Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6636
Review Article(ISSN: 2637-6636) 
Gorlin Goltz Syndrome: Report of A Clinical Case with no Genetic Background Volume 6 - Issue 2
Gabriela Elizabeth Gómez Cárdenas1, Cristóbal Landa Román2* and Francisco Javier Gómez Pamatz3
- 1Student, Faculty of Dentistry, Universidad Michoacana de San Nicolás de Hidalgo, Mexico
- 2Master in Public Health, Dental Surgeon, Graduated from Universidad Michoacana De San Nicolas Hidalgo, Private Dental Practice, Mexico
- 3Doctorate in Educational Sciences, Master’s Degree in Psychopedagogy, Maxillofacial Surgeon of the Pediatric Dentistry Service at the Children’s Hospital of Morelia, Mexico
Received:May 24, 2021 Published: June 04, 2021
*Corresponding author: Cristóbal Landa Román, Dental Surgeon, Graduated from Universidad Michoacana De San Nicolas Hidalgo, Private Dental Practice, Mexico
DOI: 10.32474/IPDOAJ.2021.06.000233
Abstract
First described in 1960 by pathologist Robert Gorlin and dermatologist Robert W Goltz of the University of Minnesota, USA, they provided the basis for the diagnosis of the syndrome, establishing basal cell carcinomas, keratocysts, and skeletal malformations as the primary criteria for the syndrome. Subsequently, authors such as Evans, Kimonis, and Bree established major and minor criteria for the correct diagnosis. According to the International Classification of Applied Diseases in Dentistry and Stomatology (ICD-AO), Gorlin Goltz Syndrome is classified within congenital anomalies and other hamartomatosis. It is caused by a mutation in chromosome 9q22.3 in the tumor suppressor Patched 1. This work presents the clinical case of a 10-year-old female patient with a 13x15cm volume increase in the maseterine region on the left side, diagnosed as Gorlin Goltz Syndrome with no hereditary antecedents.
Keywords:Syndrome; Gorlin-Goltz, Basal Cell carcinoma; Keratocysy; Chromosome 9
Background
In 1960 pathologist Robert Gorlin and dermatologist Robert W Goltz from the University of Minnesota, United States; provided the basis for diagnosing the syndrome, establishing basal cells carcinomas, keratocysts, and skeletal malformations as the primary criteria for the syndrome [1]. Subsequently, authors such as Evans, Kimonis, and Bree established major and minor criteria for the correct diagnosis [2,3]. Currently, the Bree criteria is followed to establish the diagnosis. The criteria establishes that it is necessary to comply with a direct hereditary relationship, a major and minor criterion. According to Landa RC, Gómez-Pamatz FJ1, it is possible to identify the evolution of the major and minor clinical characteristics useful for the correct diagnosis (Table 1). It is an autosomal dominant syndrome of variable expressivity, resulting from a mutation on chromosome 9q22 [3]. In the tumor suppressor Patched 1. When one allele is affected, malformations occur; when two alleles are altered, tumors and cysts happen [4-6]. According to the International Classification of Diseases to Dentistry and Stomatology (ICD-DA) Gorlin Goltz Syndrome is classified within congenital anomalies and other hamartomatosis [4-7]. In the last decade, a worldwide prevalence of 1/56,000 and 1/221,000 patients was estimated, with a predominance in Caucasians and 1:1 sexual distribution [2].
Differential Diagnosis
Ameloblastoma, dentigerous cyst, Brooke-Spiegler syndrome, Bazex syndrome, Rombo syndrome, Muir-Torre syndrome, Beckwith-Wiedemann syndrome [8].
Histopathology
The keratocyst shows a cavity with a lining composed of parakeratinized squamous epithelium with a corrugated basement membrane and a superficial zone of parakeratin [9].
Radiology
In imaging studies, we can find anomalies in the skull: calcification of the brain sickle, macrocephaly, hypertelorism; maxilla and mandible: cystic cavities and bone deformities resulting from cortical expansion, incomplete development of the premaxilla; clavicle, ribs, and vertebrae: skeletal deformity, kyphoscoliosis, short metacarpals, bifid ribs [1].
Treatment
The work should be multidisciplinary, aimed at modifying the clinical manifestations as much as possible. It should be carried out among dermatologists, plastic surgeons, medical geneticists, medical oncologists, stomatologists, maxillofacial surgeons, and psychologists.
Clinical Case
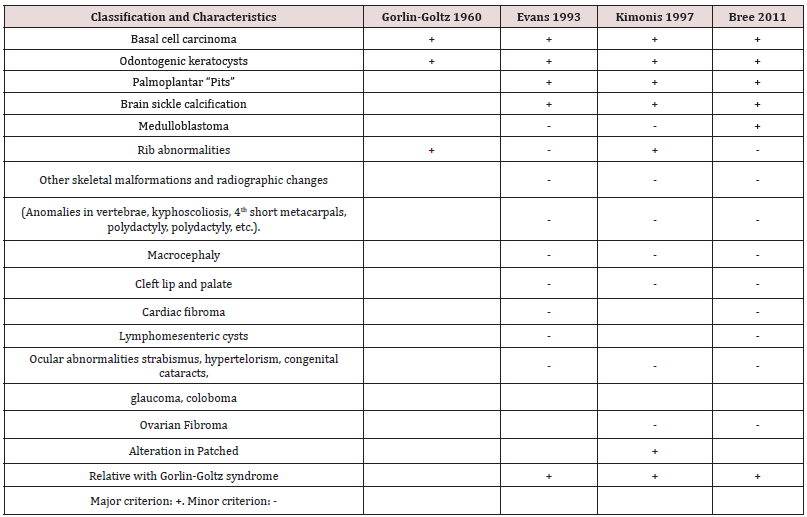
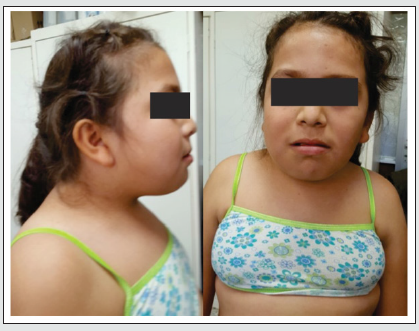
A 10-year-old female patient presents accompanied by her guardian, who refers to an increase in volume accompanied by pain on the left side of the face with 25 days of evolution. For this reason, they decided to go to the Children’s Hospital of Morelia “Eva Sámano de López Mateos” (SSA) for proper care. Extraoral physical examination showed an increase in volume of 13x15cm in the maseterine region on the left side, normothermic, normochromic, non-displaced. Mild intellectual disability, normal cephalic head, convergent strabismus in the left eye, short neck, soft abdomen, peristalsis present, no visceromegaly, with integral and symmetrical extremities with capillary filling of two seconds. External genitalia according to age and gender (Figure 1). En la palma de los pies y manos se aprecian pits (Figure 2). No relevant history for her current condition; no history of trauma or allergies. Genetically related to two clinically healthy siblings. Laboratory studies showed: hemoglobin 12.5g/dl, hematocrit 38.9% platelets 346x 10ʌ3/uL, glucose 72mg/dl, urea 19.7mg/dl, creatinine 0.7mg/dl, Alkaline Phosphatase 845u/l in imaging studies, it can be seen in the 16-detector helical tomography in single and contrasted phase. In the lower jaw to the right of the midline, a lesion expands, erodes, and remodels the body, angle and ascending ramus of the mandible; this lesion conditions a cavity inside with air, soft tissue density, and a molar, which apparently corresponds to the right first molar, the right medial wall of the mandible is communicated to the oral cavity, this lesion measures 59x25x32mm. The second lesion of similar characteristics is observed; it is located to the left and conditions a cavity occupied by soft tissue density, measuring approximately 13x15mm. A third image with the same characteristics is located in the lower jaw discreetly to the right of the midline in relation to the right lateral incisor with a diameter of 14mm in the cavity, a fourth and fifth images with the same characteristics in the upper jaw on the right and left of the midline, relative to the upper canines (Figure 3). The bones of the skull are normal; the paranasal cavity shows adequate development. There is a thickening of the mucosa of the right frontoethmoidal recess and the middle groups of the ethmoid cells.
Figure 3: CT scan, cortical expansion is observed in the mandibular ramus and mandibular body on the left side.
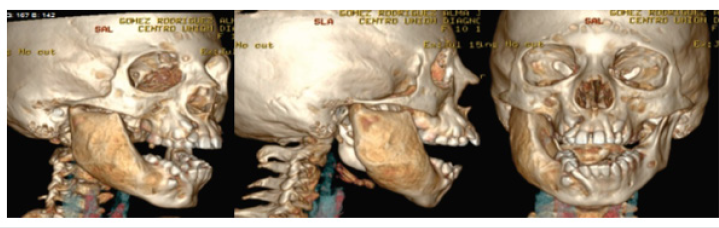
Figure 4(A): Under general and local anesthesia, cystic enucleation and biopsy were performed, showing a macroscopic structure of 9x6x2cm.
Figure 4(B): Microscopically, fragments of a wall lined by poly-stratified squamous epithelium are observed showing active inflammatory changes altering with proliferative changes of the epithelium with areas of pronounced ridge networks.
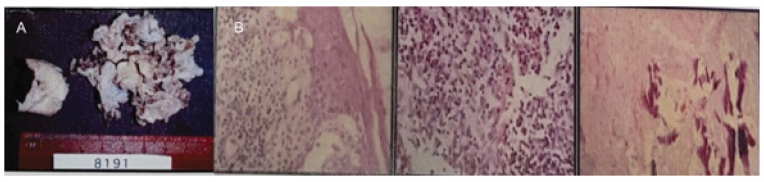
Orbits and their content present typical characteristics. Well-developed and pneumatized mastoids. Under general and local anesthesia, cystic enucleation and biopsy were performed, showing a macroscopic structure of 9x6x2cm with a gray and white membranous appearance. Among them, bone fragments and a dental organ measuring 1x1cm, with a smooth brown surface, spiculated on its surface, surrounded by soft gray tissue, were identified. Microscopically, fragments of a wall lined by poly-stratified squamous epithelium are observed showing active inflammatory changes altering with proliferative changes of the epithelium with areas of pronounced ridge networks (Figure 4A). The epithelium shows numerous ulcerated areas where there is an intense acute and chronic active inflammatory process with dense clusters of lymphocytes, histiocytes and plasma cells in addition to actively proliferating granulation tissue. There are areas of multifocal hemorrhage. There are also areas of multifocal fibrosis with bone spicules with degenerative changes, characterized by sclerosis and spicular thinning. No ameloblastic epithelium was identified, no keratin lamina was observed. It is essential to mention that the cyst is multi fragmented and has a critical inflammatory behavior. This should be noted because the differential diagnosis with other types of cysts of odontogenic origin such as dentigerous cyst and odontogenic keratocyst can present very similar morphogenic images when the inflammatory component is important (Figure 4B). Based on clinical features, histopathology, and imaging findings, Gorlin Goltz syndrome was diagnosed with no genetic history.
Discussion
In the Children’s Hospital of Morelia, an average of 103,992 consultations were made in the last five years with a ratio of 1:259,980 for maxillofacial surgery, of which 1,200 patients per year with a prevalence of 1:3,000 patients diagnosed with Gorlin Goltz syndrome in a 3:1 gender ratio with a higher frequency for the male gender. Subsequent to the literature review, it has only been mentioned that the syndrome can exist without genetic load. However, it was not feasible to find scientific articles published in PubMed related to the subject.
Conclusion
Gorlin Goltz syndrome is a nosological entity characterized by being autosomal dominant with variable expressivity. It can occur in patients who do not have a direct genetic lineage with the syndrome, resulting from a solid genetic load on Patched 1.
Recognition
Translated into English by Jazmín Michelle Hernández Jiménez.
References
- Landa RC, Gómez-Pamatz FJ (2017) Gorlin-Goltz syndrome update, about a case at the Morelia Children's Hospital. ADM Magazine 74(2): 94-99.
- Rosón GS, González GR, Naval GL, Sastre PJ, Muñoz-Guerra MF, et al. (2009) Gorlin-Goltz syndrome: 7-case series. Rev esp oral and maxillofacial surgery 31(5): 309-315.
- Larsen AK, Mikkelsen DB, Hertz JM, Bygum A (2014) Manifestations of Gorlin-Goltz Syndrome. Dan Med J 61(5): 48-59.
- Ortega GA, García AO, Zepeda NS, Acha SA, Aguirre UJ (2008) Gorlin-Goltz syndrome: clinicopathologic aspects. Med Oral Patol Oral Cir Bucal 13(6): 338-343.
- Arango-Salgado A, Arroyave-Sierra JE, Ruiz-Suárez AC (2013) Gorlin syndrome. About a case. Rev CES Med 27(1): 77-82.
- Kalogeropoulou C, Zampakis P, Kazantzi S, Kraniotis P, Mastronikolis NS (2009) Gorlin-Goltz syndrome: incidental finding on routine ct scan following car accident. Cases J 2: 9087.
- Leiva VN, Veliz MS, Gonzalez EL, Salazar PC (2015) Gorlin-Goltz syndrome associated with bilateral cleft lip and palate. Cuban Rev Stomatol 52(2): 188-195.
- Lo ML (2008) Nevoid basal cell carcinoma síndrome (Gorlin sí Orphanet Journal of Rare Diseases 31(3): 1750-1752.
- Alonso MA, Santos JE, Zeta CE, González AP, Portilla RJ (2020) Decompression treatment of an odontogenic keratocyst. Mexican dental journal 24(2): 124-133.
Editorial Manager:
Email:
pediatricdentistry@lupinepublishers.com

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...