Lupine Publishers Group
Lupine Publishers
Menu
Research Article(ISSN: 2641-6875) 
Carbon Nanomaterial and Solid-Phase Extraction: an Effective Adsorbent and Impressive Method for Measuring Norfloxacin in Biological Samples Volume 2 - Issue 3
Ali Moghimi*1 and Milad Abniki2
- 1Department of Chemistry, Branch Islamic Azad University, Iran
- 2Department of Resin and Additives, Institute for Color Science and Technology, Iran
Received:September 15, 2021; Published:October 11, 2021
*Corresponding author: Ali Moghimi, Department of Chemistry, Varamin-Pishva, Branch Islamic Azad University, Varamin, Iran
DOI: 10.32474/CTBM.2021.02.000140
Abstract
In recent years, drug use is on the rise, causing environmental pollution. Therefore, drug control is a common practice in many laboratories. This project focuses on increasing the method for determining small amounts of norfloxacin in aqueous and biological samples. Solid-phase extraction of small amounts of norfloxacin in aqueous samples using carbon nanotubes and visible and ultraviolet spectrophotometric measurements are used in biological samples. These systems include two phases, the aqueous donor phase and the acceptor phase of conjugated carbon nanomaterial.
Aqueous phase extraction and norfloxacin adsorption were performed in two experimental steps. First, methanol acidic solvent was used and the adsorbed samples were submitted to Vis-UV spectrophotometry for further analysis. Extraction parameters in this method This cheap and simple method is compatible with most tool analysis methods. These parameters include extraction time, adsorption of organic solvent effect, adsorption time, shaking time, the volume of donor phases, and optimized surfactant effect, and analysis and measurement were performed under optimal conditions. Low consumption of organic solvents, elimination of the effect of previous experiments, short extraction time, low detection threshold, and high concentration coefficient are the advantages of the mentioned technique and also the concentration factor and detection threshold for Norfloxacin were 49 and 8.96. The linear amplitude and relative standard were 1.46%.
Keywords: Norfloxacin; Extraction Method; Carbon Nanomaterial; Spectrophotometry
Introduction
In the analysis and measurement of biological samples that contain very complex compounds that often interfere and sometimes even with the strongest detection systems the number of contaminants are not detectable to a small extent or are not compatible with routine analysis systems. Some contaminants have dangerous biological effects in small amounts. Therefore, the introduction of new and sensitive methods to measure the number of such pollutants is necessary. Analytical devices such as chromatography, spectroscopy, microscopic devices, as well as sensors, and microdevices have been developed, although highly accurate and non-destructive methods are not available in most cases. Therefore, to increase the current methods, it is necessary to prepare one or more sample steps [1-5].
The use of improper preparation methods can negatively affect the process of the analysis method. In recent years, little attention has been paid to this issue. Of course, sometimes additional steps in these processes, such as derivation, seem very necessary. The most important common steps in the analysis method include the preparation steps. In this paper, the measurement of the interaction of adsorption of carbon nanomaterials and drugs using measurable and ultraviolet spectrophotometry is considered. This process is based on drug adsorbents and nanomaterials. Important parameters in this method and key parameters in this measurement are evaluated. These advantages include easy analysis, efficient methods, new capabilities, and low-dose analysis of fluoroquinolones Norfloxacin of fluoroquinolones antibiotics with the chemical formula C16H18FN3O3. Norfloxacin is a broadspectrum antibiotic that is active against both gram-positive and Gram-negative bacteria. It functions by inhibiting DNA gyrase, a type II topoisomerase, and topoisomerase IV [6-13].
The high performance of high-pressure liquid chromatography is increased by HPLC for norfloxacin and its three metabolites in the analysis of plasma, serum and urine. The HPLC method for the extraction of norfloxacin and three metabolites in urine samples was on a polystyrene column, which was quantitatively analyzed using a ultra-violating detector. The current method involves chromatographic extraction, which is suitable for plasma and serum levels and urine levels [2].
In this study, we intend to measure norfloxacin in real samples (plasma, urine) by the interaction between carbon nanomaterials and daronorfloxacin using a UV-Visible spectrophotometer. This method is based on the interaction between norfloxacin and carbon nanomaterials. Important parameters on the interaction and those that affect the measurement are examined.
Experimental
Chemicals and reagents
Norfloxacin(C16H18FN3O3) was prepared from Darmstadt, Germany of Merck, Method and dried for a week over phosphorus pentoxide in a vacuum desiccator before apply. Multiwall carbon nanotubes were purchased from Merck. All solutions were prepared with doubly distilled deionized water from Darmstadt, Germany of Merck. It was conditioned before use by suspending in 4 M nitric acid for 20 min, and then washed two times with water. Used substances and solvents for preparation of solutions and standards are highly analytically pure
Instrumentation
Double beam ultraviolet-visible spectrophotometer, Model UV1700, in Razi Laboratory, University of Science and Research. These conditions are tabulated in. The pH measurements were used by Sartorius model PB-11. Digital scale with 0.1 milligrams accuracy (AND GR-200 model).
Synthesis method of Carboxyl conjugated carbon nanotube
0.52 g of multiwall raw carbon nanotubes are added to 4 to 3 nitic acids to sulfuric acid. The solution is kept in an 11 kHz ultrasonic bath for 31 minutes, then it was refluxed in an agitator for 31 hours. The obtained product was washed with distilled water until its pH reaches 9. Solid phases are separated and are dried in a vacuum for 43 hours at 61 degrees centigrade. MWCNTCOOH, produced in the second step is mixed with 31 milliliters of ethylenediamine (mixed for 9 hours in an 11kHz ultrasonic bath). The obtained mixture was agitated for 31 hours at 61 degrees centigrade and 1.33-micron Millipore polycarbonate was separated in a membrane filter and the solid product was washed with waterfree methanol. The obtained solid product was dried in a vacuum overnight so that MWCNT-NH was produced [14-18].
Preparation of Method
In this study, new solid-phase methods are used to detect insignificant amounts of norfloxacin in aqueous samples using carbon nanotubes and UV-visible spectrophotometry in biological samples. These techniques are a biphasic system in which the donor phase is an aqueous sample and the acceptor phase is conjugated carbon nanotubes. Experiments were performed in two steps, aqueous phase extraction and norfloxacin resorption using methanol acidic solvent, and resorbed samples were provided to Vis-UV spectrophotometry for further analysis. This method is cheap, simple, fast, and compatible with most instrumental analysis methods. Extraction parameters including resorbing organic solvent effect, pH of donor and acceptor phases, extraction duration, resorption duration, shaking time, the volume of donor phases, and surfactant effect were optimized and analysis and measurements were performed under the optimized situation.
Primary experiment: absorbent effect of norfloxacin extraction
To assess the effect of amine or carboxyl conjugated carbon nanotubes, the following steps are performed. For each experiment tube, buffer in the 10-2 range was added. Since the goal is selecting appropriate absorbents, the lower absorption was considered the best value.5 ml of the drug with 50 pm concentration for norfloxacin was taken. 1.15 g of amine conjugated carbon nanotube, 5 ml of buffer in 2-10 range were taken and added to a 50 ml balloon and delivered to volume with distilled deionized water. Then the balloon is shaken for 51 minutes, centrifuged for 15 minutes, and then passed through syringe filters and finally, quantitative analysis of norfloxacin took place. The same procedure is done from carboxylconjugated carbon nanotubes. Quantitative analysis of filtered solutions for norfloxacin is done using UV-Vis spectrophotometry in 200 to 500 nm of wavelength.
After performing primary experiments and reassuring the efficacy of conjugated carbon nanotubes in norfloxacin extraction, efforts to enhance the efficacy of this method were performed as it will be described.
Optimizing wavelength in the extraction of norfloxacin This medication with carboxyl conjugated carbon nanotubes and 2-10 range of buffer undergoes quantitative analysis with UVvis spectrophotometer, after being shaken and centrifuged Figure 1.
pH effect on norfloxacin extraction
To assess the effect of pH in norfloxacin extraction, the following steps were performed initially. For each container, the desired tampon is added, since the goal is to determine the appropriate pH. The lowest absorption is considered the best value. 50 ml of the drug with 500 pm concentration for norfloxacin was taken. 1.15 g of carboxyl absorbent, 5 ml of buffer in the 2-10 range were taken and added to a 50 ml balloon and delivered to volume with distilled deionized water. Then the balloon is shaken for 15 minutes, centrifuged for 15 minutes, and then passed through syringe filters and finally, quantitative analysis of norfloxacin took place.
Absorbent amount in norfloxacin extraction
In this step, optimized pH and absorbent were used in the optimized wavelength based on previous experiments, and different amounts of absorbent (3, 5, 10, 12, 15, and 20) were used for each medication.
5 ml of the drug was taken. 0.15 g of carboxyl conjugated carbon nanotube, 5 ml of buffer in 2-10 range were taken and added to a 50 ml balloon and delivered to volume with distilled deionized water. Then the balloon is shaken for 15 minutes, centrifuged for 15 minutes, and then passed through syringe filters and finally, quantitative analysis of norfloxacin took place. Quantitative analysis of filtered solutions for norfloxacin was performed in 272 nm wavelength in a UV-vis spectrophotometer.
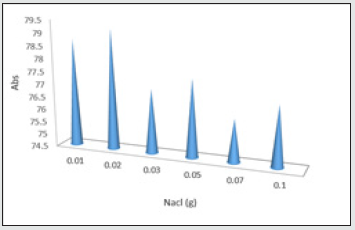
Salt effect
The other important parameter is the salt, due to paired ion function between reactants which leads to a better reaction between the substances. It is also very important in absorption intensity. 0.20 g was selected as the optimum value for norfloxacin. In this step of the experiment, optimum pH, absorbent, and wavelength were used. Different amounts of salt were used for the drug.
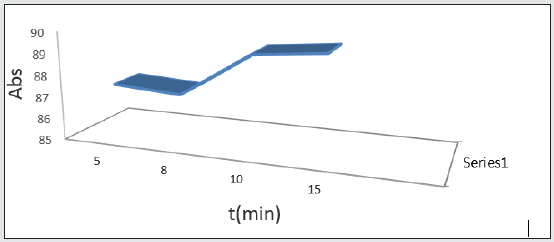
Effect of time of drug absorption in solution
Another important parameter on absorption system and drug measurement based on their extraction is reaction rate. 5 solutions were prepared with optimum properties and shaking was performed at different times. Then they were centrifuged and passed through a filter and their absorption rate at maximum wavelength was checked.
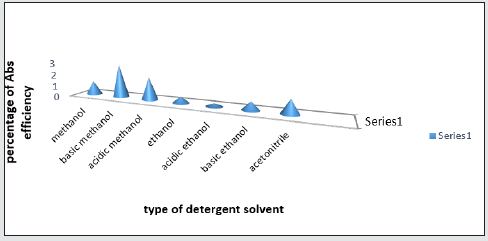
Effect of elusion solvent type
The type of elusion solvent is one of the most important parameters affecting the absorption system. In this study, for each medication, methanol, ethanol, acetonitrile, acidic and basic methanol, acidic and basic ethanol were used and the optimum solvent was detected for each medication. After choosing the optimum solvent, the acidic or basic form of the solvent was assessed. 7 balloons were used (by considering optimum conditions) and the upper water was removed after centrifuging and the solvents were added. After shaking for 20 minutes and centrifuging for 15 minutes, the solutions were filtered and their absorption at maximum wavelength was recorded and resorption took place. Due to resorption, the highest absorption should be selected.
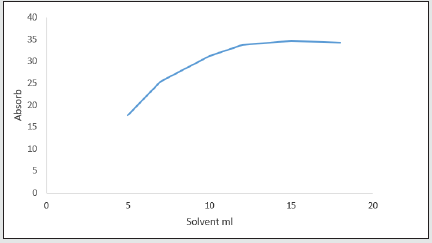
Effect of elusion solvent volume
The volume of elution solvent is another effective parameter on the absorption intensity of the system. In this study, different volumes of selected solvents have been assessed. 5, 7, 10, 12, 15, and 18 ml of the solvent have been added to the absorbent in optimum conditions. Absorption intensity was recorded with a UV-Vis spectrophotometer, after shaking for 20 minutes and centrifuging for 15 minutes.
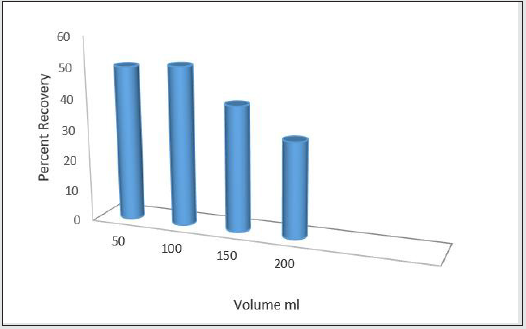
Determining limit volume and condensation factor
To determine limit volume, desperate solutions of norfloxacin were prepared with 50,100,150, and 200 ml volume. Then, 100 ml was selected as the limit volume [15].
Analytical properties
After optimizing all effective parameters on absorption intensity, a calibration curve of the method was drawn. For this purpose, a 10 ml volumetric flask was filled with different concentrations of the medication. Then sodium choroid salt was added to 0.12 g norfloxacin in percentage form and carboxyl conjugated carbon nanotube absorbents were added to the volumetric flask with optimum pH. Finally, it was delivered to volume by adding distilled deionized water. Then elusion and … steps were performed. Finally, the absorption intensity of the solutions was recorded in laboratory temperature and the calibration curve was drawn.
Calculation of Limit of Detection (LOD)
Generally, the limit of detection of a laboratory substance is considered as the concentration whose device response is significantly different from control or background. The common definition of a limit of detection in analytical chemistry is a concentration of a substance with a response equal to three times of control standard deviation (Sb) according to the following equation:
LOD=3Sb/m
To calculate the limit of detection for norfloxacin measurement, four control solutions with the optimum situation were prepared without adding the medication and absorption intensity was recorded at medication absorption peak wavelength:
LOQ10Sb LOQ m
Accuracy of %RSD method
This parameter is used to evaluate experiment accuracy and closeness of study data. To assess the accuracy of this method (based on relative standard deviation), measuring absorption intensity for 4 solutions of norfloxacin were measured in one day. For this purpose, 4 standard solutions with optimum concentrations were prepared exactly based on the purposed method:
Evaluating disturbing species and selectivity
Effects of disturbing species in the measurement of norfloxacin were evaluated based on biological matrix under optimum conditions. For this purpose, the medication sample was mixed with different concentrations of disturbing species and measurement was performed one hour later and absorption intensity was compared with pure medication same. Fluoxetine drug was a disturbing species.
Preparation of biologic samples for norfloxacin measurement
A blood sample is taken from the patient and put into EDTAcontaining tubes with 3.9 ml volume. Samples were centrifuged at 3000 RPM for 30 minutes. The yellow fluid above the sample is plasma and is taken. To make sure that there is no protein in the plasma, 10 ml of acetone is added to 10 ml of plasma and centrifuged 3000 RPM for 10 minutes so that excess proteins coagulate. For measurement with the purposed method, a definite volume of plasma is taken and measurement steps are performed.
Urine Sample for norfloxacin measurement
Huma urine samples s taken and filtered. It is stored in a black glass container. To measure with the purposed method, a definite volume of urine is taken and measurement steps are performed.
Discussion and Results
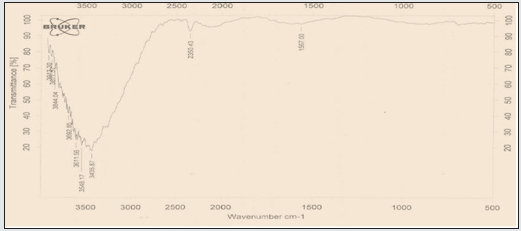
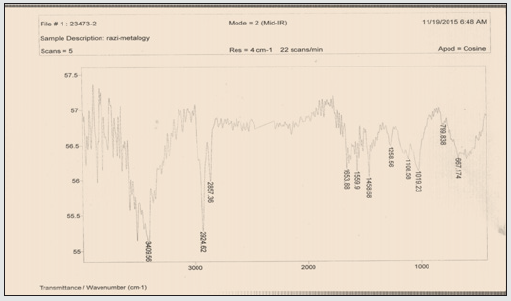
Assessment of results of FT-IR spectrum
This combination of two tensile vibration strips belongs to the C-O bond of carboxyl carbon which is observed in the 1559 cm-1 area. On the other hand, two tensile vibration strips belonging to the C=O bond on the carboxyl group can be observed in 1653 cm-1. Finally, there is a strong vibration in 3409 cm-1 belonging to O-H bond tensile vibration (Figure 1 and 2). The emergence of absorption peaks in 1653 and 1019 cm-1 introduce carboxyl groups on carbon nanotube.
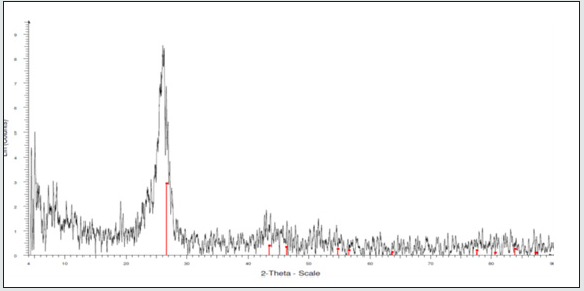
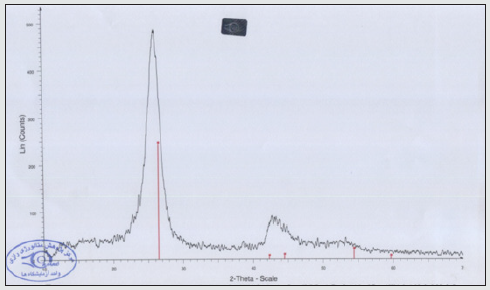
Assessment of results of XRD spectrum
In the below XRD spectrum, the combination of a very highintensity peak in Ɵ=26.8 belongs to carboxyl nanotube and a very low-intensity peak in 42.2 region having very sharp and small peaks, respectively (Figure 3 and 4).
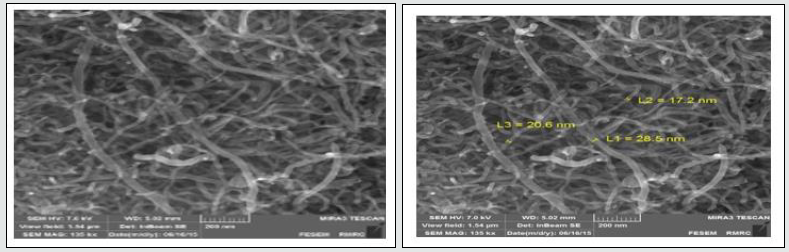
Assessment of results of SEM spectrum
In the figure below, an SEM image of carboxyl conjugated carbon nanotubes is depicted. For carboxyl nanotubes, particles with 200 nm size are produced. Moreover, the SEM picture after absorption shows the placement of desired metal on carboxyl conjugated carbon nanotubes from which it is inferred that the width of the sheets is increased. As it is seen in the picture, carboxyl functional groups are shown as more bright points on the surface of carbon nanotubes (Figures 5 and 6).
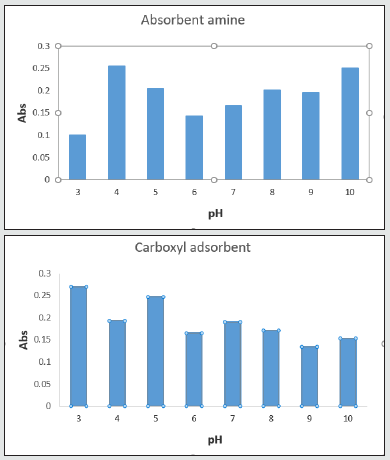
Primary experiment: absorbent effect of norfloxacin extraction
According to the charts, carboxyl conjugated carbon nanotubes showed better absorption. Consequently, a carboxyl absorbent was selected for norfloxacin extraction [16]. All spectrums were put together in 272 nm of wavelength. As a result, this wavelength was determined to be the optimum absorption wavelength (Figure 7).
pH effect on norfloxacin extraction
This chart shows that pH=6 is appropriate for protonating carbon nanotubes which is associated with higher norfloxacin absorption on carboxyl conjugated carbon nanotubes. Thus, the best electrostatic situation for absorbent and the drug for surface attraction are present at pH=6 [17] Figure 8.
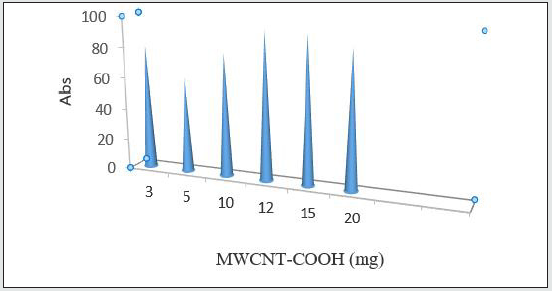
Absorbent amount in norfloxacin extraction
Another parameter affecting absorption, is the amount of absorbent. 0.12 g was selected for norfloxacin. Reported results indicate that din lower amount of absorbent, some substances may enter the solution since absorption may happen in drug maximum wave length [18] Figure 9.
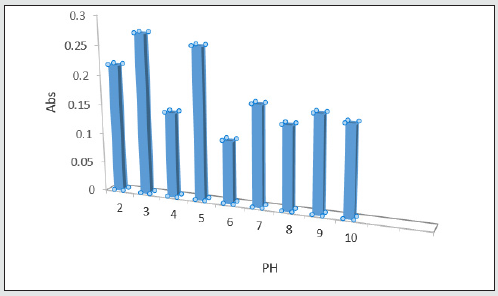
Salt effect
This chart shows that adding 0.20 g sodium chloride produces appropriate electrostatic charge on absorbent and drug sample for norfloxacin extraction and 2%W/V is the optimum salt concentration showing highest drug absorption [19] Figure 10.
Effect of time of drug absorption in solution
This chart shows that as the duration of exposure of absorbent and drug increase, equilibrium condition gets better and after that no changes take place on the concentration of the medication. 20 minutes was selected as the optimum reaction time for norfloxacin [19] Figure 11.
Effect of elusion solvent type
According to this chart, basic solvents show best conditions in the equilibrium between absorbent and elusion solvent. Consequently, basic methanol was selected as optimum solvent for the highest absorption [20] Figure 12.
Effect of elusion solvent volume
According to this chart, for volumes above 10 ml, all the medication enters the elusion solvent and the equilibrium goes to the side of elusion solvent and complete resorption takes place. Thus, 10 ml was selected as optimum volume for norfloxacin [21] Figure 13.
Determining limit volume and break through volume
According to this chart, as the medication is diluted, the possibility of complete absorption on the absorbent decreases. Thus, 100 ml was selected as limit volume for norfloxacin Figure 14.
Numerator V = limit volume
Denominator V = elusion solvent volume
F = condensation factor
F = V/V
F =100/10 = 10
Condensation factor for norfloxacin
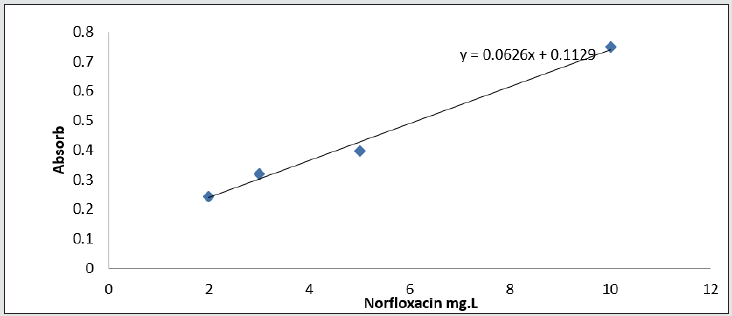
Calibration curve for norfloxacin medication method
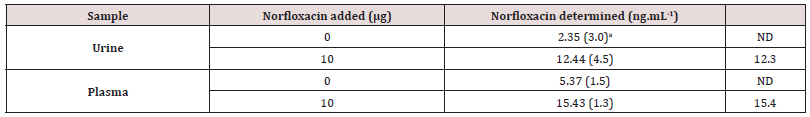
According to the obtained results in optimum conditions, there is a linear relationship between absorption intensity and the concentration of the drug [22]. Fluoxetine is the disturbing specie and more disturbance is observed in higher concentrations (table 1). The absolute amount of disturbing specie is decreased by dilution. In this step we reassure that the amount of added drug and the amount of found drug in plasma and urine are the same which shows that accuracy of this method is acceptable [23] Figure 15.
a: Values in parentheses are %RSDs based on five individual replicate analysis.
b: Not detected.
Conclusion
In the research method and the results presented in the previous chapters, solid phase extraction method and UV-vis spectrophotometer, extraction and measurement of small amount of norfloxacin in biological samples have been used. The aim of this study was to increase the efficiency, selective, inexpensive and simple method for evaluating the amount of norfloxacin in biological samples. The development of solid phase extraction methods in recent years has shown the need for an effective adsorbent. Therefore, in this study, carboxyl-conjugated carbon nanotubes were used to increase the extraction performance of norfloxacin. Parameters including effect including pH, type of buffer and concentration, amount of adsorbent, type and volume of solvent, time of reaction and effect of salt were investigated. This method has good reproducibility and a wide linear range (1- 11 ppm) and a suitable density coefficient for the determination of norfloxacin. Also, as a good linear range, 7.4 ppb detection limit and high reproducibility are other properties of this method. According to the results, the advantage of this method compared to other methods is that the adsorbent used in high profile surfaces, which is a key factor in choosing this material to be used as) adsorbent used in the method. The proposal can be retrieved, many can be tested. Another advantage of the proposed method compared to other methods, the detection limit is lower than most of the proposed methods and has a better concentration factor than many other methods and easy technique and high accuracy.
References
- Abniki M, Moghimi A, Azizinejad F (2021) Synthesis of calcium‐layered double hydroxide based nanohybrid for controlled release of an anti‐inflammatory drug. J Chin Chem Soc (Taipei, Taiwan) 68(2): 343.
- Eskandarinezhad S, Khosravi R, Amarzadeh M, Mondal P, Magalhães Filho FJC (2021) Application of different Nanocatalysts in industrial effluent treatment. A review. Journal of Composites and Compounds 3: 43.
- Dixit H, Nguyen VM, Dixit, Varian (1991) Sample Preparation Products. Harbor City, CA 90710, J Chromatogr 15 563(2): 379.
- Abniki M, Moghimi A, Azizinejad F (2020) Fabrication of bionanocomposite based on LDH using biopolymer of gum arabic and chitosan-coating for sustained drug-release. Journal of the Serbian Chemical Society 85: 1223.
- Pourshamsi T, Amri F, Abniki MA (2021) comprehensive review on application of the syringe in liquid-and solid-phase microextraction methods. Journal of the Iranian Chemical Society: 1.
- Abniki M, Moghimi A (2021) Synthesis of chitosan functionalized magnetic carbon nanotubes for dispersive solid‐phase extraction of bromocresol green. Micro & Nano Letters 16(2): 455.
- Drlica K, Zhao X (1997) "DNA gyrase, topoisomerase IV, and the 4-quinolones". Microbiol Mol Biol 61 (3): 377-92.
- Moghimi A, Qomi M, Yari M, Abniki M (2019) Solid phase extraction of Hg (П) in water samples by nano-Fe. Int J Bio-Inorg Hybr Nanomater 8:163.
- Moghimi A, Abniki M (2021) Removal and measurement of bromocresol purple dye in aqueous samples by β-cyclodextrin-modified magnetic carbon nanotube with dispersive solid-phase extraction technique. Journal of Color Science and Technology.
- Moghimi A, Abniki M (2021) The Dispersive Solid-Phase Extraction of Fluoxetine Drug from Biological Samples by the Amine-Functionalized Carbon Nanotubes with HPLC Method. Chemical Methodologies 5(3): 250-258.
- Moghimi A, Abniki M (2021) Preconcentration and Separation of Ultra-Trace Cu (II) with Disks of Octadecyl Silica Membrane Modified Nano-Fe3O4-Encapsulated-Dioctyl Phthalate and Linked-Diethylenetriamine. Advanced Journal of Chemistry-Section A 4(2):78-86.
- Abniki M, Azizi Z, Panahi HA (2021) Design of 3‐aminophenol‐grafted polymer‐modified zinc sulphide nanoparticles as drug delivery system. IET Nanobiotechnology 15(8): 664-673.
- Moghimi A, Yari M (2014) Preconcentration of trace Ni (II) using C18 disks nano graphene with amino propyltriethoxysilane (APTES). Merit Research Journal of Environmental Science and Toxicology 2(5): 110-119.
- Moghimi A, Akbarieh SP (2014) Evaluation of Solid-phase Extraction Sorbent with Octadecane-functionalized Nano Graphene (ODG) for the Preconcentration of Chromium Species in Water. International Journal of Scientific Research in Knowledge 2(1): 8.
- Moghimi A (2013) Detection of trace amounts of Pb (II) by schiff base-chitosan-grafted multi-walled carbon nanotubes. Russian J Physic Chem A 87(7): 1203.
- Soylak M, Karatepe AU, Elci L, Dogan M (2003) Column preconcentration/separation and atomic absorption spectrometric determinations of some heavy metals in table salt samples using amberlite XAD-1180. Turkish Journal of Chemistry 27(2): 235.
- Moghimi A (2014) Extraction of Ni (II) on micro crystalline naphthalene modified with organic-solution-processable functionalized nano graphene. Russian Journal of Physical Chemistry A 88(7): 1177.
- Moghimi A, Abdouss M (2013) Extraction of Co (II) by Isocyanate Treated Graphite Oxides (iGOs) Adsorbed on Surfactant Coated C18 Before Determination by FAAS. Int J Bio-Inorg Hybd Nanomat 2(1): 319-327.
- Moghimi A, Sabertehrani M, Waquif-Husain S (2007) Preconcentration and determination of chromium species using octadecyl silica membrane disks and flame atomic absorption spectrometry. Chinese Journal of Chemistry 25(12): 1859-1865.
- Moghimi A, Poursharifi MJ (2012) Perconcentration of Ni (II) from Sample Water by Modified Nano Fiber. Oriental Journal of Chemistry 28(1): 353-361.
- Moghimi A (2014) Separation and extraction of Co (II) using magnetic chitosan nanoparticles grafted with β-cyclodextrin and determination by FAAS. Russ. J Phys Chem A 88(12): 2157-2164.
- Moghimi A, Siahkalrodi SY (2013) Extraction and Determination of Pb (II) by Organic Functionalisation of Graphenes Adsorbed on Surfactant Coated C18 in Environmental Sample. Journal of Chemical Health Risks 3 (3): 01.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...