes, joints, kidneys, brain, serous membranes, lungs, heart, and sometimes the gastrointestinal tract. Organ systems can be affected either alone or in combination. The main features of SLE are the overproduction of autoantibodies and a rash of induration (Figure 1). The SLE classification requires that patients meet four of the 11 criteria established by the American College of Rheumatology (ACR). Clinical manifestations that appear are identified by clinical examination, laboratory tests, and patient records [3]. The prevalence of SLE is nine times more common in women, but the main cause of the sex differences remains unclear. The prevalence of SLE is 31 per 100,000 women in a population of European descent, which is 50-75% lower than in other populations. SLE predominantly affects women in a 9: 1 ratio. The prevalence of women is 90% in African Americans, compared with men [4]. The decreased expression of IL-2 and IFNγ can prolong chronic inflammation so that it can aggravate both SLE and periodontitis (Marques et al, 2016; Fu et al, 2013). IL-10 causes an increase in autoantibodies due to failure of immunoregulation so that when the amount increases, there will be a persistent inflammatory response. (Marques et al, 2016). Immunohistochemical staining studies with specific antibodies to IL-2, IL-10 and monospecific anti-IFN-γ antigens have also shown increased severity of chronic inflammation [5,6].
Discussion
Impact of Immunoscenesens on Humoral Immunity
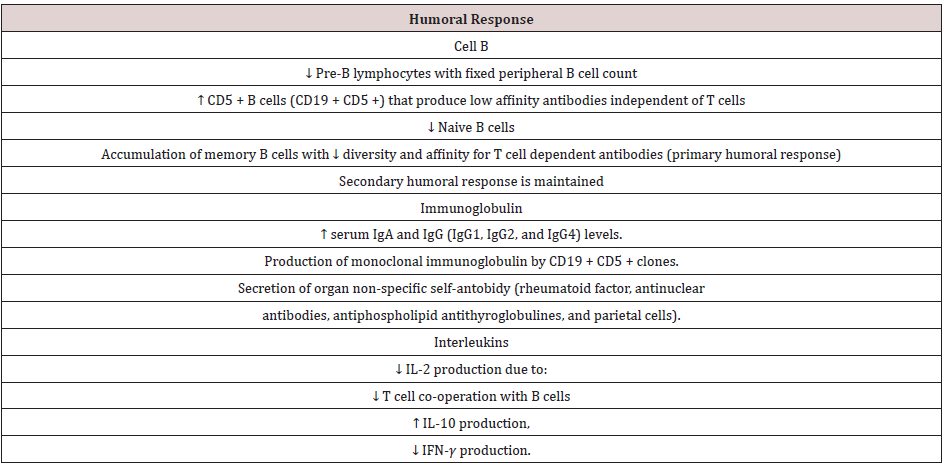
B cells also undergo age-related changes which further exacerbate functional defects of the adaptive immune response. Similar to the aging T cell system, the number of naïve B cells decreases, and effector B cells accumulate in old age. This leads to a reduction in the diversity of antibody responses. There is a defect in isotype switching and somatic mutation which is an important process in the production of IgG antibodies, thus triggering a weak antibody response with low affinity in the elderly. During the aging process, the total number of B cells decreases, with some increase in total memory B cells (CD27+), while other subsets have decreased. IgD-CD27 + B-cells, and B cell precursors were consistently reduced. There was an accumulation of A CD11c + CD21- B cells identified associated with aging [2]. Humoral immunity in the elderly has decreased as a result of two main mechanisms, namely:
a) A decrease in the number of B lymphocytes that produce long-term immunoglobulins due to intrinsic defects and microenvironment defects, and
b) Loss of diversity and affinity of immunoglobulins as an effect of disruption of germinal center formation.
The effects of aging on peripheral B cells vary, the number of B cells from the bone marrow has decreased, exacerbating peripheral defects. Despite a four- to fivefold decrease in B cell production in old mice, the number of peripheral B cells remained relatively constant. In addition, oligoclonal expansion of B cells is associated with CD5 expression, independent immunoglobulin production and production of low affinity auto-antibodies known to occur in the elderly, as a result of which other B cell populations cannot occupy their positions [2,7]. This condition in humoral immunity causes the aging of the immune system, which is marked by expansion of memory T cells, clonal exhausting occurs so that it produces dominant cytokines, namely IL-2, IL-10 and IFNγ. The production of inflammatory cytokines in the LES also plays a role in the periodontitis mechanism, IL-2, IL-10 and IFNγ can stimulate chronic inflammation so as to stimulate osteoclasts and resorption of alveolar bone, and periodontitis occurs [8]. The disruption of T cell proliferation causes an imbalance in the number of naive T cells and memory. The number of naïve T production in the periphery decreases, and there is a decrease in function leading to decreased IL-2, IFNγ and increased IL-10 (Table 1). There is a decrease in IL-2 and IFNγ production due to T lymphocytes, both in their latent and active form in SLE patients [9].
Immunosenescence related periodontal disease in autoimmune.
Studies show, IL-2 decreases with the severity of periodontitis. This reduction was associated with advanced periodontitis, with a greater clinical severity. These results show the same results as previous studies, IL-2 has decreased. Interleukin-2 is a cytokine that has many functions, being the main regulator of the body’s resistance mechanism against various pathogens and plays an active role in the pathogenesis of periodontal disease [10]. Decreased IL-2 can inhibit the proliferation and signaling of T cells. This failure causes robus of calcium inlux, hyperphosphorylation and an increase in Fc receptors. This condition reduces immune dysregulation, the mechanism of CREM and CREB resulting in severe inflammation. IL-2 is also able to cause T cells to experience anergy and induce autoantibodies in B cells. This situation also affects the fimbria protein of Porphyromonas gingivalis at the protein level, namely by suppressing the transcription factor activator protein 1 (AP- 1) and mediating bacteria through leupeptin so that bacteria can damage the wider periodontal tissue [7,8]. The higher the amount of IL-10 indicates the worse periodontal condition. This condition is in accordance with previous studies that a positive correlation with the cytokine IL-10 was found in periodontal disease which was exacerbated by smoking. In vivo studies showed an increase in IL-10 in severe gingival inflammation and an increase in IL-10 in gingival crevicular fluid in patients with periodontitis [11].
Interleukin-10 functions as both proinflammatory and antiinflammatory to control the production of several cytokines and other mediators. IL10 has the main target, namely myeloid cells that can inhibit cytokine production and regulate inflammation. The regulation of T cells in alveolar bone inflammation is impaired due to increased IL-10 (Goncalves et al, 2010). IL-10 is important in regulating balance and is a modulator of infection response to JAKSTAT signaling. IL-10 was found to have strong anti-inflammatory activity and a broad spectrum, which has been proven in various models of infection, chronic inflammation, autoimmune and even cancer [12]. IL10 can induce T cell proliferation and cytotoxic activity. have a function since higher levels of these mediators are associated with a decreased likelihood of experiencing periodontitis. IL-10 is able to increase metalloproteinase production. In addition, IL-10 through the GM-CSF pathway can mediate pro-inflammatory cytokines and increase the differentiation and chemotaxic of cytotoxic T cells so that inflammation becomes severe [13]. IL- 10 was found to trigger RANKL production, thereby triggering osteoclasts. IL-10 can trigger periodontitis and regulate other proinflammatory cytokines, including those involved in alveolar bone resorption. Individuals with high levels of IL-10 were found to have severe chronic periodontitis by examining the mouse HPA. The role of IL-10 can increase inflammation leading to bone resorption, and in this study, it was shown that higher levels of IL-10 were found in patients with more severe periodontitis status [14].
Research shows that examination of the gingival crevicular fluid a decrease in the amount of IFN-γ indicates a manifestation of periodontal disease. IFN-γ is a cytokine that plays a role in increasing the expression of toll-like receptors (TLRs), increasing antigen presentation by MHC class I and II, triggering chemokine secretion, phagocytosis, activation of CD8 + T cells and radically fighting pathogenic microorganisms and tumor cells [15,16]. The effect of interferon in the gingival crevicular fluid and saliva of periodontitis patients suggests that IFNγ, an immunoregulatory cytokine, plays an important role in periodontal disease. Stem cells in the periodontal ligament and stem cells from other tissues, have an immunomodulatory capacity that is IFNγ regulated. IFNγ plays a role in systemic disease through its receptors consisting of two polypeptide chains and is called IFNGR1 and IFNGR2. IFN-γ can regulate the production of T cells and B cells to produce autoantibodies through the NETs pathway. (Extracellular Neutrophil Traps). Decreased IFN-γ causes neutrophil activation, forming antibody DNA consisting of immune complexes resulting in severe inflammation and tissue damage [17]. Both in vitro and in vivo studies on lupus mice have shown that the IFN-γ cytokine has a low rate of periodontal disease and contributes to the onset and progression of periodontitis. IFN-γ can activate the ubiquitineproteasome pathway in osteoclasts, resulting in TRAF6 degradation and inhibiting RANKL signaling, so its main function is to control bone resorption in the T cell response. The mechanism that occurs is when the IFN-γ state is low, the RANKL barrier function is reduced so that the induction of osteoclast genesis is high and bone resorption occurs [18].
Conclusion
Defect production of T cells and B cells in autoimmune disease could results immune aging. This phenomenon causes neutrophil activation, forming antibody DNA consisting of immune complexes resulting in severe inflammation and tissue damage, as periodontal disease in oral cavity. The mechanism that occurs is when the IFN-γ induce the RANKL barrier function is reduced so that the induction of osteoclast genesis is high and bone resorption occurs.
References
- Montoya-ortiz G (2013) Immunosenescence , Aging , and Systemic Lupus Erythematous.
- Van den Hoogen LL, Sims GP, van Roon JAG, Fritsch-Stork RDE (2015) Aging and Systemic Lupus Erythematosus - Immunosenescence and Beyond. Current Aging Science 8(2): 158–177.
- Gómez-Martín D, Mariana Díaz-Zamudio, José Carlos Crispín, Jorge Alcocer-Varela (2009) Interleukin 2 and systemic lupus erythematosus :Beyond the transcriptional regulatory net abnormalities. J Autoimmunity Reviews 9: 34–39.
- Ganguly D (2013) The role of dendritic cells in autoimmunity. Macmillan Publisher 13(8): 566-577.
- Ebersole JL, Taubman MA (1994) The protective nature of host responses in periodontal diseases. Periodontol 2000 5: 112–141.
- Mesia R, Gholami F, Huang H, et al. (2016) Systemic inflammatory responses in patients with type 2 diabetes with chronic periodontitis BMJ Open Diabetes Research and Care 4: e000260.
- Andrukhov O, Ulm C, Reischl H, Nguyen P, Matejka M, et al. (2011)) Serum cytokine levels in periodontitis patients in relation to the bacterial load. J Periodontal 82(6): 885-892.
- Mizraji (2017) Porphyromonas gingivalis Promotes Unrestrained Type I Interferon Production by Dysregulating TAM Signaling via MYD88 Degradation 18: 419–431.
- Müller L, Pawelec G (2014) Aging and immunity - Impact of behavioral intervention. Brain, Behavior, and Immunity 39: 8–22.
- Batool H, Al-Ghurabei, Zahraa F Shaker, Raghed Fadhel, Nahla G Al-Khayli, Leen K Mustafa (2012) Serum Levels of Interlukine-1Beta and Interlukine-2 in Chronic Periodontitis. Al- Mustansiriya J Sci 23(3).
- Gonçalves TO, Costa DJ, Brodskyn CI, Duarte PM, Cesar Neto JB, et al. (2010) Release of cytokines by stimulated peripheral blood mononuclear cells in chronic periodontitis. J Arch Oral Biol 55: 975-980.
- Ebersole JL, Steffen MJ, Thomas MV (2013) Smoking-related cotinine levels and host responses in chronic periodontitis. Journal of Periodontal Research 49(5): 642-651.
- Khalaf H, Bengtsson T (2012) Altered T-Cell Responses by the Periodontal Pathogen Porphyromonas gingivalis. PLoS ONE 7(9): e45192.
- Kobayashi R, Kono T, Bolerjack BA, Fukuyama Y, Gilbert RS, et al. (2011) Induction of IL-10-producing CD4+ T-cells in Chronic Periodontitis. Journal of Dental Research 90(5): 653–658.
- Leibbrandt A, dan Penninger JM (2010) Novel Function of RANK(L) Signalling in the Immune System. J Osteoimmunology : Springer 658: 77-94.
- Pawelec G (2012) Hallmarks of human “immunosenescence”: adaptation or dysregulation? Immunity & Ageing 9(1): 15.
- Souto GR, Queiroz-Junior CM, de Abreu MHNG, Costa FO, Mesquita RA (2014) Pro-inflammatory, Th1, Th2, Th17 Cytokines and Dendritic Cells: A Cross sectional Study in Chronic Periodontitis. PLoS ONE 9(3): e91636.
- Tsai CC (2007) Changes in Gingival Crevicular Fluid Interleukin-4 And Interferon-Gamma in Patients with Chronic Periodontitis Before and After Periodontal Initial Therapy. Kaohsiung J Med Sci 23: 1–7.
Editorial Manager:
Email:
pediatricdentistry@lupinepublishers.com

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...