
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4579
Research Article(ISSN: 2637-4579) 
Neutron Activation and X-Ray Fluorescent Analysis in Study of Association Between Age and Chemical Element Contents in Thyroid of Males Volume 2 - Issue 4
Dr. Vladimir Zaichick1* and FRS Sofia Zaichick2
- 1 PhD, DSc, CChem, Professor, Radionuclide Diagnostics Department, Medical Radiological Research Centre, Russia
- 2 MD, PhD Laboratory of Dr. Gabriela Caraveo Piso, Feinberg School of Medicine, Northwestern University, Chicago, USA
Received: June 11, 2018; Published: July 17, 2018
Corresponding author: Dr. Vladimir Zaichick, PhD, DSc, CChem, Professor, Radionuclide Diagnostics Department, Medical Radiological Research Centre, 249036 Kaluga Region, Russia, Tel: +7 (48439) 60289; Email: vzaichick@gmail.com/vezai@obninsk.com
DOI: 10.32474/OAJBEB.2018.02.000144
Abstract
A prevalence of thyroid dysfunction is higher in the elderly as compared to the younger population. An excess or deficiency of chemical element contents in thyroid may play important role in goitro- and carcinogenesis of gland. The variation with age of the mass fraction of twenty chemical elements (Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn) in intact (normal) thyroid of 72 males (mean age 37.8 years, range 2-80 years) was investigated by energy dispersive X-ray fluorescent analysis and instrumental neutron activation analysis with high resolution spectrometry of short- and long-lived radionuclides. This work revealed that there is a statistically significant increase in Ca, I, and Se mass fraction, as well as a decrease in K and Mn mass fraction in the normal thyroid of male during a lifespan. Moreover, a disturbance of intra-thyroidal chemical element relationships with increasing age was found. Therefore, a goitrogenic and carcinogenic effect of inadequate Ca, I, K, Mn, and Se level in the thyroid of old males and a harmful impact of disturbance in intra-thyroidal chemical element relationships with increasing age may be assumed.
Keywords: Thyroid, Chemical elements, Age-related changes, X-ray fluorescent analysis, Neutron activation analysis.
Abbrevations: ROS: Reactive Oxygen Species, CRM/SRM: Certified/Standard Reference Materials, PEDXRF: Energy Dispersive X-Ray Fluorescent Analysis, INAA-SLR: Instrumental Neutron Activation Analysis with High Resolution Spectrometry of Short-Lived Radionuclides, INAA-LLR: Instrumental Neutron Activation Analysis with High Resolution Spectrometry of Short- and Long-Lived Radionuclides
Introduction
The endocrine organs, including the thyroid gland, undergo important functional changes during aging and a prevalence of thyroid dysfunction is higher in the elderly as compared to the younger population [1,2]. Advancing age is known to influence the formation of adenomatous goiter and thyroid cancer [3]. The prevalence of thyroid nodules is increased in the elderly, reaching a frequency of nearly 50% by the age of 65 [4]. Both prevalence and aggressiveness of thyroid cancer increase with age [1]. Women are affected by thyroid nodule and cancer two to five times more often than men, but in age over 65 years a prevalence of thyroid cancer may be higher in men [1,3-5]. Aging is characterized by progressive impairment of body functions caused by the accumulation of molecular damage in DNA, proteins and lipids, is also characterized by an increase in intracellular oxidative stress due to the progressive decrease of the intracellular reactive oxygen species (ROS) scavenging [6,7]. Oxidative damage to cellular macromolecules which induce age-related diseases, including cancer, can also arise through overproduction of ROS and faulty antioxidant and/or DNA repair mechanisms [8]. Overproduction of ROS is associated with stress, inflammation, radiation, and some other factors, including overload of certain chemical elements, in both blood and certain tissues, or deficiency of other chemical elements with antioxidant properties [9-15]. The imbalance in the composition of chemical elements in cells, tissues and organs may cause different types of pathology. The importance of appropriate levels of many chemical elements is indisputable, due to their beneficial roles when present in specific concentration ranges, while on the other hand they can cause toxic effects with excessively high or low concentrations [12].
In our previous studies [16-23] the high mass fraction of iodine and some other chemical element were observed in intact human thyroid gland when compared with their levels in non-thyroid soft tissues of the human body. However, the age-dependence of chemical element mass fraction in thyroid of adult and, particularly, elderly males is still need to be evaluated. One valuable way to elucidate the situation is to compare the mass fractions of chemical elements in young adult (the control group) with those in older adult and geriatric thyroid. The findings of the excess or deficiency of chemical element contents in thyroid and the perturbations of their relative proportions in glands of adult and elderly males may indicate their roles in a higher prevalence of thyroid dysfunction in the elderly population [24].
The reliable data on chemical element mass fractions in normal geriatric thyroid is apparently extremely limited. There are multiple studies reporting chemical element content in human thyroid, using chemical techniques and instrumental methods [25-42]. However, majority of the analytical methods currently used and validated for the determination of major and trace elements in thyroid and other human organs are based on techniques requiring sample digestion. The most frequently used digestion procedures are the traditional dry ashing and high-pressure wet digestion that cause destruction of organic matter of the sample. Sample digestion is a critical step in elemental analysis and due to the risk of contamination and analytes loss, a digestion step contributes to the systematic uncontrolled analysis errors [43-45]. Moreover, only a few of the previous studies employed quality control using certified/standard reference materials (CRM/SRM) for determination of the chemical element mass fractions. Therefore, sample-nondestructive technique like energy dispersive X-ray fluorescent analysis (EDXRF) as well as instrumental neutron activation analysis with high resolution spectrometry of short- and long-lived radionuclides (INAA-SLR and INAA-LLR, respectively) combined with a quality assurance using CRM/SRM is good alternatives for multi-element determination in the samples of thyroid parenchyma.
There were three aims in this study. The primary purpose of the study was to determine reliable values for chemical element mass fractions in the normal (intact) thyroid of subjects ranging from children to elderly males using EDXRF, INAA-SLR, and INAALLR. The second aim was to compare the chemical mass fractions determined in thyroid gland of age group 2 (adults and elderly persons aged 36 to 80 years), with those of group 1 (from 2 to 35 years) as well as to find the correlations between age and chemical element contents, and the final aim was to find the inter-correlations of chemical elements in normal thyroid of males and their changes with age. All studies were approved by the Ethical Committee of the Medical Radiological Research Centre. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Materials and Methods
Samples of the human thyroid were obtained from randomly selected autopsy specimens of 72 males (European-Caucasian) aged 2 to 80 years. All the deceased were citizens of Obninsk and had undergone routine autopsy at the Forensic Medicine Department of City Hospital, Obninsk. Subjects were divided into two age groups, group 1 with 2-35 years (22.3±1.4 years, M±SEM, n=36) and group 2 with 36–80 years (53.3±2.5 years, M±SEM, n=36). These groups were selected to reflect the condition of thyroid tissue in the children, teenagers, young adults and first period of adult life (group 1) and in the second period of adult life as well as in old age (group 2). The available clinical data were reviewed for each subject. None of the subjects had a history of an intersex condition, endocrine disorder, or other chronic disease that could affect the normal development of the thyroid. None of the subjects were receiving medications or used any supplements known to affect thyroid chemical element contents. The typical causes of sudden death of most of these subjects included trauma or suicide and also acute illness (cardiac insufficiency, stroke, embolism of pulmonary artery, alcohol poisoning).
All right lobes of thyroid glands were divided into two portions using a titanium scalpel [46]. One tissue portion was reviewed by an anatomical pathologist while the other was used for the chemical element content determination. A histological examination was used to control the age norm conformity as well as the unavailability of micro adenomatosis and latent cancer. After the samples intended for chemical element analysis were weighed, they were transferred to -20°C and stored until the day of transportation in the Medical Radiological Research Center, Obninsk, where all samples were freeze-dried and homogenized [47-49]. For EDXRF the pounded sample weighing about 8 mg was applied to the piece of Scotch tape serving as an adhesive fixing backing [50,51]. To determine the contents of the elements by comparison with a known standard, aliquots of commercial, chemically pure compounds were used [52]. The microliter standards prepared from aliquots of commercially available pure compounds were placed on disks made of thin, ashfree filter papers fixed on the Scotch tape pieces and dried in a vacuum.
The sample weighing about 100 mg was used for chemical element measurement by INAA-SLR. The samples for INAASLR were sealed separately in thin polyethylene films washed beforehand with acetone and rectified alcohol. The sealed samples were placed in labeled polyethylene ampoules. Biological synthetic standards (BSS) prepared from phenol-formaldehyde resins were used as standards [52]. In addition to BSS, aliquots of commercially available pure compounds were also used. The sample weighing about 50 mg was used for trace element measurement by INAALLR. The samples for INAA-LLR were wrapped separately in a highpurity aluminum foil washed with rectified alcohol beforehand and placed in a nitric acid-washed quartz ampoule. BSS were used as standards [52]. Ten subsamples of the Certified Reference Materials (CRM) IAEA H-4 (animal muscle) and IAEA HH-1 (human hair) were analyzed to estimate the precision and accuracy of results obtained by EDXRF, INAA-SLR, and INAA-LLR. In each method the CRMs subsamples were prepared and analyzed in the same way as the samples of thyroid tissue.
The facility for EDXRF included an annular 109Cd source with an activity of 2.56 G Bq, Si (Li) detector and portable multichannel analyzer combined with a PC. Its resolution was 270 eV at the 5.9 keV line of 55Fe-source. The duration of the Br, Cu, Fe, Rb, Sr, and Zn measurements was 60 min. The intensity of Kα-line of Br, Cu, Fe, Rb, Sr, and Zn for samples and standards was estimated on calculation basis of the total area of the corresponding photopeak in the spectra. The trace element content was calculated by the relative way of comparing between intensities of Kα-lines for samples and standards. More details of the facility and method of analysis were presented in our previous publication [50,51]. A horizontal channel equipped with the pneumatic rabbit system of the WWR-c research nuclear reactor was used for INAA-SLR. The neutron flux in the channel was 1.7 × 1013n cm−2 s−1. Ampoules with thyroid tissue samples, SSB, intra laboratory-made standards, and certified reference material were put into polyethylene rabbits and then irradiated separately for 180s. Copper foils were used to assess neutron flux. The measurement of each sample was made twice, 1 and 120 min after irradiation. The duration of the first and second measurements was 10 and 20 min, respectively. Spectrometric measurements were performed using a coaxial 98-cm3 Ge (Li) detector and a spectrometric unit (NUC 8100), including a PCcoupled multichannel analyzer. Resolution of the spectrometric unit was 2.9-keV at the 60Co 1,332-keV line. Details of used nuclear reactions, radionuclides, and gamma-energies were reported in our earlier publications concerning the INAA chemical element contents in human scalp hair [53].
A vertical channel of nuclear reactor was applied to determine the content of trace elements by INAA-LLR. The quartz ampoule with thyroid samples, standards, and certified reference material was soldered, positioned in a transport aluminum container and exposed to a 24-hour neutron irradiation in a vertical channel with a neutron flux of 1.3⋅1013 n⋅cm-2⋅s-1. Ten days after irradiation samples were reweighed and repacked. The samples were measured for period from 10 to 30 days after irradiation. The duration of measurements was from 20 min to 10 hours subject to pulse counting rate. The gamma spectrometer included the 100 cm3 Ge (Li) detector and on-line computer-based MCA system. The spectrometer provided a resolution of 1.9 keV on the 60Co 1332 keV line. Details of used nuclear reactions, radionuclides, and gammaenergies were presented in our earlier publications concerning the INAA chemical element contents in human prostate and scalp hair [53,54]. A dedicated computer program for INAA mode optimization was used [55]. All thyroid samples were prepared in duplicate, and mean values of chemical element contents were used in final calculation. Using Microsoft Office Excel, a summary of the statistics, including, arithmetic mean, standard deviation, standard error of mean, minimum and maximum values, median, percentiles with 0.025 and 0.975 levels was calculated for chemical element contents. The difference in the results between two age groups was evaluated by the parametric Student’s t-test and non-parametric Wilcoxon-Mann-Whitney U-test. For the construction of “age – chemical element mass fraction” diagrams (including lines of trend with age) and the estimation of the Pearson correlation coefficient between age and chemical element mass fraction as well as between different chemical elements the Microsoft Office Excel programs were also used. To identify the trend of the age dependency of chemical element contents, we applied approximation methods using exponential, linear, polynomial, logarithmic and power function. The maximum of corresponding values of R2 parameter, reflecting the accuracy of approximation, was used for the selection of function.
Results and Discussion
A good agreement of our results for the Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn mass fractions with the certified values of CRM IAEA H-4 and CRM IAEA HH-1 (human hair) (Table 1) as well as the similarity of the means of the Br, Fe, Rb, and Zn mass fractions in the normal thyroid of male determined by both EDXRF and INAA methods (Table 2) demonstrates an acceptable precision and accuracy of the results obtained in the study and presented in Tables 3-8 and Figure 1
Table 1: EDXRF, INAA-SLR and INAA-LLR data of chemical element contents in certified reference material IAEA H-4 (animal muscle) and IAEA HH-1 (human hair) compared to certified values ((mg/kg, dry mass basis).
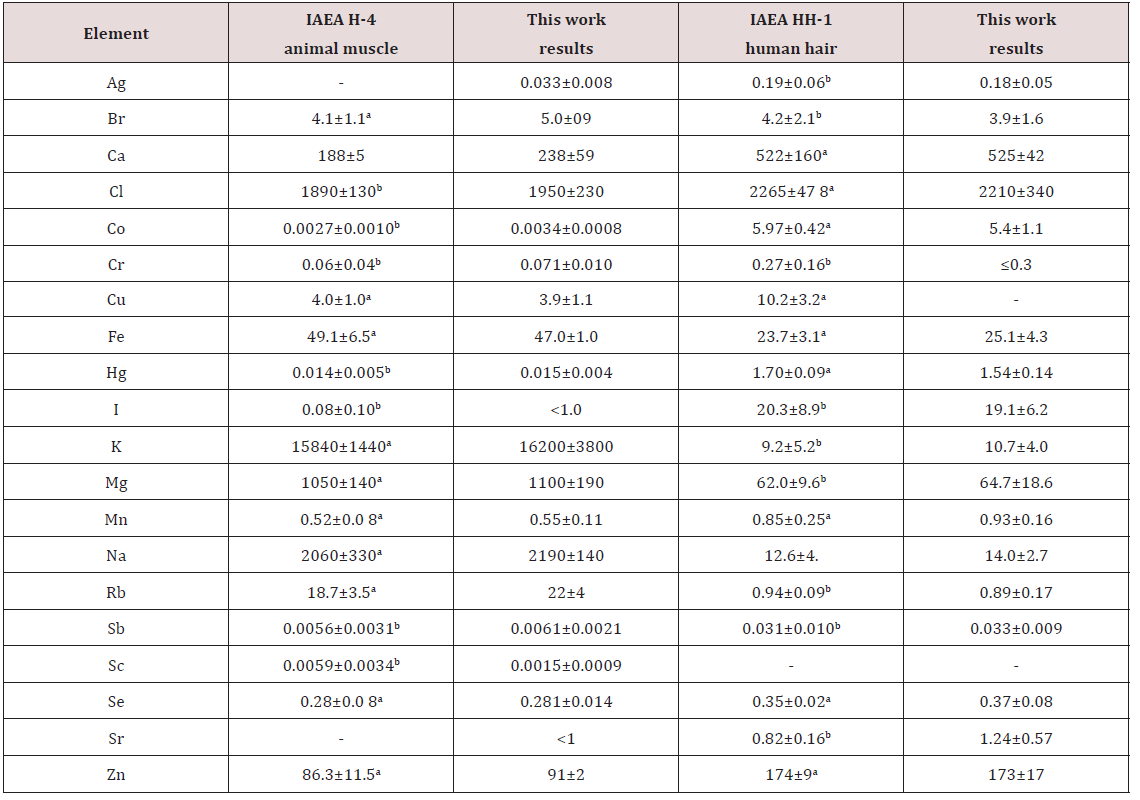
Table 2: Comparison of the mean values (MSEM) of the chemical element mass fractions (mg/kg, dry mass basis) in the normal thyroid of male obtained by both EDXRF and INAA methods.
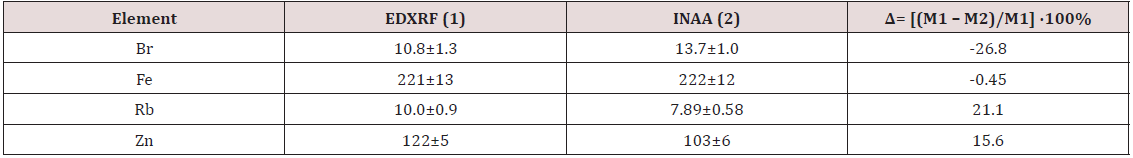
Figure 1: Data sets of individual Ca, I, K, Mn, and Se mass fraction values in the normal thyroid of males and their trend lines.
Table 3 represents certain statistical parameters (arithmetic mean, standard deviation, standard error of mean, minimal and maximal values, median, percentiles with 0.025 and 0.975 levels) of the Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn mass fractions in intact (normal) thyroid of males.
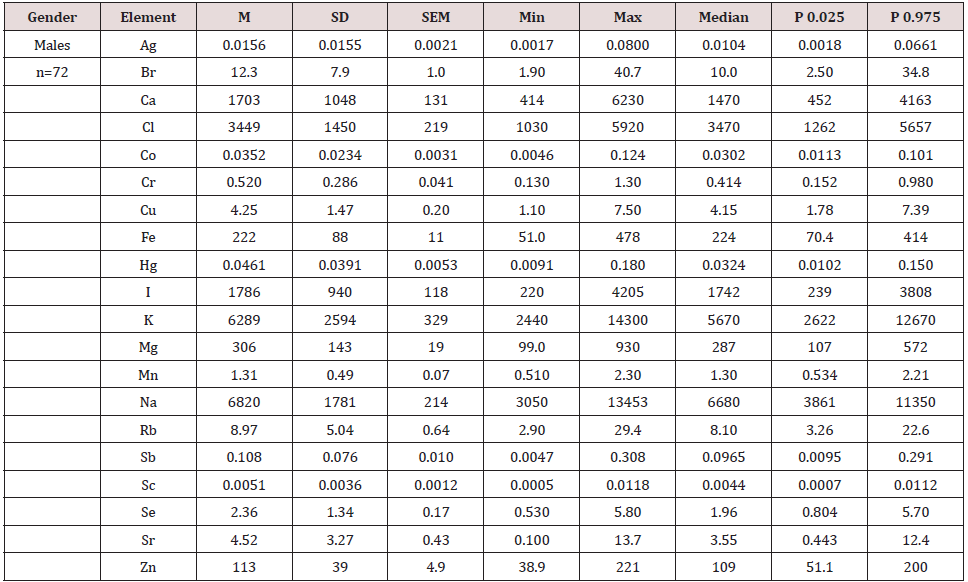
Table 3: Some statistical parameters of Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn mass fraction (mg/kg, dry mass basis) in the normal thyroid of male.
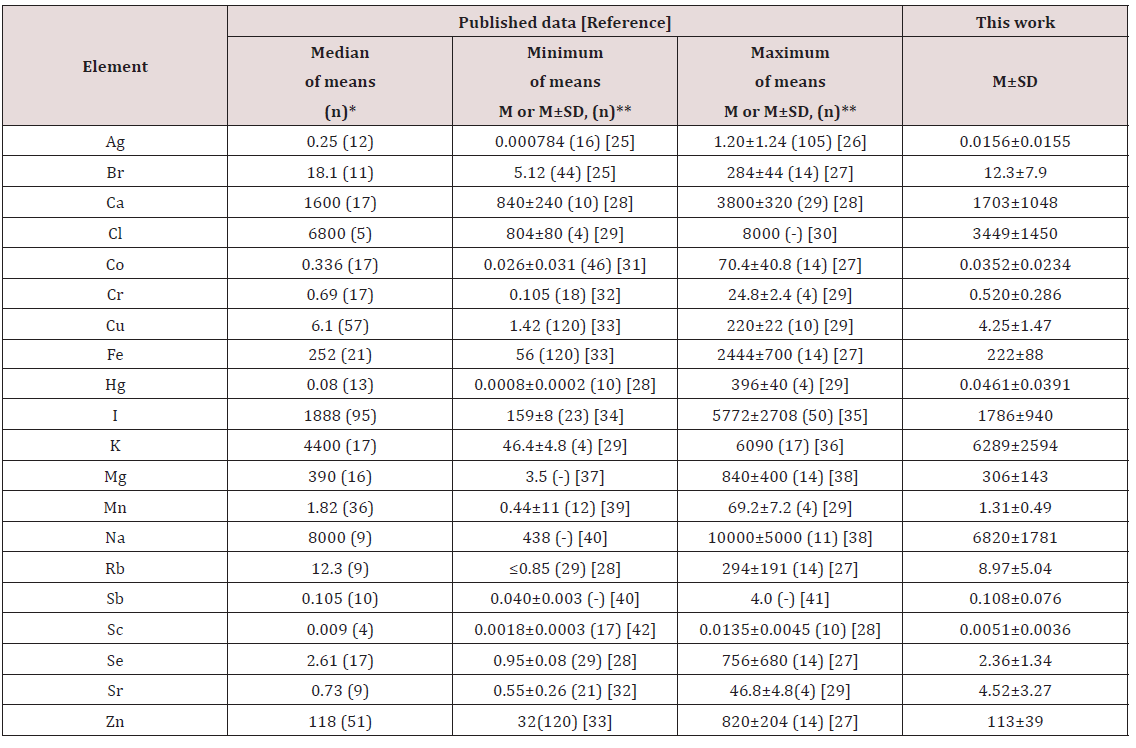
The obtained means for Br, Ca, Cl, Cr, Cu, Fe, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, and Zn mass fraction, as shown in Table 4, agree well with the medians of mean values reported by other researches for the human thyroid, including samples received from persons who died from different non-thyroid diseases [25-42]. The obtained means for Ag and Co are an order of magnitude lower while the mean for Sr is an order of magnitude higher than the median of previously reported data. However, they are inside the ranges of previously reported data. A number of values for chemical element mass fractions were not expressed on a dry mass basis by the authors of the cited references. Hence we calculated these values using published data for water 75% [31] and ash 4.16% on dry mass basis [56] contents in thyroid of adults.
Table 4: Median, minimum and maximum value of means Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, J, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zncontents in the normal thyroid according to data from the literature in comparison with our results (mg/kg, dry mass basis).
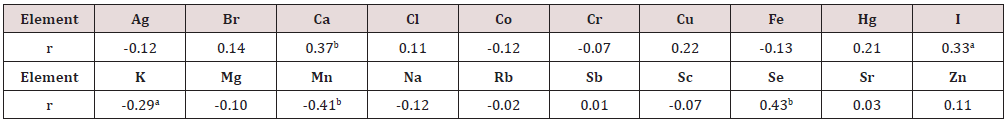
To estimate the effect of age on the chemical element contents we examined two age groups, described above (Table 5). In addition, the Pearson correlation coefficient between age and chemical element mass fraction was calculated (Table 6). Figure 1 shows the individual data sets for the Ca, I, K, Mn, and Se mass fraction in all samples of thyroid, and also lines of trend with age. Since the age dependency of these element contents was best described by a polynomial function, this approximation was reflected in Figure 1. A statistically significant age-related increase in Se as well as decrease in K and Mn mass fraction was observed in thyroid of males when two age groups were compared (Table 5). In second group of males with mean age 53.3 years the mean of K and Mn mass fraction in thyroids were 19% and 24%, respectively, lower while the mean of Se was 44% higher than in thyroids of the first age group (mean age 22.3 years). A statistically significant increase in Se and also decrease in K and Mn mass fraction with age was confirmed by the Pearson’s coefficient of correlation between age and mass fractions of these elements (Table 6). Moreover, a statistically significant increase in Ca, and I mass fraction with increasing of age was shown by the positive Pearson’s coefficient of correlation between age and mass fractions of these elements (Table 6 & Figure 1). As per author’s current information, no published data referring to agerelated changes of Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn mass fractions in human thyroid is available.
Table 5: Differences between mean values (MSEM) of Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn mass fraction (mg/kg, dry mass basis) in the normal male thyroid of two age groups (AG).
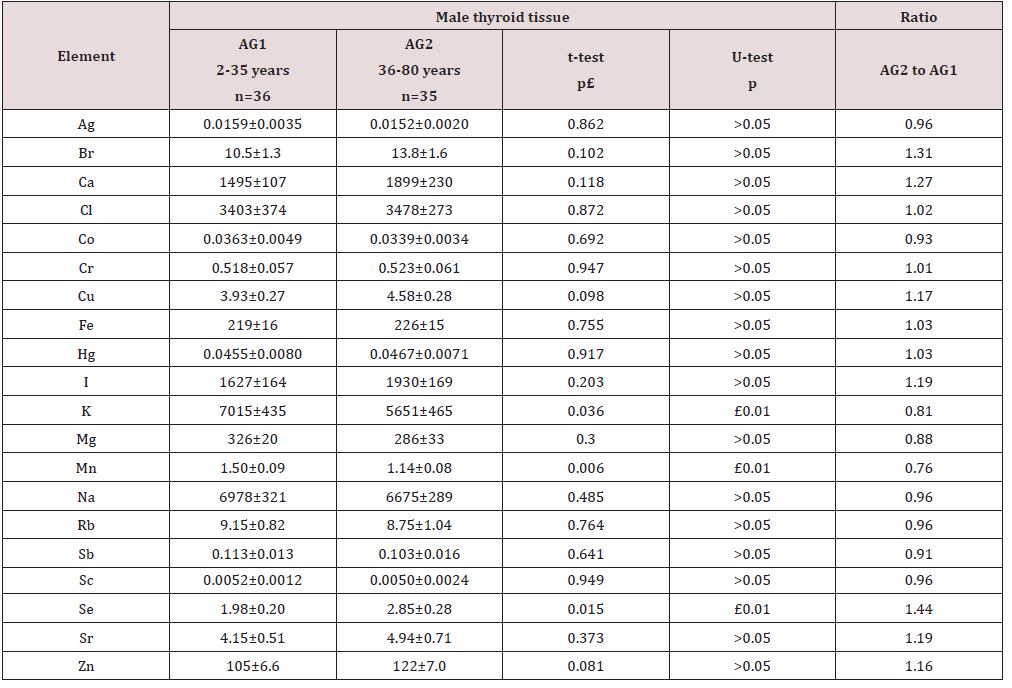
Table 6: Correlations between age (years) and chemical element mass fractions (mg/kg, dry mass basis) in the normal thyroid of male (r – coefficient of correlation).
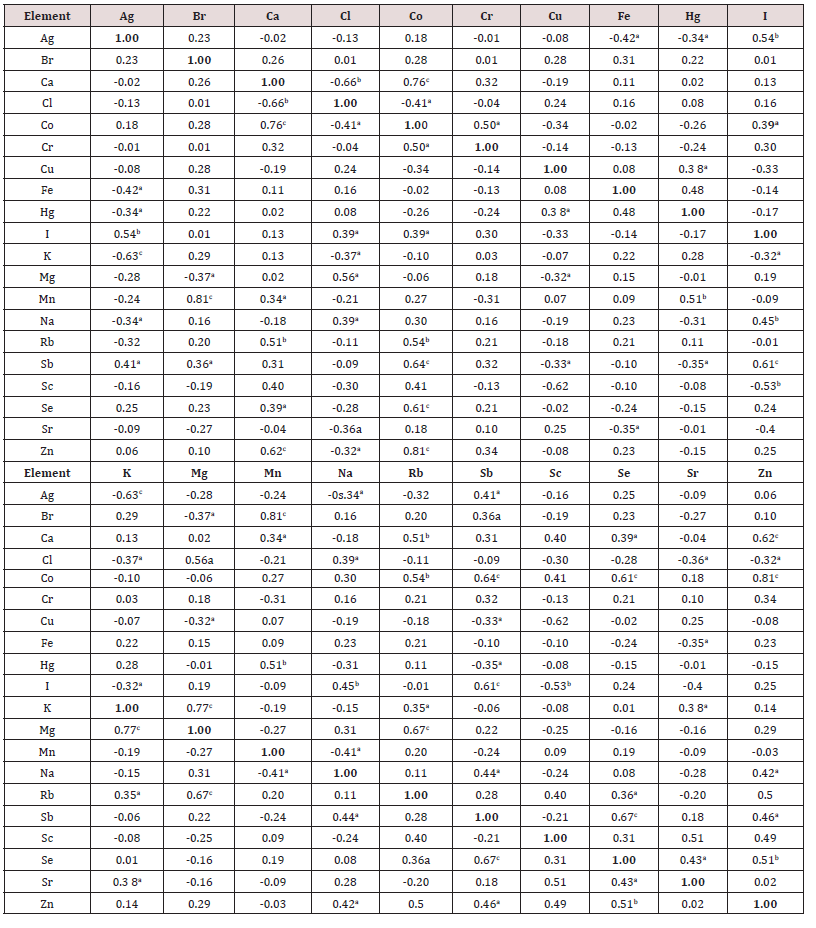
The data of inter-correlation calculations (values of r – coefficient of correlation) including all chemical elements identified by us in the normal thyroid of males aged 2-35 and 36-80 years are presented in Table 7 and Table 8, respectively. A significant direct correlation, for example, between the I and Br, I and Ca, I and Sb, I and Se, I and Zn mass fractions as well as an inverse correlation between I and Hg, I and Mg, I and Na mass fractions was seen in male thyroid of the first age group (Table 7). In age group 2 many correlations between chemical elements in thyroid found in the age group 1 are no longer evident (Table 8). For example, correlations between I-Br, I-Ca, I-Hg, I-Mg, I-Se, I-Zn, existed in the age from 2 to 35 years, disappeared but new direct I-Ag and I-Co as well as new inverse correlation I-K, I-Sc, and I-Sr were arisen. Thus, if we accept the levels and relationships of chemical element mass fraction in thyroid glands of males in the age range 2 to 35 years as a norm, we must conclude that after age 35 years the level of Ca, I, K, Mn and Se, as well as relationships of chemical elements in thyroid significantly changed. If some positive correlations between the elements in the group 1 were predictable (e.g., I-Br), the interpretation of other observed relationships and their perturbation with age requires further study for a complete understanding. No published data on inter-correlations of Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn mass fractions in human thyroid and agerelated changes of these inter-correlations was found.
Table 7: Intercorrelations of the chemical element mass fractions in the normal thyroid of male aged 2-35 years (r – coefficient of correlation).
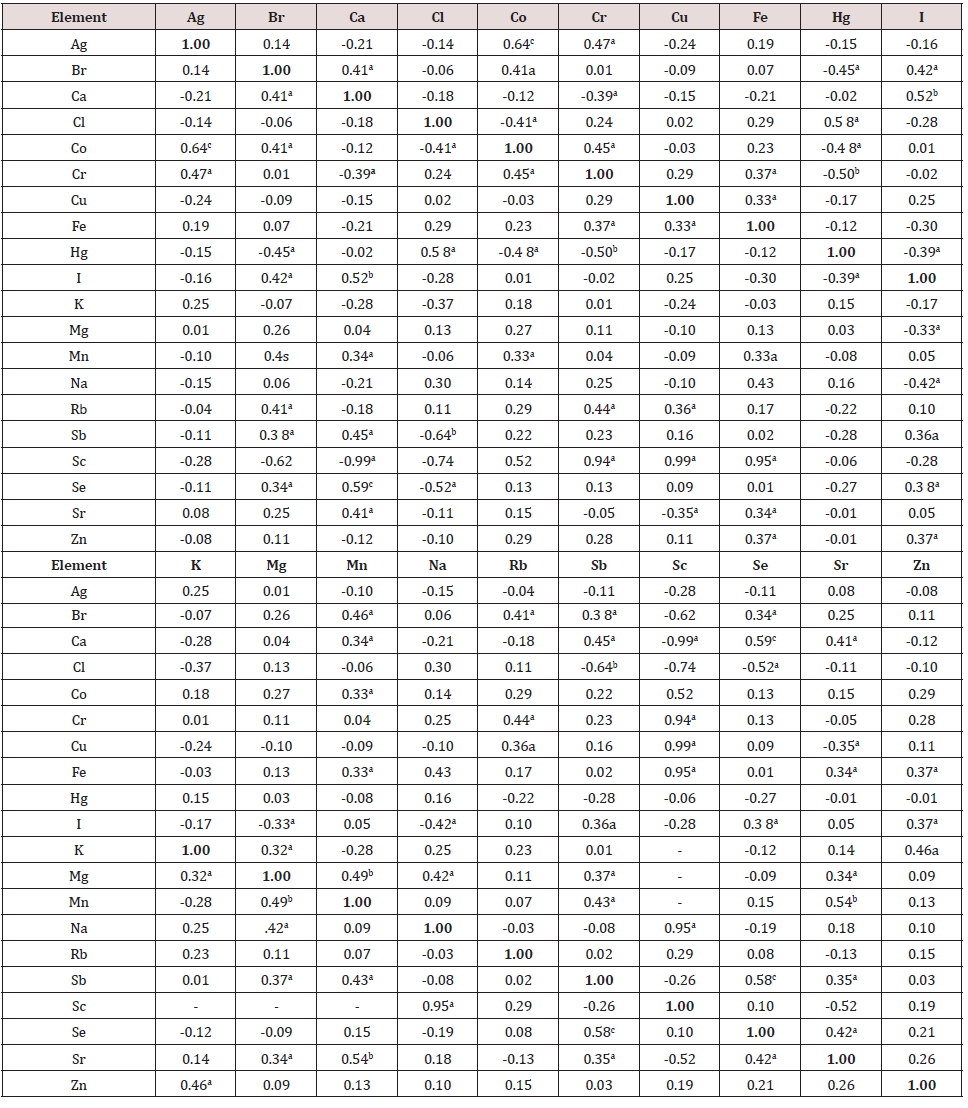
Table 8: Intercorrelations of the chesmical element mass fractions in the normal thyroid of male aged 36-80 years (r – coefficient of correlation).
An age-related increase and excess in Ca mass fractions in thyroid tissue may contribute to harmful effects on the gland. There are good reasons for such speculations since many reviews and numerous papers raise the concern about role of Ca in the prostate, breast, lung and other organ malignant transformation [57-67]. Calcium ions Ca2+ are central to both cell proliferation and cell death [60]. Changes in cytosolic Ca2+ trigger events critical for tumorigenesis, such as cellular motility, proliferation and apoptosis [61]. An increased growth rate of cells is correlated with an increase in the intracellular calcium pool content [57,58]. Moreover, increases in cytosolic free Ca2+ represent a ubiquitous signaling mechanism that controls a variety of cellular processes, including not only proliferation, but also cell metabolism and gene transcription [60]. Indeed, an increased level of Ca content in the thyroid tissue of old males reflects an increase in the intracellular calcium pool. Thus, an increase of Ca content in tissue and organs with age is a key feature in etiology of many benign and malignant tumors, including thyroid goiter and cancer. It is well known that excess in I mass fractions in thyroid tissue may contribute to harmful effects on the gland [19,21,68-71]. Because K+ is mainly an intracellular electrolyte, a decreased level of K content in the thyroid tissue of old males might indicate an age-related decrease of ratio “thyroid cell mass – follicular colloid mass”. From the other hand, mass fraction of Na does not change during a lifespan (Table 5). From this it follows that an intracellular Na+K+ ratio in thyroid of old males may be higher normal level. In turn, increasing intracellular Na+K+ ratio is associated with a depolarization of the cell membrane [72]. The sustained depolarization of the cell membrane results in an increased rate of cell division and in that way with an increased risk of goiter, benign and malignant tumor of thyroid.
It was reported that intracellular Mn content was positively correlated with manganese-containing superoxide dismutase (Mn- SOD), suggesting that the intracellular Mn level is associated with Mn-SOD activity [73]. Thus, a decrease of Mn content in thyroid parenchyma with age indicates the deficiency of antioxidant enzymes in the gland of old males. The high level of Se content found just in the thyroid gland of old males cannot be regarded as pure chance. The seleno-protein characterized as Se-dependent glutathione peroxidase (Se-GSH-Px) is involved in protecting cells from peroxidative damage. This enzyme may reduce tissue concentration of free radicals and hydroperoxides. It is particular important for the thyroid gland, because thyroidal functions involve oxidation of iodide, which is incorporated into thyroglobulin, the precursor of the thyroid hormones. For oxidation of iodide thyroidal cells produce a specific thyroid peroxidase using of physiologically generated hydrogen-peroxide (H2O2) as a cofactor [74]. It follows that the thyroid parenchyma must be continuously exposed to a physiological generation of H2O2 and in normal conditions must be a balance between levels of Se (as Se-GSH-Px) and H2O2. Thus, it might be assumed that the elevated level of Se in thyroid of old males reflects an increase in concentration of free radicals and hydroperoxides in male gland at age above 60 years.
Conclusion
The combination of energy dispersive X-ray fluorescent analysis with instrumental neutron activation analysis with high resolution spectrometry of short- and long-lived radionuclides is a useful analytical tool for the non-destructive determination of chemical element content in the thyroid tissue samples. This combination allows determine the mean of content for 20 chemical elements: Ag, Br, Ca, Cl, Co, Cr, Cu, Fe, Hg, I, K, Mg, Mn, Na, Rb, Sb, Sc, Se, Sr, and Zn. Our data elucidate that there is a statistically significant increase in Ca, I, and Se mass fraction, as well as a decrease in K and Mn mass fraction in the normal thyroid of male during a lifespan. Moreover, a disturbance of intra-thyroidal chemical element relationships with increasing age was found. Therefore, a goitrogenic and carcinogenic effect of inadequate Ca, I, K, Mn, and Se level in the thyroid of old males and a harmful impact of disturbance in intra thyroidal chemical element relationships with increasing age may be assumed.
Acknowledgement
Acknowledgement. Yu. Choporov, Head of the Forensic Medicine Department of City Hospital, Obninsk, for supplying thyroid samples.
References
- Mitrou P, Raptis SA, Dimitriadis G (2011) Thyroid disease in older people. Maturitas 70(1): 5-9.
- Gesing A (2015) The thyroid gland and the process of aging. J Thyroid Res (Suppl 1): A8.
- Kwong N, Medici M, Angell TE (2015) The influence of patient age on thyroid nodule formation, multinodularity, and thyroid cancer risk. J Clin Endocrinol Metab 100: 434-440
- Mazzaferri E (1993) Management of a solitary thyroid nodule. NEJM 328(8): 553-559
- Smailyte G, Miseikyte-Kaubriene E, Kurtinaitis J (2006) Increasing thyroid cancer incidence in Lithuania in 1978-2003. BMC Cancer 11(6): 284.
- Olinski R, Siomek A, Rozalski R (2007) Oxidative damage to DNA and antioxidant status in aging and age-related diseases. Acta Biochim Pol 54(1): 11-26.
- Minelli A, Bellezza I, Conte C (2009) Oxidative stress-related aging: A role for prostate cancer. Biochim Biophys Acta 1795(2): 83-91.
- Klaunig JE, Kamendulis LM, Hocevar BA (2010) Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 38(1): 96-109.
- Zaichick V, Zaichick S (1999) Role of zinc in prostatecancerogenesis. In: Anke M, et al, editors. Mengen und Spurenelemente, 19 Arbeitstagung. Jena: Friedrich-Schiller-Universitat 104-115.
- Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68: 167-182.
- Zaichick V (2004) INAA and EDXRF applications in the age dynamics assessment of Zn content and distribution in the normal human prostate. J Radioanal Nucl Chem 262: 229-234.
- Zaichick V (2006) Medical elementology as a new scientific discipline. J Radioanal Nucl Chem 269(2): 303-309.
- Toyokuni S (2008) Molecular mechanisms of oxidative stress-induced carcinogenesis: from epidemiology to oxygenomics. IUBMB Life 60(7): 441-447.
- Gupte A, Mumper RJ (2009) Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev 35(1): 32-46.
- Lee JD, Wu SM, Lu LY (2009) Cadmium concentration and metallothionein expression in prostate cancer and benign prostatic hyperplasia of humans. Taiwan yi zhi 108(7): 554-559.
- Zaichick V, Tsyb A, Vtyurin BM (1995) Trace elements and thyroid cancer. Analyst 120(3): 817-821.
- Zaichick V, Choporov Yu (1996) Determination of the natural level of human intra-thyroid iodine by instrumental neutron activation analysis. J Radioanal Nucl Chem 207: 153-161.
- Zaichick V, Zaichick S (1997) Normal human intrathyroidal iodine. Sci Total Environ 206(1): 39-56.
- Zaichick V (1998) Iodine excess and thyroid cancer. J Trace Elem Exp Med 11: 508-509.
- Zaichick V (1998) In vivo and in vitro application of energy-dispersive XRF in clinical investigations: experience and the future. J Trace Elem Exp Med 11: 509-510.
- Zaichick V, Iljina T (1998) Dietary iodine supplementation effect on the rat thyroid 131I blastomogenic action. In: Anke M editors. Die Bedentung der Mengen- und Spurenelemente. 18. Arbeitstangung. Jena: Friedrich- Schiller-Universität, Germany, pp. 294-306.
- Zaichick V, Zaichick S (1999) Energy-dispersive X-ray fluorescence of iodine in thyroid puncture biopsy specimens. J Trace Microprobe Tech 17(2): 219-232.
- Zaichick V (1999) Human intrathyroidal iodine in health and nonthyroidal disease. In: Abdulla M [Eds.]. New aspects of trace element research. London and Tokyo: Smith-Gordon and Nishimura, pp. 114-119.
- Zaichick V (2000) Relevance of, and potentiality for in vivo intrathyroidal iodine determination. In: In Vivo Body Composition Studies. Ann N Y Acad Sci 904: 630-632.
- Zhu H, Wang N, Zhang Y (2010) Element contents in organs and tissues of Chinese adult men. Health Phys 98(1): 61-73.
- Vlasova ZA (1969) Dynamics of trace element contents in thyroid gland in connection with age and atherosclerosis. Proceedings of the Leningrad Institute of Doctor Advanced Training 80:135-144.
- Salimi J, Moosavi K, Vatankhah S (2004) Investigation of heavy trace elements in neoplastic and non-neoplastic human thyroid tissue: A study by proton - induced X-ray emissions. Iran J Radiat Res 1(4): 211-216.
- Boulyga, SF, Zhuk IV, Lomonosova EM (1997) Determination of microelements in thyroids of the inhabitants of Belarus by neutron activation analysis using the k0-method. J Radioanal Nucl Chem 222: 11-14.
- Reddy SB, Charles MJ, Kumar MR (2002) Trace elemental analysis of adenoma and carcinoma thyroid by PIXE method. Nucl Instrum. Methods Phys Res B 196(3-4): 333-339.
- Woodard HQ, White DR (1986) The composition of body tissues. Brit J Radiol 708: 1209-1218.
- Katoh Y, Sato T, Yamamoto Y (2002) Determination of multielement concentrations in normal human organs from the Japanese. Biol Trace Elem Res 90(1-3): 57-70.
- Tipton IH, Cook MJ (1963) Trace elements in human tissue. Part II. Adult subjects from the United States. Health Phys 9:103-145.
- Ataulchanov IA (1969) Age-related changes of manganese, cobalt, coper, zinc, and iron contents in the endocrine glands of females. Problemy Endocrinologii 15(2): 98-102.
- Neimark II, Timoschnikov VM (1978) Development of carcinoma of the thyroid gland in person residing in the focus of goiter endemic. Problemy Endocrinilogii 24(3): 28-32.
- Zabala J, Carrion N, Murillo M (2009) Determination of normal human intrathyroidal iodine in Caracas population. J Trace Elem Med Biol 23(1): 9-14.
- Forssen A (1972) Inorganic elements in the human body. Ann Med Exp Biol Fenn 50(3): 99-162.
- Kortev AI, Dontsov GI, Lyascheva AP (1972) Bio-elements in human pathology. Middle Ural publishing-house, Sverdlovsk.
- Soman SD, Joseph KT, Raut SJ (1970) Studies of major and trace element content in human tissues. Health Phys 19: 641-656.
- Teraoka H (1981) Distribution of 24 elements in the internal organs of normal males and the metallic workers in Japan. Arch Environ Health 36(4): 155-165.
- Boulyga SF, Becker JS, Malenchenko AF (2000) Application of ICP-MS for multielement analysis in small sample amounts of pathological thyroid tissue. Microchimica Acta 134: 215-222.
- Fuzailov YuM (1981) Reaction of human and animal thyroids in the conditions of antimony sub-region of the Fergana valley. In: IX All-Union Conference on Trace Elements in Biology Kishinev, p. 58-62.
- Kvicala J, Havelka J, Zeman J (1991) Determination of some trace elements in the thyroid gland by INAA. J Radioanal Nucl Chem 149(2): 267-274.
- Zaichick V (1997) Sampling, sample storage and preparation of biomaterials for INAA in clinical medicine, occupational and environmental health. In: Harmonization of Health-Related Environmental Measurements Using Nuclear and Isotopic Techniques. Vienna: IAEA, pp. 123-133.
- Zaichick V (2004) Losses of chemical elements in biological samples under the dry ashing process. Trace Elements in Medicine 5: 17-22.
- Zaichick V, Zaichick S (2000) INAA applied to halogen (Br and I) stability in long-term storage of lyophilized biological materials. J Radioanal Nucl Chem 244: 279-281.
- Zaichick V, Zaichick S (1996) Instrumental effect on the contamination of biomedical samples in the course of sampling. The Journal of Analytical Chemistry 51: 1200-1205.
- Zaichick V, Tsislyak YuV (1978) A simple device for biosample lyophilic drying. Lab. Laboratornoe Delo 2: 109-110.
- Zaichick V, Tsislyak YuV (1981) A modified adsorptive and cryogenic lyophilizer for biosample concentrations. Laboratornoe Delo 2: 100-101.
- Zaichick V, Zaichick S (1997) A search for losses of chemical elements during freeze-drying of biological materials. J Radioanal Nucl Chem 218: 249-253.
- Zaichick S, Zaichick V (2010) The effect of age and gender on 37 chemical element contents in scalp hair of healthy humans. Biol Trace Elem Res 134(1): 41-54.
- Zaichick S, Zaichick V (2011) The effect of age on Ag, Co, Cr, Fe, Hg, Sb, Sc, Se, and Zn contents in intact human prostate investigated by neutron activation analysis. J Appl Radiat Isot 69(6): 827-833.
- Zaichick V (1995) Applications of synthetic reference materials in the medical Radiological Research Centre. Fresenius J Anal Chem 352(1-2): 219-223.
- Zaichick S, Zaichick V (2010) Method and portable facility for energydispersive X-ray fluorescent analysis of zinc content in needle-biopsy specimens of prostate. X-Ray Spectrom 39: 83-89.
- Zaichick S, Zaichick V (2011) The Br, Fe, Rb, Sr, and Zn content and interrelation in intact and morphologic normal prostate tissue of adult men investigated by energy dispersive X-ray fluorescent analysis. X-Ray Spectrom 40: 464-469.
- Korelo AM, Zaichick V (1993) Software to optimize the multielement INAA of medical and environmental samples. In: Activation Analysis in Environment Protection. Dubna, Joint Institute for Nuclear Research, Russia 326-332.
- Schroeder HA, Tipton IH, Nason AP (1972) Trace metals in man: strontium and barium. J Chron Dis 25: 491-517.
- Legrand G, Humez S, Slomianny C (2001) Ca2+ pools and cell growth. Evidence for sarcoendoplasmic Ca2+-ATPases 2B involvement in human prostate cancer cell growth control. J Biol Chem 276(50): 47608-47614.
- Munarov L (2002) Calcium signalling and control of cell proliferation by tyrosine kinase receptors (Review). Int J Mol Med 10(6): 671-676.
- Capiod T, Shuba Y, Skryma R (2007) Calcium signalling and cancer cell growth. Calcium Signalling and Disease. Subcell Biochem 45: 405-427.
- Roderick HL, Cook SJ (2008) Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer 8(5): 361-375.
- Flourakis M, Prevarskaya N (2009) Insights into Ca2+ homeostasis of advanced prostate cancer cells. Biochim Biophys Acta 1793(6): 1105- 1109.
- Yang H, Zhang Q, He J (2010) Regulation of calcium signaling in lung cancer. J Thorac Dis 2(1): 52-56.
- Mc Andrew D, Grice DM, Peters AA (2011) ORAI1-mediated calcium influx in lactation and in breast cancer. Mol Cancer Ther 10(3): 448-460.
- Zaichick V, Zaichick S (2014) INAA application in the assessment of chemical element mass fractions in adult and geriatric prostate glands. J Appl Radiat Isot 90: 62-73.
- Zaichick V, Zaichick S, Davydov G (2015) Differences between chemical element contents in hyperplastic and nonhyperplastic prostate glands investigated by neutron activation analysis. Biol Trace Elem Res 164(1): 25-35.
- Zaichick V, Zaichick S (2016) Age-related changes in concentration and histological distribution of Br, Ca, Cl, K, Mg, Mn, and Na in nonhyperplastic prostate of adults. European Journal of Biology and Medical Science Research 4: 31-48.
- Zaichick V, Zaichick S, Rossmann M (2016) Intracellular calcium excess as one of the main factors in the etiology of prostate cancer. AIMS Molecular Science 3: 635-647
- Camargo RYA, Tomimori EK, Neves SC (2008) Thyroid and the environment: Exposure to excessive nutritional iodine increases the prevalence of thyroid disorders in São Paulo, Brazil. Eur J Endocrinol 159(3): 293-299.
- Sun X, Shan Z, Teng W (2014) Effects of increased iodine intake on thyroid disorders. Endocrinology and Metabolism 29(3): 240-247.
- Miranda DMC Massom JN, Catarino RM (2015) Impact of nutritional iodine optimization on rates of thyroid hypoechogenicity and autoimmune thyroiditis: A cross-sectional, comparative study. Thyroid 25(1): 118-124.
- Shan Z, Chen L, Lian X (2016) Iodine Status and Prevalence of Thyroid Disorders after Introduction of Mandatory Universal Salt Iodization for 16 Years in China: A Cross-Sectional Study in 10 Cities. Thyroid 26(8): 1125-1130.
- Nagy I, Lustyik G, Lukács G (1983) Correlation of malignancy with the intracellular Na+:K+ ratio in human thyroid tumors. Cancer Res 43(11): 5395-5402.
- Hasegawa S, Koshikawa M, Takahashi I (2008) Alterations in manganese, copper, and zinc contents, and intracellular status of the metalcontaining superoxide dismutase in human mesothelioma cells. J Trace Elem Med Biol 22(3): 248-255.
- Aaseth J, Frey H, Glattre E (1990) Selenium concentrations in the human thyroid gland. Biol Trace Elem Res 24(2-3): 147-152.
Editorial Manager:
Email:
biomedicalengineering@lupinepublishers.com

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...












