
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4544
Research Article(ISSN: 2637-4544) 
Content of Eleven Trace Elements in Thyroid Malignant Nodules and Thyroid Tissue adjacent to Nodules Volume 5 - Issue 1
Vladimir Zaichick*
- Radionuclide Diagnostics Department, Medical Radiological Research Centre, Russia
Received:January 13, 2021Published: January 20, 2021
Corresponding author: Radionuclide Diagnostics Department, Medical Radiological Research Centre, Russia
DOI: 10.32474/IGWHC.2021.05.000204
Abstract
Background: Thyroid malignant nodules (TMNs) are the most common endocrine cancer and the fifth most frequently occurring
type of malignancies. Women are at particular risk for this thyroid disease The etiology and pathogenesis of TMNs must be
considered as multifactorial. The present study was performed to clarify the role of some trace elements (TEs) in the etiology of
these thyroid disorders.
Methods: Thyroid tissue levels of silver (Ag), cobalt (Co), chromium (Cr), iron (Fe), mercury (Hg), iodine (I), rubidium (Rb),
antimony (Sb), scandium (Sc), selenium (Se), and zinc (Zn) were prospectively evaluated in malignant tumor and thyroid tissue
adjacent to tumor of 41 patients with TMNs. Measurements were performed using non-destructive instrumental neutron activation
analysis. Tissue samples were divided into two portions. One was used for morphological study while the other was intended for
TEs analysis. Results of the study were additionally compared with previously obtained data for the same TEs in “normal” thyroid
tissue.
Results: It was observed that in malignant tissue the mass fraction of I was 25.6 times lower, whereas mass fractions of Ag,
Co, Cr, Hg, and Rb were approximately 13, 1.4, 1.6, 20 and 1.7 times, respectively, higher than in normal tissues of the thyroid. In
a general sense Cr, Fe, Sb, Sc, and Zn contents found in the “normal” and “adjacent” groups of thyroid tissue samples were similar.
However, in the “adjacent” group mean mass fractions of Ag, Co, Hg, I, Rb, and Se were approximately 33, 1.8, 52, 1.7, 2.6, and 1.3
times, respectively, higher, than in the “normal” group. Significant reduced levels of tumor TEs in comparison with thyroid tissue
adjacent to tumor were found for Ag, Hg, I, and Se. In malignant tumor Ag, Hg, I, and Se contents were approximately 2.6, 2.6, 43, 1.5,
and 1.5 times, respectively, lower than in “adjacent” group of tissue samples.
Conclusions: Thus, from results obtained, it was possible to conclude that the drastically reduced level of I, as well as elevated
levels of Ag, Co, Cr, Hg, and Rb in cancerous tissue could possibly be explored for differential diagnosis of benign and malignant
thyroid nodules.
Keywords: Thyroid; Thyroid malignant nodules; Trace elements; Neutron activation analysis
Introduction
Thyroid malignant nodules (TMNs) are the most common
endocrine cancer and the fifth most frequently occurring type of
malignancies [1-3]. Women are at particular risk for this thyroid
disease with 22.2/100,000 individuals affected every year [2].
The incidence of TMNs has increased worldwide over the past
four decades. TMNs are divided into three main histological
types: differentiated (papillary and follicular thyroid cancer),
undifferentiated (poorly differentiated and anaplastic thyroid
cancer, and medullary thyroid cancer, arising from C cells of
thyroid [3]. For over 20th century, there was the dominant opinion
that TMNs is the simple consequence of iodine deficiency [4].
However, it was found that TMNs is a frequent disease even in
those countries and regions where the population is never exposed
to iodine shortage. Moreover, it was shown that iodine excess has
severe consequences on human health and associated with the
presence of TMNs [5-8]. It was also demonstrated that besides the
iodine deficiency and excess many other dietary, environmental,
and occupational factors are associated with the TMNs incidence
[9-11]. Among these factors a disturbance of evolutionary stable
input of many trace elements (TEs) in human body after industrial
revolution plays a significant role in etiology of TMNs [12].
Besides iodine, many other TEs have also essential physiological
functions [13]. Essential or toxic (goitrogenic, mutagenic,
carcinogenic) properties of TEs depend on tissue-specific need or tolerance, respectively [13]. Excessive accumulation or an
imbalance of the TEs may disturb the cell functions and may result
in cellular proliferation, degeneration, death, benign or malignant
transformation [13-15]. In our previous studies the complex of
in vivo and in vitro nuclear analytical and related methods was
developed and used for the investigation of iodine and other
TEs contents in the normal and pathological thyroid [16-22].
Iodine level in the normal thyroid was investigated in relation to
age, gender and some non-thyroidal diseases [23,24]. After that,
variations of many TEs content with age in the thyroid of males and
females were studied and age- and gender-dependence of some
TEs was observed [25-41]. Furthermore, a significant difference
between some TEs contents in colloid goiter, thyroiditis, and thyroid
adenoma in comparison with normal thyroid was demonstrated
[42-46]. To date, the etiology and pathogenesis of TMNs must be
considered as multifactorial. The present study was performed to
find out differences in TEs contents between the group of cancerous
tissue and thyroid visually intact tissue adjacent to tumor, as well
as to clarify the role of some TEs in the etiology of TMNs. Having
this in mind, the aim of this exploratory study was to examine
differences in the content of silver (Ag), cobalt (Co), chromium (Cr),
iron (Fe), mercury (Hg), iodine (I), rubidium (Rb), antimony (Sb),
scandium (Sc), selenium (Se), and zinc (Zn) in tumor and adjacent
to tumor tissues of thyroids with TMNs, using a combination of
non-destructive instrumental neutron activation analysis with high
resolution spectrometry of short-lived radionuclides (INAA-SLR)
and long-lived radionuclides (INAA-LLR), and to compare the levels
of these TEs in two groups (tumor and adjacent to tumor thyroid
tissues) of the cohort of TMNs samples. Moreover, for understanding
a possible role of TEs in etiology and pathogenesis of TMNs results
of the study were compared with previously obtained data for the
same TEs in “normal” thyroid tissue [42-46].
Materials and Methods
All patients with TMNs (n=41, mean age M±SD was 46±15 years,
range 16-75) were hospitalized in the Head and Neck Department
of the Medical Radiological Research Centre (MRRC), Obninsk.
Thick-needle puncture biopsy of suspicious nodules of the thyroid
was performed for every patient, to permit morphological study
of thyroid tissue at these sites and to estimate their trace element
contents. In all cases the diagnosis has been confirmed by clinical
and morphological results obtained during studies of biopsy and
resected materials. Histological conclusions for malignant tumors
were: 25 papillary adenocarcinomas, 8 follicular adenocarcinomas,
7 solid carcinomas, and 1 reticulosarcoma. Tissue samples of
tumor and visually intact tissue adjacent to tumor were taken
from resected materials. “Normal” thyroids for the control group
samples were removed at necropsy from 105 deceased (mean age
44 ± 21 years, range 2-87), who had died suddenly. The majority
of deaths were due to trauma. A histological examination in the
control group was used to control the age norm conformity, as well
as to confirm the absence of micro-nodules and latent cancer. All
studies were approved by the Ethical Committees of MRRC. All the
procedures performed in studies involving human participants
were in accordance with the ethical standards of the institutional
and/or national research committee and with the 1964 Helsinki
declaration and its later amendments, or with comparable ethical
standards. Informed consent was obtained from all individual
participants included in the study. All tissue samples obtained from
tumors and visually intact tissue adjacent to tumors were divided
into two portions using a titanium scalpel to prevent contamination
by TEs of stainless steel [47]. One was used for morphological study
while the other was intended for TEs analysis. After the samples
intended for TEs analysis were weighed, they were freeze-dried
and homogenized [48].
To determine contents of the TEs by comparison with a
known standard, biological synthetic standards (BSS) prepared
from phenol-formaldehyde resins were used [49]. In addition to
BSS, aliquots of commercial, chemically pure compounds were
also used as standards. Ten certified reference material IAEA H-4
(animal muscle) and IAEA HH-1 (human hair) sub-samples were
treated and analyzed in the same conditions that thyroid samples to
estimate the precision and accuracy of results. The content of I were
determined by INAA-SLR using a horizontal channel equipped with
the pneumatic rabbit system of the WWR-c research nuclear reactor
(Branch of Karpov Institute, Obninsk). Details of used nuclear
reaction, radionuclide, gamma-energies, spectrometric unit, sample
preparation, and the quality control of results were presented in
our earlier publications concerning the INAA-SLR of I contents in
human thyroid [27,28] and scalp hair [50]. A vertical channel of the
same nuclear reactor was applied to determine the content of Ag,
Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn by INAA-LLR. Details of used
nuclear reactions, radionuclides, gamma-energies, spectrometric
unit, sample preparation and procedure of measurement were
presented in our earlier publications concerning the INAA-LLR
of TEs contents in human thyroid [29,30], scalp hair [50], and
prostate [51,52]. A dedicated computer program for INAA-SLR and
INAA-LLR mode optimization was used [53]. All thyroid samples
for TEs analysis were prepared in duplicate, and mean values of TEs
contents were used in final calculation. Using Microsoft Office Excel
software, a summary of the statistics, including, arithmetic mean,
standard deviation of mean, standard error of mean, minimum and
maximum values, median, percentiles with 0.025 and 0.975 levels
was calculated for TEs contents in nodular and adjacent tissue of
thyroids with TMNs. Data for “normal” thyroid were taken from our
previous publications [42-46]. The difference in the results between
three groups of samples (“normal”, “tumor”, and “adjacent”) was
evaluated by the parametric Student’s t-test and non-parametric
Wilcoxon-Mann-Whitney U-test.
Results
Table 1 presents certain statistical parameters (arithmetic mean, standard deviation, standard error of mean, minimal and maximal values, median, percentiles with 0.025 and 0.975 levels) of the Ag, Co, Cr, Fe, Hg, I, Rb, Sb, Sc, Se, and Zn mass fraction in “normal”, “tumor”, and “adjacent” groups of thyroid tissue samples. The ratios of means and the comparison of mean values of Ag, Co, Cr, Fe, Hg, I, Rb, Sb, Sc, Se, and Zn mass fractions in pairs of sample groups such as “normal” and “tumor”, “normal” and “adjacent”, and also “adjacent” and “tumor” are presented in Table 2, 3, and 4, respectively.
Table 1: Some statistical parameters of Ag, Co, Cr, Fe, Hg, I, Rb, Sb, Sc, Se, and Zn mass fraction (mg/kg, dry mass basis) in normal thyroid and thyroid cancer (tumor and adjacent to tumor “intact” thyroid tissue).
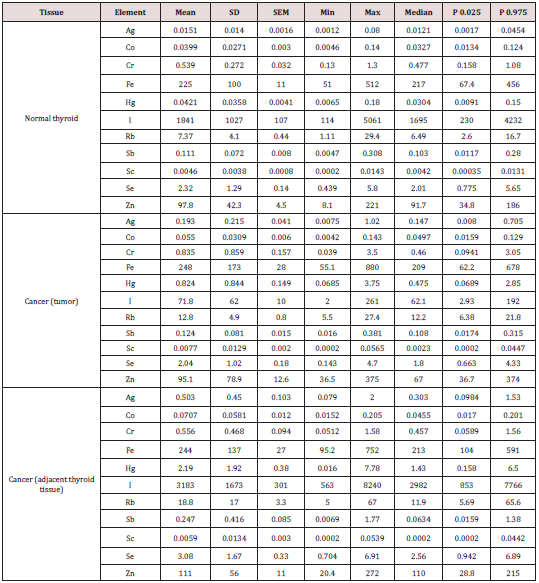
M – arithmetic mean, SD – standard deviation, SEM – standard error of mean, Min – minimum value, Max – maximum value, P 0.025 – percentile with 0.025 level, P 0.975 – percentile with 0.975 level.
Table 2: Differences between mean values (M ± SEM) of Ag, Co, Cr, Fe, Hg, I, Rb, Sb, Sc, Se, and Zn mass fraction (mg/kg, dry mass basis) in normal thyroid and thyroid cancer ((tumor).
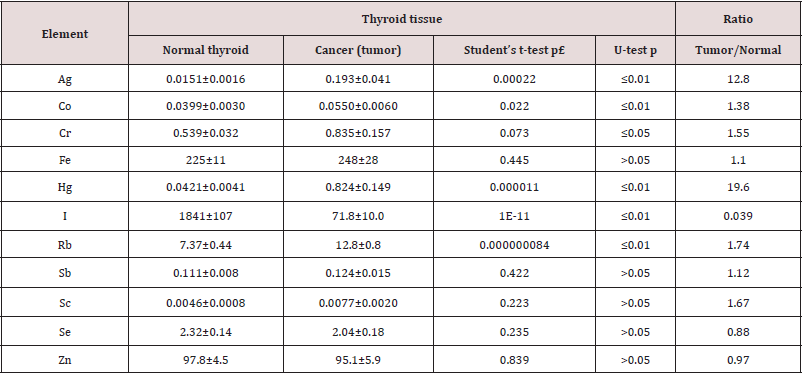
M – arithmetic mean, SEM – standard error of mean, statistically significant values are in bold.
Table 3: Differences between mean values (M± SEM) of Ag, Co, Cr, Fe, Hg, I, Rb, Sb, Sc, Se, and Zn mass fraction (mg/kg, dry mass basis) in normal thyroid and “intact” thyroid tissue adjacent to tumor.
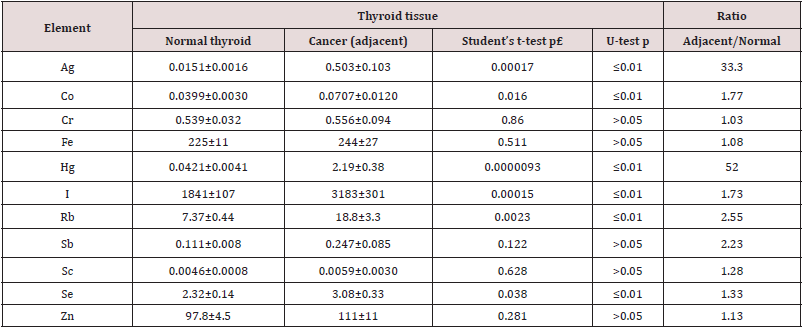
M – arithmetic mean, SEM – standard error of mean, statistically significant values are in bold.
Table 4: Differences between mean values (M ± SEM) of Ag, Co, Cr, Fe, Hg, I, Rb, Sb, Sc, Se, and Zn mass fraction (mg/kg, dry mass basis) in thyroid cancer and “intact” thyroid tissue adjacent to tumor.
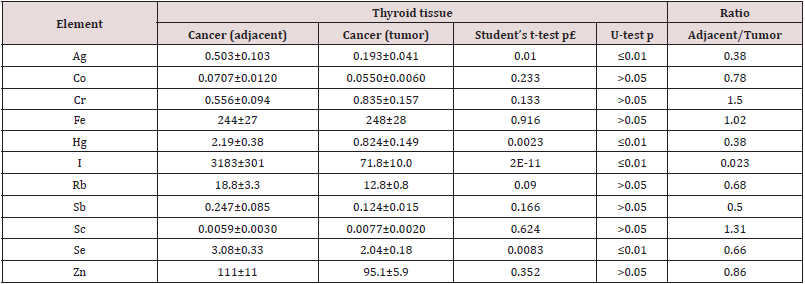
M – arithmetic mean, SEM – standard error of mean, statistically significant values are in bold.
Discussion
As was shown before [27-30,50-52] good agreement of the TEs
contents in CRM IAEA H-4 and CRM IAEA HH-1 samples analyzed
by instrumental neutron activation analysis with the certified
data of these CRMs indicates acceptable accuracy of the results
obtained in the study of “normal”, “tumor”, and “adjacent” groups
of thyroid tissue samples presented in Tables 1-4 From Table 2, it
was observed that in cancerous tissue the mass fraction of I was
25.6 times lower, whereas mass fractions of Ag, Co, Cr, Hg, and Rb
were approximately 13, 1.4, 1.6, 20 and 1.7 times, respectively,
higher than in normal tissues of the thyroid. Thus, if we accept the
TEs contents in thyroid glands in the “normal” group as a norm, we
have to conclude that with a malignant transformation the Ag, Co,
Cr, Hg, I, and Rb in thyroid tissue significantly changed. In a general
sense Cr, Fe, Sb, Sc, and Zn contents found in the “normal” and
“adjacent” groups of thyroid tissue samples were similar (Table 3).
However, in the “adjacent” group mean mass fractions of Ag, Co, Hg,
I, Rb, and Se were approximately 33, 1.8, 52, 1.7, 2.6, and 1.3 times,
respectively, higher, than in the “normal” group. Significant reduced
levels of tumor TEs in comparison with thyroid tissue adjacent
to tumor were found for Ag, Hg, I, and Se. In malignant tumor Ag,
Hg, I, and Se contents were approximately 2.6, 2.6, 43, 1.5, and 1.5
times, respectively, lower than in “adjacent” group of tissue samples
(Table 4).
Characteristically, elevated or reduced levels of TEs observed in
thyroid nodules are discussed in terms of their potential role in the
initiation and promotion of these thyroid lesions. In other words,
using the low or high levels of the TEs in affected thyroid tissues
researchers try to determine the role of the deficiency or excess of
each TE in the etiology and pathogenesis of thyroid diseases. In our
opinion, abnormal levels of many TEs in TMNs could be and cause,
and also effect of thyroid tissue transformation. From the results of
such kind studies, it is not always possible to decide whether the
measured decrease or increase in TEs level in pathologically altered
tissue is the reason for alterations or vice versa. According to our
opinion, investigation of TEs contents in thyroid tissue adjacent
to malignant nodules and comparison obtained results with TEs
levels typical of “normal” thyroid gland may give additional useful
information on the topic because these data show conditions of
tissue in which TMNs were originated and developed. Thus, from
results obtained, it was possible to conclude that the common
characteristics of TMNs in comparison with “normal” thyroid and
visually “intact” thyroid tissue adjacent to malignant tumors were
drastically reduced level of I (Tables 2-4). It meant that thyroid
tissue adjacent to malignant nodules kept the main function of
thyroid gland, while malignantly transformed thyroid cells lost its
capacity to accumulate I. However, the TEs composition of thyroid
tissue adjacent to tumor did not equal TEs contents of “normal”
thyroid (Table 3). Moreover, contents of such elements as Ag, Hg, I,
and Se in adjacent tissue were higher than in tumor (Table 4). From
here, the excessive accumulation of Ag, Hg, I, and Se by thyroid
tissue is likely to precede the TMNs origination and development.
Silver
Ag is a TE with no recognized trace metal value in the human body [54]. Food is the major intake source of Ag and this metal is authorized as a food additive (E174) in the EU [55]. Another source of Ag is contact with skin and mucosal surfaces because Ag is widely used in different applications (e.g., jewelry, wound dressings, or eye drops) [56]. Ag in metal form and inorganic Ag compounds ionize in the presence of water, body fluids or tissue exudates. The silver ion Ag+ is biologically active and readily interacts with proteins, amino acid residues, free anions and receptors on mammalian and eukaryotic cell membranes [57]. Besides such the adverse effects of chronic exposure to Ag as a permanent bluish-gray discoloration of the skin (argyria) or eyes (argyrosis), exposure to soluble Ag compounds may produce other toxic effects, including liver and kidney damage, irritation of the eyes, skin, respiratory, and intestinal tract, and changes in blood cells [58]. Experimental studies shown that Ag nanoparticles may affect thyroid hormone metabolism [59]. More detailed knowledge of the Ag toxicity can lead to a better understanding of the impact on human health, including thyroid function.
Cobalt
Health effects of high Co occupational, environmental, dietary and medical exposure are characterized by a complex clinical syndrome, mainly including neurological, cardiovascular and endocrine deficits, including hypothyroidism [60,61]. Co is genotoxic and carcinogenic, mainly caused by oxidative DNA damage by reactive oxygen species, perhaps combined with inhibition of DNA repair [62]. In our previous studies it was found a significant agerelated increase of Co content in female thyroid [29]. Therefore, a goitrogenic and, probably, carcinogenic effect of excessive Co level in the thyroid of old females was assumed. Elevated level of Coin TMNs, observed in the present study, supports this conclusion. Anyway, the accumulation of Coin malignant thyroid tumors could possibly be explored for diagnosis of TMNs.
Chromium
The general population can be exposed to low levels of Cr primarily through consumption of food and to a lesser degree through inhalation of ambient air and ingestion of drinking water [63]. Cr-compounds are cytotoxic, genotoxic, and carcinogenic in nature. Some Cr forms, including hexavalent chromium (Cr6+), are toxicants known for their carcinogenic effect in humans. They have been classified as certain or probable carcinogens by the International Agency for Research on Cancer [64]. The lung cancer risk is prevalent in pigment chromate handlers, ferrochromium production workers, stainless steel welders, and chrome-platers [65]. Except in Cr-related industries and associated environments, Cr intoxication from environmental exposure is not common. However, it was found, that drinking water supplies in many geographic areas contain chromium in the +3 and +6 oxidation states. Exposure of animals to Cr6+ in drinking water induced tumors in the mouse small intestine [66]. Many other animal experiments and in vitro studies demonstrate also that Cr can induce oxidative stress and exert cytotoxic effects [67]. Besides reactive oxygen species (ROS) generation, oxidative stress, and cytotoxic effects of Cr exposure, a variety of other changes like DNA damage, increased formation of DNA adducts and DNA-protein cross-links, DNA strand breaks, chromosomal aberrations and instability, disruption of mitotic cell division, chromosomal aberration, premature cell division, S or G2/M cell cycle phase arrest, and carcinogenesis also occur in humans or experimental test systems [65]. Anyway, the accumulation of Cr in malignant thyroid tumors could possibly be explored for diagnosis of TMNs.
Mercury
In the general population, potential sources of Hg exposure include the inhalation of this metal vapor in the air, ingestion of contaminated foods and drinking water, and exposure to dental amalgam through dental care [68]. Hg is one of the most dangerous environmental pollutants [69]. The growing use of this metal in diverse areas of industry has resulted in a significant increase of environment contamination and episodes of human intoxication. Many experimental and occupational studies of Hg in different chemical states shown significant alterations in thyroid hormones metabolism and thyroid gland parenchyma [70,71]. Moreover, Hg was classified as certain or probable carcinogen by the International Agency for Research on Cancer [72]. For example, in Hg polluted area thyroid cancer incidence was almost 2 times higher than in adjacent control areas [73].
Iodine
To date, it was well established that iodine deficiency or excess has severe consequences on human health and associated with the presence of TMNs [4-8,74-76]. In present study elevated level of I in thyroid tissue adjacent to malignant tumor and drastically reduced I mass fraction in cancerous tissue was found in comparison with “normal” thyroid. Compared to other soft tissues, the human thyroid gland has higher levels of I, because this element plays an important role in its normal functions, through the production of thyroid hormones (thyroxin and triiodothyronine) which are essential for cellular oxidation, growth, reproduction, and the activity of the central and autonomic nervous system. As was shown in present study, malignant transformation is accompanied by a significant loss of tissue-specific functional features, which leads to a drastically reduction in I content associated with functional characteristics of the human thyroid tissue. On the one hand, significantly high level of I in thyroid tissue adjacent to malignant tumor may indicate an involvement of I excess in carcinogenesis. But, on the other hand, because the malignant part of gland stopped to produce thyroid hormones, the rest “intact” part of thyroid tries to compensate thyroid hormones deficiency and work more intensive than usual. The intensive work may explain elevated level of I in thyroid tissue adjacent to malignant tumor in comparison with thyroid tissue of “normal” gland. Drastically reduced level of I content in cancerous tissue could possibly be explored for differential diagnosis of benign and malignant thyroid nodules, because, as was found in our earlier studies, thyroid benign transformation (goiter, thyroiditis, and adenoma) is accompanied by a little loss of I accumulation [42-46].
Rubidium
There is very little information about Rb effects on thyroid function. Rb as a monovalent cation Rb+ is transferred through membrane by the Na+K+-ATPase pump like K+ and concentrated in the intracellular space of cells. Thus, Rb seems to be more intensively concentrated in the intracellular space of cells. The source of Rb elevated level in tumor and adjacent to tumor tissue may be Rb environment overload. The excessive Rb intake may result a replacement of medium potassium by Rb, which effects on iodide transport and iodoaminoacid synthesis by thyroid [77]. The source of Rb increase in TMNs tissue may be not only the excessive intake of this TE in organism from the environment, but also changed Na+K+ -ATPase or H+K+ - ATPase pump membrane transport systems for monovalent cations, which can be stimulated by endocrine system, including thyroid hormones [78]. It was found also that Rb has some function in immune responce [79] and that elevated concentration of Rb could modulate proliferative responses of the cell, as was shown for bone marrow leukocytes [80]. These data partially clarify the possible role of Rb in etiology and pathogenesis of TMNs.
Selinium
The high level of Se content found just in thyroid tissue adjacent to malignant tumor cannot be regarded as pure chance. The selenoprotein characterized as Se-dependent glutathione peroxidase (Se- GSH-Px) is involved in protecting cells from peroxidative damage. This enzyme may reduce tissue concentration of free radicals and hydroperoxides. It is particular important for the thyroid gland, because thyroidal functions involve oxidation of iodide, which is incorporated into thyroglobulin, the precursor of the thyroid hormones. For oxidation of iodide thyroidal cells produce a specific thyroid peroxidase using of physiologically generated hydrogenperoxide (H2O2) as a cofactor [81]. It follows that the thyroid parenchyma must be continuously exposed to a physiological generation of H2O2 and in normal conditions must be a balance between levels of Se (as Se-GSH-Px) and H2O2. The elevated level of Se in thyroid tissue adjacent to malignant nodules was accompanied excessive accumulation of Ag, Co, Hg, I, and Rb in comparison with “normal” values for these elements. Moreover, contents of Ag, Co, Hg, I, and Rb in adjacent tissue were higher than in malignant nodules. Thus, it might be assumed that the elevated level of Se is reaction of adjacent tissue on an increase in concentration of free radicals and hydroperoxides in thyroid gland and that this increase preceded the TMNs origination and development.
Limitations
This study has several limitations. Firstly, analytical techniques employed in this study measure only eleven TEs (Ag, Co, Cr, Fe, Hg, I, Rb, Sb, Sc, Se, and Zn) mass fractions. Future studies should be directed toward using other analytical methods which will extend the list of TEs investigated in “normal” thyroid and in pathologically altered tissue. Secondly, the sample size of TMNs group was relatively small and prevented investigations of TEs contents in this group using differentials like gender, histological types of TMNs, tumor functional activity, stage of disease, and dietary habits of patients with TMNs. Lastly, generalization of our results may be limited to Russian population. Despite these limitations, this study provides evidence on many TEs level alteration in malignant tumor and adjacent to tumor tissue and shows the necessity to continue TEs research of TMNs.
Conclusion
In this work, TEs analysis was carried out in the tissue samples of TMNs using neutron activation analysis. It was shown that neutron activation analysis is an adequate analytical tool for the non-destructive determination of Ag, Co, Cr, Fe, Hg, I, Rb, Sb, Sc, Se, and Zn content in the tissue samples of human thyroid in norm and pathology, including needle-biopsy specimens. It was observed that in malignant tissue the mass fraction of I was 25.6 times lower, whereas mass fractions of Ag, Co, Cr, Hg, and Rb were approximately 13, 1.4, 1.6, 20 and 1.7 times, respectively, higher than in normal tissues of the thyroid. In a general sense Cr, Fe, Sb, Sc, and Zn contents found in the “normal” and “adjacent” groups of thyroid tissue samples were similar. However, in the “adjacent” group mean mass fractions of Ag, Co, Hg, I, Rb, and Se were approximately 33, 1.8, 52, 1.7, 2.6, and 1.3 times, respectively, higher, than in the “normal” group. Significant reduced levels of tumor TEs in comparison with thyroid tissue adjacent to tumor were found for Ag, Hg, I, and Se. In malignant tumor Ag, Hg, I, and Se contents were approximately 2.6, 2.6, 43, 1.5, and 1.5 times, respectively, lower than in “adjacent” group of tissue samples. It was supposed that the drastically reduced level of I, as well as elevated levels of Ag, Co, Cr, Hg, and Rb in cancerous tissue could possibly be explored for differential diagnosis of benign and malignant thyroid nodules.
Acknowledgements
The author is extremely grateful to Profs. B.M. Vtyurin and V.S. Medvedev, Medical Radiological Research Center, Obninsk, as well as to Dr. Yu. Choporov, former Head of the Forensic Medicine Department of City Hospital, Obninsk, for supplying thyroid samples.
Funding
There were not any sources of funding that have supported this work.
Conflict of Interest
The author has not declared any conflict of interests.
References
- Laha D, Nilubol N, Boufraqech M (2020) New therapies for advanced thyroid cancer. Front Endocrinol (Lausanne) 11: 82.
- Buczyńska A, Sidorkiewicz I, Rogucki M, Siewko K, Adamska A, et al. (2021) Oxidative stress and radioiodine treatment of differentiated thyroid cancer. Sci Rep 11: 17126.
- Prete A, Borges de Souza P, Censi S, Muzza M, Nucci N, et al. (2020) Update on Fundamental Mechanisms of Thyroid Cancer. Front Endocrinol (Lausanne) 11: 102.
- Barrea L, Gallo M, Ruggeri RM, Di Giacinto P, Sesti F, et al. (2021) Nutritional status and follicular-derived thyroid cancer: An update. Crit Rev Food Sci Nutr 61(1): 25-59.
- Zaichick V (1998) Iodine excess and thyroid cancer. J Trace Elem Exp Med 11(4): 508-509.
- Zaichick V, Iljina T (1998) Dietary iodine supplementation effect on the rat thyroid 131I blastomogenic action. In: Die Bedentung der Mengen-und Spurenelemente.18. Arbeitstangung. Jena:Friedrich-Schiller-Universität pp: 294-306.
- Kim K, Cho SW, Park YJ, Lee KE, Lee D W, et al. (2021) Association between iodine intake, thyroid function, and papillary thyroid cancer: A case-control study. Endocrinol Metab (Seoul) 36(4): 790-799.
- Vargas Uricoechea Р, Pinzón Fernández MV, Bastidas Sánchez BE, Jojoa Tobar E, Ramírez Bejarano LE, et al. (2019) Iodine status in the colombian population and the impact of universal salt iodization: a double-edged sword? J Nutr Metab 2019:6239243.
- Stojsavljević A, Rovčanin B, Krstić D, Borković Mitić S, Paunović I, et al. (2019) Risk assessment of toxic and essential trace metals on the thyroid health at the tissue level: The significance of lead and selenium for colloid goiter disease. Expo Health.
- Fahim YA, Sharaf NE, Hasani IW, Ragab EA, Abdelhakim HK (2020) Assessment of thyroid function and oxidative stress state in foundry workers exposed to lead. J Health Pollut 10(27): 200903.
- Liu M, Song J, Jiang Y, Lin Y, Peng J, et al. (2021) A case-control study on the association of mineral elements exposure and thyroid tumor and goiter. Ecotoxicol Environ Saf 208: 111615.
- Zaichick V (2006) Medical elementology as a new scientific discipline. J Radioanal Nucl Chem 269: 303-309.
- Moncayo R, Moncayo H (2017) A post-publication analysis of the idealized upper reference value of 2.5 mIU/L for TSH: Time to support the thyroid axis with magnesium and iron especially in the setting of reproduction medicine. BBA Clin 7: 115-119.
- Beyersmann D, Hartwig A (2008) Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol 82(8): 493-512.
- Martinez Zamudio R, Ha HC (2011) Environmental epigenetics in metal exposure. Epigenetics 6(7): 820-827.
- Zaĭchik V, Raibukhin YuS, Melnik AD, Cherkashin VI. (1970) Neutron-activation analysis in the study of the behavior of iodine in the organism. Med Radiol (Mosk) 15(1): 33-36.
- Zaĭchik V, Matveenko EG, Vtiurin BM, Medvedev VS (1982) Intrathyroid iodine in the diagnosis of thyroid cancer. Vopr Onkol 28(3): 18-24.
- Zaichick V, Tsyb AF, Vtyurin BM (1995) Trace elements and thyroid cancer. Analyst 120(3): 817-821.
- Zaichick V, Choporov YuYa (1996) Determination of the natural level of human intra-thyroid iodine by instrumental neutron activation analysis. J Radioanal Nucl Chem 207(1): 153-161.
- Zaichick V (1998) In vivo and in vitro application of energy dispersive XRF in clinical investigations: experience and the future. J Trace Elem Exp Med 11(4): 509-510.
- Zaichick V, Zaichick S (1999) Energy-dispersive X-ray fluorescence of iodine in thyroid puncture biopsy specimens. J Trace Microprobe Tech 17(2): 219-232.
- Zaichick V (2000) Relevance of, and potentiality for in vivo intrathyroidal iodine determination. Ann N Y Acad Sci 904: 630-632.
- Zaichick V, Zaichick S (1997) Normal human intrathyroidal iodine. Sci Total Environ 206(1): 39-56.
- Zaichick V (1999) Human intrathyroidal iodine in health and non-thyroidal disease. In: New aspects of trace element research (Eds: M.Abdulla, M.Bost, S.Gamon, P.Arnaud, G.Chazot). London: Smith-Gordon; and Tokyo: Nishimura 1999:114-119.
- Zaichick V, Zaichick S (2017) Age-related changes of some trace element contents in intact thyroid of females investigated by energy dispersive X-ray fluorescent analysis. Trends Geriatr Healthc 1(1): 31-38.
- Zaichick V, Zaichick S (2017) Age-related changes of some trace element contents in intact thyroid of males investigated by energy dispersive X-ray fluorescent analysis. MOJ Gerontol Ger 1(5): 00028.
- Zaichick V, Zaichick S (2017) Age-related changes of Br, Ca, Cl, I, K, Mg, Mn, and Na contents in intact thyroid of females investigated by neutron activation analysis. Curr Updates Aging 1: 5-1.
- Zaichick V, Zaichick S (2017) Age-related changes of Br, Ca, Cl, I, K, Mg, Mn, and Na contents in intact thyroid of males investigated by neutron activation analysis. J Aging Age Relat Dis 1(1): 1002.
- Zaichick V, Zaichick S (2017) Age-related changes of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn contents in intact thyroid of females investigated by neutron activation analysis. J Gerontol Geriatr Med 3: 015.
- Zaichick V, Zaichick S (2017) Age-related changes of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn contents in intact thyroid of males investigated by neutron activation analysis. Curr Trends Biomedical Eng Biosci 4(4): 555644.
- Zaichick V, Zaichick S (2018) Effect of age on chemical element contents in female thyroid investigated by some nuclear analytical methods. Micro Medicine 6(1): 47-61.
- Zaichick V, Zaichick S (2018) Neutron activation and X-ray fluorescent analysis in study of association between age and chemical element contents in thyroid of males. Op Acc J Bio Eng Bio Sci 2(4): 202-212.
- Zaichick V, Zaichick S (2018) Variation with age of chemical element contents in females’ thyroids investigated by neutron activation analysis and inductively coupled plasma atomic emission spectrometry. J Biochem Analyt Stud 3(1): 1-10.
- Zaichick V, Zaichick S (2018) Association between age and twenty chemical element contents in intact thyroid of males. SM Gerontol Geriatr Res 2(1): 1014.
- Zaichick V, Zaichick S (2018) Associations between age and 50 trace element contents and relationships in intact thyroid of males. Aging Clin Exp Res 30(9): 1059-1070.
- Zaichick V, Zaichick S (2018) Possible role of inadequate quantities of intra-thyroidal bromine, rubidium and zinc in the etiology of female subclinical hypothyroidism. EC Gynaecology 7(3): 107-115.
- Zaichick V, Zaichick S (2018) Possible role of inadequate quantities of intra-thyroidal bromine, calcium and magnesium in the etiology of female subclinical hypothyroidism. Int Gyn and Women’s Health 1(3): IGWHC.MS.ID.000113.
- Zaichick V, Zaichick S (2018) Possible role of inadequate quantities of intra-thyroidal cobalt, rubidium and zinc in the etiology of female subclinical hypothyroidism. Women’s Health Sci J 2(1): 000108.
- Zaichick V, Zaichick S (2018) Association between female subclinical hypothyroidism and inadequate quantities of some intra-thyroidal chemical elements investigated by X-ray fluorescence and neutron activation analysis. Gynaecology and Perinatology 2(4): 340-355.
- Zaichick V, Zaichick S (2018) Investigation of association between the high risk of female subclinical hypothyroidism and inadequate quantities of twenty intra-thyroidal chemical elements. Clin Res: Gynecol Obstet 1(1): 1-18.
- Zaichick V, Zaichick S (2018) Investigation of association between the high risk of female subclinical hypothyroidism and inadequate quantities of intra-thyroidal trace elements using neutron activation and inductively coupled plasma mass spectrometry. Acta Scientific Medical Sciences 2(9): 23-37.
- Zaichick V (2021) Comparison between Trace Element Contents in Macro and Micro Follicular Colloid Goiter using Neutron Activation Analysis. Journal of Clinical Research and Clinical Case Reports 2(2): 1-7.
- Zaichick V (2021) Trace Element Contents in Thyroid of Patients with Diagnosed Nodular Goiter Investigated by Instrumental Neutron Activation Analysis. Journal of Medical Research and Health Sciences 4(8): 1405-1417.
- Zaichick V (2021) Comparison of Trace Element Contents in Normal and Adenomatous Thyroid investigated using Instrumental Neutron Activation Analysis. Saudi J Biomed Res 6(11): 246-255.
- Zaichick V (2021) Evaluation of Ten Trace Elements in Riedel’s Struma using Neutron Activation Analysis. Mod Res Clin Canc Prev 1(1): 1-6.
- Zaichick V (2021) Comparison of Trace Element Contents in Normal Thyroid and Thyroid with Hashimoto's thyroiditis using Neutron Activation Analysis. World Journal of Advanced Research and Reviews 12(01): 503-511.
- Zaichick V, Zaichick S (1996) Instrumental effect on the contamination of biomedical samples in the course of sampling. The Journal of Analytical Chemistry 51(12): 1200-1205.
- Zaichick V, Zaichick S (1997) A search for losses of chemical elements during freeze-drying of biological materials. J Radioanal Nucl Chem 218(2): 249-253.
- Zaichick V (1995) Applications of synthetic reference materials in the medical Radiological Research Centre. Fresenius J Anal Chem 352: 219-223.
- Zaichick S, Zaichick V (2010) The effect of age and gender on 37 chemical element contents in scalp hair of healthy humans. Biol Trace Elem Res 134(1): 41-54.
- Zaichick S, Zaichick V (2011) The effect of age on Ag, Co, Cr, Fe, Hg, Sb, Sc, Se, and Zn contents in intact human prostate investigated by neutron activation analysis. Appl Radiat Isot 69(6): 827-833.
- Zaichick V, Zaichick S (2013) INAA application in the assessment of Ag, Co, Cr, Fe, Hg, Rb, Sb, Sc, Se, and Zn mass fraction in pediatric and young adult prostate glands. J Radioanal Nucl Chem 298(3): 1559-1566.
- Korelo AM, Zaichick V (1993) Software to optimize the multielement INAA of medical and environmental samples. In: Activation Analysis in Environment Protection. Dubna, Russia: Joint Institute for Nuclear Research 1993: 326-332.
- Lansdown AB (2007) Critical observations on the neurotoxicity of silver. Crit Rev Toxicol 37(3): 237-250.
- De Vos S, Waegeneers N, Verleysen E, Smeets K, Mast J (2020) Physico-chemical characterisation of the fraction of silver (nano)particles in pristine food additive E174 and in E174-containing confectionery. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 37(11): 1831-1846.
- Hadrup N, Sharma AK, Loeschner K (2018) Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul Toxicol Pharmacol 98: 257-267.
- Lansdown AB (2006) Silver in health care: antimicrobial effects and safety in use. Curr Probl Dermatol 33: 17-34.
- Drake PL, Hazelwood KJ (2005) Exposure-related health effects of silver and silver compounds: a review. Ann Occup Hyg 49(7):575-585.
- Katarzyńska Banasik D, Grzesiak M, Kowalik K, Sechman A (2021) Administration of silver nanoparticles affects ovarian steroidogenesis and may influence thyroid hormone metabolism in hens (Gallus domesticus). Ecotoxicol Environ Saf 208: 111427.
- Leyssens L, Vinck B, Van Der Straeten C, Wuyts F, Maes L (2017) Cobalt toxicity in humans-A review of the potential sources and systemic health effects. Toxicology 387: 43-56.
- Yu R (2017) Cobalt Toxicity, An overlooked Cause of Hypothyroidism. J Endocrinol Thyroid Res 1(3):1-4.
- Simonsen LO, Harbak H, Bennekou P (2012) Cobalt metabolism and toxicology--a brief update. Sci Total Environ 432: 210-215.
- Linos A, Petralias A, Christophi CA, Christoforidou E, Kouroutou P, et al. (2011) Oral ingestion of hexavalent chromium through drinking water and cancer mortality in an industrial area of Greece--an ecological study. Environ Health 10: 50.
- Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68: 167-182.
- Nigam A, Priya S, Bajpai P, Kumar S (2014) Cytogenomics of hexavalent chromium (Cr 6+) exposed cells: a comprehensive review. Indian J Med Res 139(3): 349-370.
- Zhitkovich A (2011) Chromium in drinking water: sources, metabolism, and cancer risks. Chem Res Toxicol 4(10): 1617-1629.
- Ding SZ, Yang YX, Li XL, Michelli Rivera A, Han SY, et al. (2013) Epithelial-mesenchymal transition during oncogenic transformation induced by hexavalent chromium involves reactive oxygen species-dependent mechanism in lung epithelial cells. Toxicol Appl Pharmacol 269(1): 61-71.
- Kim S A, Kwon YM, Kim S, Joung H (2016) Assessment of dietary mercury intake and blood mercury levels in the Korean population: Results from the Korean National Environmental Health Survey 2012–2014. Int J Environ Res Public Health 13(9): 877.
- Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36: 609-662.
- Correia MM, Chammas MC, Zavariz JD, Arata A, Martins LC, et al. (2020) Evaluation of the effects of chronic occupational exposure to metallic mercury on the thyroid parenchyma and hormonal function. Int Arch Occup Environ Health 93(4): 491-502.
- Hu O, Han X, Dong G, Yan W, Wang X, et al. (2021) Association between mercury exposure and thyroid hormones levels: A meta-analysis. Environ Res 196: 110928.
- Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68: 167-182.
- Malandrino P, Russo M, Ronchi A, Minoia C, Cataldo D, et al. (2016) Increased thyroid cancer incidence in a basaltic volcanic area is associated with non-anthropogenic pollution and biocontamination. Endocrine 53(2): 471-479.
- Leung AM, Braverman LE (2014) Consequences of excess iodine. Nat Rev Endocrinol 10(3): 136-142.
- Lee J H, Hwang Y, Song R Y, Yi JW, Yu HW, et al. (2017) Relationship between iodine levels and papillary thyroid carcinoma: A systematic review and meta-analysis. Head Neck 39(8): 1711-1718.
- Aakre I, Evensen LT, Kjellevold M, Dahl L, Henjum S, et al. (2020) Iodine status and thyroid function in a group of seaweed consumers in Norway. Nutrients 12(11): 3483.
- Haibach H, Greer MA (1973) Effect of replacement of medium potassium by sodium, cesium or rubidium on in vitro iodide transport and iodoamino acid synthesis by rat thyroid. Proc Soc Exp Biol Med 143(1): 114-117.
- York DA, Bray GA, Yukimura Y (1978) An enzymatic defect in the obese (ob/ob) mouse: Loss of thyroid-induced sodium- and potassium-dependent adenosinetriphosphatase. Proc Natl Acad Sci USA 75(1): 477-481.
- Jones JM, Yeralan O, Hines G, Maher M, Roberts DW, et al. (1990) Effects of lithium and rubidium on immune responses of rats. Toxicology Letters 52(2): 163-168.
- Petrini M, Vaglini F, Carulli G, Azzarà A, Ambrogi F, et al. (1990) Rubidium is a possible supporting element for bone marrow leukocyte differentiation. Haematologica 75(1): 27-31.
- Aaseth J, Frey H, Glattre E, Norheim G, Ringstad J, Thomassen Y (1990) Selenium concentrations in the human thyroid gland. Biol Trace Elem Res 24(2-3): 147-152.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...





