Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6636
Research Article(ISSN: 2637-6636) 
Antimicrobial Susceptibility Pattern of Coconut Oil Extract on Selected Bacterial and Fungi Volume 1 - Issue 3
Effiong Edet Bassey1*, Gwana Dam Mohammed1 and Okaro Cynthia2
- 1Department of Applied Microbiology and Brewing, Nnamdi Azikiwe University, Awka, Nigeria
- 2Department of Animal Health and Production Technology, Mahoment Lawan College of Agriculture Maiduaguri, Nigeria
Received: April 10, 2018; Published: April 30, 2018
*Corresponding author: Effiong Edet Bassey, Department of Applied Microbiology and Brewing, Nnamdi Azikiwe University, Awka, Nigeria
DOI: 10.32474/IPDOAJ.2018.01.000115
Abstract
The study is aimed at evaluating the phytochemical content of coconut oil and determines the susceptibility pattern of coconut oil against bacteria and fungi. Coconut oil is a high value coconut product and some positive health benefits have currently been attributed to coconut intake such as its antioxidant and anticancer properties. Coconut oil has also been shown to have antibacterial and antifungal properties and can be used in the treatment of infectious diseases. The findings revealed that the coconut oil sample contains phytochemicals such as alkaloid, glycosides, Terpenoids and considered to be responsible for the many benefits attributed to coconut consumption. This susceptibility testing was conducted by agar diffusion method and the zones inhibition indicated the antimicrobial properties of coconut oil. The assey of antibacterial activity of standard bacteria organisms showed that Staphyloccus aureus had the highest susceptibility to coconut oil while Pseudomonas aeroginosa had the least. this was concluded from their average zones of inhibition 14.55mm (32%) for Staphyloccocus Aureus, 12.1mm (27%) for Streptocccus pneumonia, 10.95 (24%) for Escherichia coli and 7.7mm (17%) for Pseudomonas aeroginosa. Candida albicans had a higher susceptibility to coconut oil more than aspergillus in antifungal testing. This was also concluded from their average zones of inhibition 18.5mm (55%) for Candida albicans and 15.1mm (45%) for Aspergillus fumigatus. The utilization of coconut oil should be promoted as a functional food in Nigeria and the use of coconut seed flesh in our diets should be encouraged for health supporting functions.
Keywords: Antimicrobial; Susceptibility; Coconut Oil; Bacteria; Fungi
Abbreviations: NAFDAC: National Agency for Food and Drug Administration and Control, NA: Nutrient Agar, SDA: Sabaurauds dextrose agar, MA: MacConkey agar, NB: Nutrient broth, SDB: Sabaurauds dextrose broth, MB: MacConkey broth, AST:Antimicrobial Susceptiblity Testing
Introduction
Coconut (cocos nucifera) is a monocytledon belonging to the Arecqceae Family and the order Arecales [1]. It is referred to as the “Tree of Life” because of its many uses. Cocos nucifera is a fruit tree found in warm, humid climates with well drained soils. Coconut oil is made from fresh coconuts. The oil retains its naturally occurring photochemical which produce a distinctive coconut taste and smell since chemical solvents are not used [2]. Coconut oil contains potential medium chain fatty acids (lauric acid, capric acid, caprylic and myristic acid) which have an inhibitory effect against bacterial.The edible oil exhibit antimicrobial (activity against bacteria and fungi such as Eschericha coli, Staphylococcus, Pseudomonas, Streptococcus, Candida albicans, Aspergillus etc [3]. Some Photochemicals with significance health potentials include flavonids, carotenoids, phenolic acids, saponins, glycosides, tannins, alkaloids and coconut has been found a typical example of plants possessing such quality [3].The antimicrobial susceptibility testing of coconut oil in a rapid means of determining the response of microorganisms to coconut oil. It utilizes the diffusion the diffusion method which is simple and widely used for sensitivity testing.
This test resist actively identities organisms that were susceptible or coconut to certain antimicrobial agents such as the nut oil by visual means [4] chemical are few techniques for oil extraction such as physical, includes, fermentation or enzymatic process using microbial as remains enzymatic starter. The benefits of coconut oil theraported by [5] include quick energy, falling hair stress management, treatment of tropical disease, premature aging, weight loss, digestion related problem, wound healing, cardiac disorder. Immunity helps fighting harmful bacterial, infection control due to antimicrobial properties. It reduces incidence of liver diseases, kidney and Gall bladder diseases are control, diabetes, coconut oil helps in controlling blood sugar and bones improvement. The aim of this study is to evaluate the photochemical components of coconut oil and determine the susceptibility of coconut oil extract against selected bacteria and fungi.
Materials and Methods
a) Materials for the Study
All materials, reagents and media used for this analysis were of analytical grade and were all obtained from National Agency for Food and Drug Administration and Control (NAFDAC), Zonal Laboratory Agulu, Anambra State.
b) Area of Study
All research was carried out at the general microbiology laboratory at Nnamdi Azikiwe University Awka Anambra State from June to December, 2017.
c) Sample used for the Study
Coconut oil was used for the study
d) Sample Collection
The fresh coconut fruits (Cocos nucifera) were bought or obtained from Eke-Awka market, Anambra State (Nigeria).
Sample Preparation
Procedure
a) The working area was thoroughly cleaned.
b) The coconut fruits were broken and dehusked.
c) The seed flesh was removed from the shell with a kitchen knife and cut into small pieces or grated.
d) The grated flesh was grinded with warm water in a blender.
e) After the grinding, the coconut milk was separated from the chaff by pouring in a sieve.
f) The bowl of coconut milk was covered and kept in a fridge overnight at 200C.
Caked white coconut oil was separated from the water and placed in a clean dry stainless steep pot and stirred for a period of time to reduce the moisture content giving an aqueous extract of coconut oil. The stirring was stopped when charred bits come were see in the oil and the pot was set aside to cool to a comfortable temperature.
a) Coconut oil was sieved with a chiffon cloth to remove the charred bits and this was collected using a sterile bottle for analysis.
b) This was stored at 40C for further analysis.
Bacterial Isolates
Standard bacterial organisms were obtained from National Agency for Food and Drug Administration and Control (NAFDAC), Zonal Laboratory Agulu, Anambra State. The bacteria strains used for the study include:
a) Gram Positive Bacteria: Staphylococcus aureus
Streptococcus pneumoniae
b) Gram Negative Bacteria: Escherichia soli
Pseudomonas aeroginosa
Fungal Isolate
Standard fungal organisms were obtained from National Agency for Food and Drug Administration and Control (NAFDAC), Zonal Laboratory Agulu, Anambra State. The bacteria strains used for the research include Candida albicans and Aspergillus fumigatus
Media used for Study
The following media were used for the analysis which includes:
a) Nutrient agar (NA)
b) Sabaurauds dextrose agar (SDA)
c) MacConkey agar (MA)
d) Nutrient broth (NB)
e) Sabaurauds dextrose broth (SDB)
f) MacConkey broth (MB)
Preparation and Dispensing Media
Preparation of media was done according to the manufacturer’s instruction which is used for culturing of microorganisms in the microbiology laboratory. Required amounts of media were accurately weighed in a beaker and distilled water was poured to make up to mark. The growth medium was dispensed into appropriate containers i.e. Petri-dishes for agar and McCartney bottles for broth. For the broth, 9ml of it was dispensed into the McCartney bottles. It was tightly covered and autoclaves at 121 0C for 15 minutes according to manufacturer’s instruction. For the agar, the process was a little different. The agar was covered with foil and autoclaved before dispensing or pouring into the Petri-dish.
Controls
Controls are sanitary and sterility measures taken to make sure there are no pre-existing contamination in the environment and growth media, but it is not always like that. The introduction of control in analysis involves dispensing a well prepared media into the respective containers without the inoculation of the sample. There are two (2) types of control:
a) Open Control:In the open control, the Petri dishes containing the well prepared media is kept open throughout the analysis. The reason for leaving them open is to checkmate the environment because during analysis, environmental contaminants enter together with the air.
b) Closed Control:In closed control, the Petri dish containing the well prepared media will never be opened all through the analysis. This type of control evaluates the integrity of the media used for analysis.
These two controls were used as measuring yardsticks for judging the results of the analysis.
Purity Test
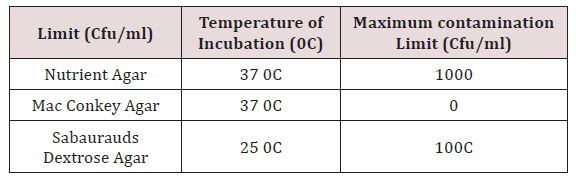
Purity test is a quantitative test carried out on samples to evaluate their microbial quantity and to ensure that the sample complies with the specifications for the test. Extracted coconut oil was cultured on Sabourauds dextrose agar (SDA), McConkey agar (MA) and Nutrient agar (NA) plates and incubated for seven (7) days to ensure that the extract was completely pure.
Materials
Media [Sabarauds dextrose agar (SDA), Sabourauds dextrose broth (SDB), McConkey agar (MA), McConkey broth (MB), Nutrient agar (NA), Nutrient broth (NB), Petri-dishes, sterile syringe, 2% phenol, 70% alcohol, beaker, distilled water, measuring cylinder, spatula, cotton wool, weighing balance, autoclave, nose mask, hand gloves, incubator, masking tape.
Procedure
a) The working area was cleaned thoroughly with 70% alcohol and 2% phenol.
b) The media was prepared according to the manufacturer’s instruction. Media and include: Sabourauds dextrose broth (SDB), MacConkey broth (MB), and Nutrient broth (NB).
c) Using a sterile syringe, IMI each of the coconut oil sample were inoculated into the McCartney bottles containing 9ml each of Sabourauds dextrose broth (SDB), McConkey broth (MB), Nutrient broth (NB). It was capped and shaken to mix.
d) 1ml each of the broth containing the sample were inoculated into the corresponding agar plates i.e. NB to NA, SDB to SDA and MB to MA by pour plate method.
e) The agar plates were incubated for 7 days to check for growth (NA at 370C for aerobic mesophilic bacteria, MA at 370C for pathogenic bacteria and SDA at 250C for fungi).
Phytochemical Analysis
For the phytochemical analysis is the extraction method involved non polar solvent. The tests were carried out to determine the active constituents according to the procedures and method outlined by Trease and Evans 1983 [6]. These phytochemical tests were done to detect the presence of secondary metabolites such as Alkaloids, tannins, saponins, flavonoids, steroid, glycosides and terpenoids in the coconut oil. These phytochemicals are chemical substances that occur naturally in plants which have antimicrobial properties.
Alkaloids
2ml of the coconut oil sample was stirred with 5ml of aqueous HCL. 1ml each of the extract was tested with Dragendorff’s reagents, Mayer’s reagent, Wagner’s reagent, Picric acid (1%). The presence of precipitate and various color changes as seen in the result indicated the presence and level of intensity of alkaloids.
Flavonoids
About 5ml of dilute NH3 solution was added to 3ml of coconut oil extract followed by the addition of concentrated tetraoxosulphate (VI) i.e. H2S04. The various color changes as seen in the result indicated the presence and level of intensity of flavonoids.
Glycosides
5ml of dilute sulphuric was added to 1m1 of coconut oil extract and boiled for 15min in a water bath. It was cooled and neutralized with 20% potassium hydroxide solution (KOH). 10ml of equal parts of Fehling’s solution A and B was added and boiled for 5 min. The color change as seen in the result indicated the presence, absence or intensity.
Saponins (Frothing Test)
About 5ml of the coconut oil extract were shaken ant boiled with equivalent amount of water for 5 minutes. Frothing that persists on warming was taken as evidence of the presence of saponins.
Resins
5ml of the coconut oil extract was added to 20ml of distilled water and the mixture was shaken vigorously. The presence of precipitate and various color changes as seen in the result indicated the presence and level of intensity of resins.
Tannins
2ml of coconut oil extract were boiled with 5ml of 45% ethanol for 5 min. The mixture was cooled and filtered. The filtrates were subjected to the following tests:
a) Lead sub acetate test and
b) Ferric chloride test.
The result indicated presence and the intensity of tannins or the absence of tannins.
Acidic Compounds
Blue litmus paper was used to test the extracts. Color changes indicated the presence or absence of the compounds.
Steroids and Terpernoids
9ml of ethanol was added to 1g of the coconut oil extract and filtered. The filtrate was concentrated to 2.5ml in a boiling water bath. 5ml of distilled water was added and the mixture was allowed to stand for 1 hour and the waxy matter was filtered off. The filtrate was extracted with 2.5ml of chloroform using a separating funnel. To 0.5ml of the extract, 1ml conc. H2SO4 was carefully added. To another 0.5ml of extract, it was evaporated to dryness on a water bath and heated with 3ml of conc. H2SO4 for 10min in a water bath. To color changes in both tests indicated the presence, absence and intensity of steroids and Terponoids.
Antimicrobial Susceptibility Testing (AST)
This is a rapid means of determining the response of microorganisms to different antimicrobial agents. Antimicrobial susceptibility testing (AST) mostly utilizes the Kirby-Bauer agar diffusion method [1]. This test simply, rapidly and effectively identifies organisms that are susceptible or resistant to certain antimicrobial agent through visual means. This method is used when the test substance can diffuse through the agar gel. Generally, this is preferred to serial dilution methods because of ease with which quantitative results can be obtained. This leads to the successful treatment of bacterial and fungal infection [7].
The antibacterial susceptibility testing was carried out on bacterial strains both gram positive (Staphylococcus aureus, Streptococcus pneumoniae) and grain negative (Escherichia coli, Pseudomonas aeroginosa) to check the effectiveness of coconut oil on bacterial infections. The antibacterial activity of the test agent was determined by measuring the diameter of the zone of inhibition in terms of millimeter. The zone of inhibition of the coconut oil was compared to that of commercially produced antibiotic (Ciprofloxacin 500mg).
Ciprofloxacin is a broad spectrum antibiotic that has antibacterial activity against Staphylococci, Streptococci, Escherichia coli, Pseudomonas, Salmonella and used for treatment of bacterial infections such as respiratory infections urinary tract infections, genital infections, gastrointestinal infections and wound infections. The reconstitution and dilution of the commercial antibiotic (Ciprofloxacin 500mg) needed for the analysis was carried out according to National Agency for Food and Drug Admission and Control (Nigeria) method book from (USP 28).
This 50μg/ml is taken to be the lowest concentration of the antibiotic at which the organism cannot produce visible growth after 24hrs. This is the lowest concentration that is effective against bacterial infections.
Procedure
a) The Sabaurauds dextrose agar (SDA) was prepared according to the manufacturer’s instruction, poured into the Petri dishes and allowed to gel.
b) The Petri-dishes were labeled accordingly for the analysis for the coconut oil sample and commercially produced antifungal (Ketoconazole).
c) A perpendicular line crossing the middle of the Petri dish was made using a marker and a mark was made in the centre of each of the two sectors (X and Y).
d) Ten-fold serial dilution of the already subculture test organisms.
e) Different swab sticks were dipped into the bottles of the 10-2 dilutions of the test organisms and smeared on the surface of the gelled agar.
f) A well/hole was created in the centre of each sector using a sterile cork borer (10mm).
g) 1ml each of the coconut oil sample was dispensed into the two holes using the sterile syringes. For ketoconazole, the topical cream is pressed directly into the hole.
h) The plates were kept for 30 minutes for the inoculums to diffuse into a considerable area of the medium.
i) Plates were incubated for 24 hours at 250C.
j) After incubation, the susceptibility of the sample or organism is indicted by the diameter of the zone of inhibition in mm, subtracting the diameter of the hole and finding average of the two zones.
Results
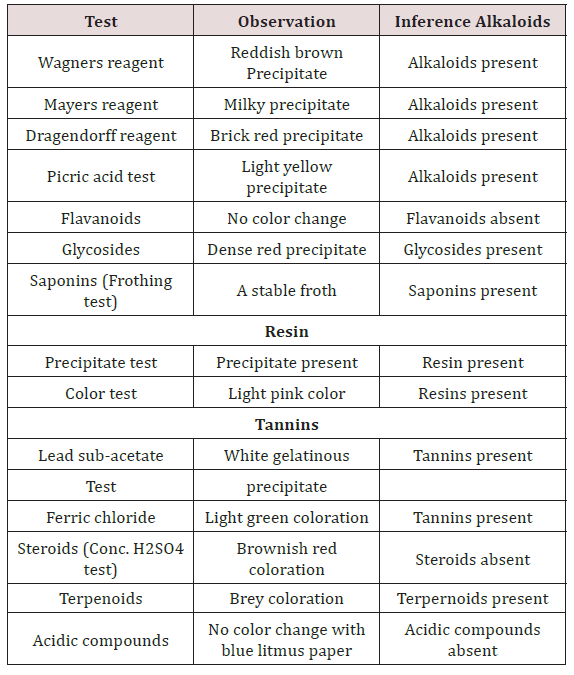
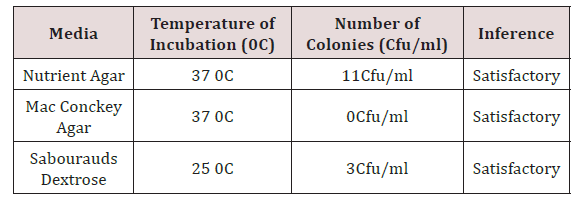
Various phytochemicals were detected in the coconut oil sample (Table 2). Using polar solvent for analysis, the following phytochemicals were present such as alkaloids, glycosides, saponins, resins, tannins and terpenoids while flavonoids, steroids and acidic compounds were absent. Table 3 shows the result of the purity test after 7 days incubation as shown below. Extracted coconut oil cultured on Nutrient agar, MacConkey agar and Sabourauds dextrose agar incubated at 250C and 370C complied with the specifications for purity testing. The coconut oil showed satisfactory results.
Assay of Antibacterial Activity
The antimicrobial properties lauric acid and its derivative monolaurin from coconut oil have shown promise in this study. Lauric acid, which is present in high concentration in coconut oil, forms monolaurin in the animal body and this derivative of lauric acid can inhibit the growth of pathogenic microorganisms. The research focused on Pseudomonas aeroginosa, Escherichia coli, Staphylococcus aureus and Streptococcus pneumonia. Antimicrobial activity was observed by the existence of clear zone formed on the media grown with colonies of tested microbial strains. It was found that the clear zone on the media fully grown with strains of Escherichia coli, Staphylococcus aureus indicated that this oil had activity against the growth of tested strains. It is now clear and scientifically validated that the inclusion of coconut oil in the diet could and should be utilized for its preventive and healing properties.
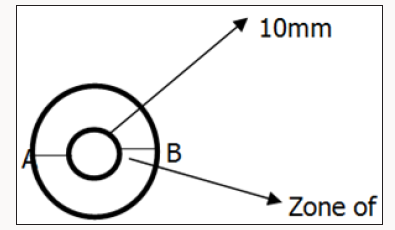
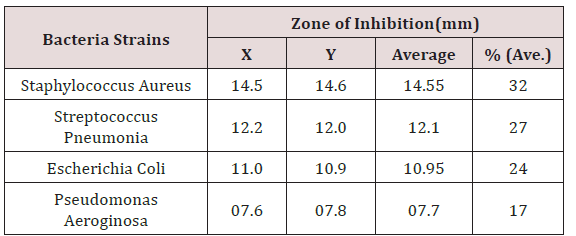
After 24hrs incubation, the antibacterial activity of the coconut oil extract on the test organisms was determined by measuring the diameter of zone of inhibition in millimeter with a meter rule. Ciprofloxacin was used as positive control to compare the sensitivity of the strains to coconut oil (Figure 1). Diameter of Zone of inhibition was gotten from measuring the diameter of A to B and subtracting the diameter of the well i.e. [A to B] - 10mm. After this, the average of the two sectors were determined by dividing the sum by 2 i.e. [X + Y]/2 (Table 4). It was shown that coconut oil produced the highest zone against Staphylococcus aureus while the zone of inhibition was lowest for Pseudomonas aeroginosa.
Antifungal Testing
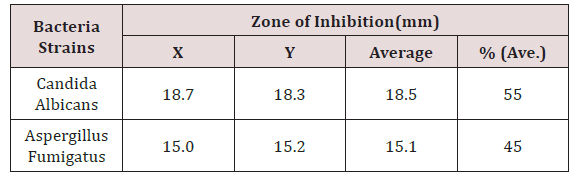
The antifungal susceptibility was determined by using the agar-well diffusion technique. The research focused on Candida albicans and Aspergillus. The solutions were allowed to diffuse for 30 minutes in the agar medium, after which the plates were then incubated at 250C for 24hrs. The zones of inhibition were measured in millimeters. The susceptibility pattern measures as zones of inhibition of the organisms to the coconut oil extract was compared with those of ketoconazole, a topical cream that is also effective against Candida albicans and Aspergillus (Table 1).
The maximum contamination limit indicates whether the test sample complies with specifications for the analysis. The result is termed ‘unsatisfactory’ if the colony forming unit exceeds the maximum limit while the result is satisfactory’ if the test complies with the specifications. (Table 2-4) shows the result of the purity test after 7 days incubation. Extracted coconut oil cultured on Nutrient agar, MacConkey agar and Sabourauds dextrose agar incubated at 250C and 370C complied with the specifications for purity testing. The coconut oil showed satisfactory results. X and Y represent the diameter zones of inhibition in mm of the two agar well which contains the coconut oil on standard bacteria organisms.
‘Average’ shows the sum of the two zones of inhibition divided by two i.e. [X + Y] /2. The percentage (%) zone of inhibition gotten from the average in Table 4 showed that Staphylococcus aureus has the highest sensitivity to coconut oil at 32% while Pseudomonas aeroginosa was the least sensitive to coconut oil at 17%. % (Average) shows the average zones of inhibition in percentage (Table 5). X and Y represent the diameter zones of inhibition in mm of the two agar well which contains the coconut oil on standard bacteria organisms.
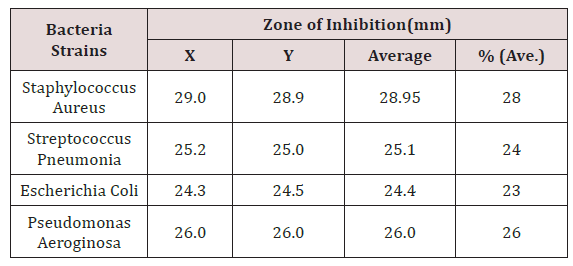
‘Average’ shows the sum of the two zones of inhibition divided by two i.e. [X + Y] /2. The percentage (%) zone of inhibition gotten from the average in Table 5 showed that Staphylococcus aureus has the highest sensitivity to coconut oil at 28% while Escherichia coli was the least sensitive to coconut oil at 23%. % (Average) shows the average zones of inhibition in percentage (Figure 2) (Table 6). X and Y represent the diameter zones of inhibition in mm of the two agar well which contains the coconut oil on standard bacteria organisms.
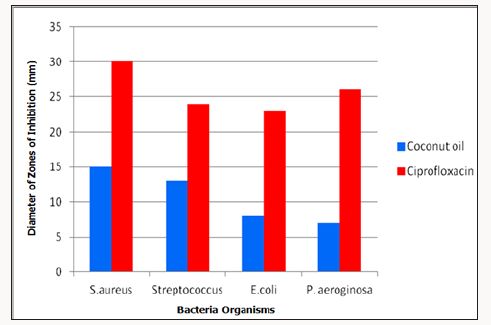
Figure 2: Graphical Presentation showing the Diameter of Zones of Inhibition of Coconut oil to ciprofloxacin as a positive control tested on standard bacteria organisms.
‘Average’ shows the sum of the two zones of inhibition divided by two i.e. [X + Y]/2. The percentage (%) zone of inhibition gotten from the average in Table 6 shows that Candida albicans is highly sensitive to ketocobazole at 55% while Aspergillus was moderately sensitive to ketoconazole. % (Average) shows the average zones of inhibition in percentage.
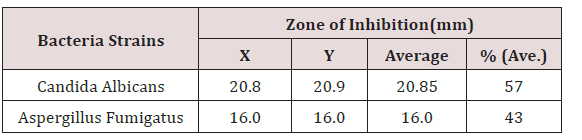
‘Average’ shows the sum of the two zones of inhibition divided by two i.e. [X + Y]/2.The percentage (%) zone of inhibition gotten from the average in Table 6 shows that Candida albicans is highly sensitive to coconut oil at 55% while Aspergillus was moderately sensitive to coconut oil. % (Average) shows the average zones of inhibition in percentage (Table 7). X and Y represent the diameter zones of inhibition in mm of the two agar well which contains the coconut oil on standard bacteria organisms.
Discussion
The presence of various phytochemicals is these coconut oil samples revealed the potentials of coconut and coconut oil as a functional food. The alkaloids, glycosides and other phytochemicals found in this study as shown in Table 2 is similar with the findings of Obiora et al. 2010. The absence of steroids, flavonoids and acidic compounds in these studied samples implies that Nigerian coconut seed flesh is not a good dietary source of steroids which agrees with the study of Obiora et al. 2010.
In this study, the assay of antibacterial activity of standard bacteria organisms showed that Staphylococcus aureus had the highest susceptibility to coconut oil while Pseudomonas aeroginosa had the least (Table 4). The antibacterial activity of the coconut oil against gram positive and gram negative organisms was consistent with previous research by [8]. The antibacterial activity of ciprofloxacin as a control gave higher zone of inhibition than coconut oil (Figure 1).
The antifungal testing studied showed that Candida albicans had a higher susceptibility to coconut oil more than Aspergillus fumigates (Table 6). The effect of coconut oil on fungal organism is as effective as that of ketoconazole (Figure 2). This correlates with the work of [9-23] in which Candida species were sensitive to 100% coconut oil. Other studies have shown that there might be better activity when these naturals are made into creams.
References
- Bauer AW and Kirby WMM (1996) Antibiotic susceptibility testing by a standardized method. American Journal of Clinical Pathology 45(4): 493-496.
- Konan JL, Bourdeix R, George ML (2008) Regeneration guidelines: Crop specific regeneration guidelines [CD-ROM]. CGIAR System-wide Genetic Resource Programme Rome, Italy p.10.
- Obidoa O, Joshua PE, Eze NJ (2010) Phytochemical Analysis of Cocos Nucifera. Journal of Pharmacy 3 (2): 280-286.
- Jorgensen J, Ferraro M (2009) Antimicrobial susceptibility testing: a review of General principles and contemporary practices. Clinical Infectious Disease 49(11):1749-1755.
- Enig MG (1998) Health and Nutritional Benefits from Coconut Oil PricePottenger Nutrition. Foundation Health Journal 20(1): 1-6.
- Harborne IB (1973) Phytochemical methods: A guide to modern techniques of plant analysis. 2nd edn, Chapman and Hall, New York, USA pp. 88-185.
- (2009) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Clinical and Laboratory Standards Institute Approved standard (8th edn.).
- Crapo T, VeralloRowell VM, Kabara J (2007) Novel antibacterial activity of monolaurin compared with conventional antibiotics against organisms from skin infections. Journal of Drugs Dermatology 6(10): 991-998.
- Ogbolu DO, Oni AA, Oloko AP (2007) In Vitro Antimicrobial Properties of Coconut oil on candida species in Ibadan, Nigeria American Journal of Medicinal Food 10(2): 384-387.
- Odenigbo UM, and Otisi CAO (2011) Fatty acids and phytochemical contents of different coconut seed flesh in Nigeria. International Journal of Plant Physiology and Biochemistry 3(11): 176-182.
- ManishaDeb Mandal, Shyamapada Mandal (2011) Coconut (Cocos nucifera L.: Arecaceae): In health promotion and disease prevention. Asian Pacific Journal of Tropical Medicine 4(3): 241-247.
- Kindersley D (2006) Nutrition for life. Lark and Deen Publishers, UK pp. 213
- James TK, Rahman A (2005) Efficacy of several organic herbicides and glyphosate formulations under simulated rainfall. New Zealand Plant Protection 58: 157-163.
- Fischer CL, Drake DR, Dawson DV, Blanchette DR, Brogden KA, et al. (2012) Antibacterial activity of sphingoid bases and fatty acids against Gram-positive and Gram-negative bacteria. Antimicrobial Agents Chemotherapy 56(3): 1157-116
- Enig M.G (2003) Coconut oil for Health and Vitality Antiviral Antimicrobial and Antiobesity. Coconut Oil-Health and Nutritional Benefits Shirley’s Wellness Café
- Enig M and Fallon (1999) Coconut: In support of good health in the 12st century. The Skinning on fats pp 1-30.
- Edeoga HO, Okwu DE, Mbaebie BO (2005) Phytochemical Constituents of some Nigerian Medicinal Plants. African Journal of Biotechnology 4(7): 685-688.
- Dilhuydy JM (2003) Patients attraction to complementary and alternative Medicine (CAM) A reality which physicians can neither ignore nor deny. Bull Cancer 90(7): 623-628.
- Dichter MA, Delanty N (2000) Antioxidant therapy in neurological disease. Archive of Neurology 57(9): 1265-1270.
- DC Abeysundara, Weerakoon C, Lucas JR, Gunatunga KAI, Obadagee KC (2001) Coconut Oil As An Alternative To Transformer Oil.
- Calbom C, Calbom J (2005) The coconut Diet. Warner Books. USA, P. 7-47.
- British Pharmacopoeia (2001) Introduction general notices and medicinal and pharmaceutical sciences. Her majesty stationary United Kingdom (1): 720-736.
- Brady GS, Clauser HR, Vaccari JA (2002) Materials Handbook An encyclopedia for managers, technical, professional purchasing and production managers, technicians and supervisor (15th edn.): McGraw- Hill, USA, pp. 250-251.
Editorial Manager:
Email:
pediatricdentistry@lupinepublishers.com

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...