Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4676
Review Article(ISSN: 2637-4676) 
Crop-based Alcohols and Phenols as Bioresources for Environmentally-friendly Organophosphorus Flame Retardants Volume 9 - Issue 2
Getnet Muche Abebele*
- Department of Plant Pathology, Kulumsa Agricultural Research Center, Ethiopian Institute of Agricultural Research, Ethiopia
Received: December 02, 2021; Published: December 16, 2021
Corresponding author: Bob A Howell, Science of Advanced Materials Center for Applications in Polymer Science Department of Chemistry and Biochemistry Central Michigan University, USA
DOI: 10.32474/CIACR.2021.09.000308
Abstract
Organophosphorus compounds derived from crop-based alcohols and phenols represent attractive alternatives to traditional flame retardants which persist as environmental contaminants and act as a source of human disease. Isosorbide, a cyclic diether diol, may be readily obtained from starch produced by many cereal grains. It serves as a biobased for the generation of several families of effective phosphorus flame retardants. Crop-based phenolics, 3,4,5-triydroxybenzoic acid (gallic acid) and 3,5-dihydroxybenzoic acid, also serve as important BioSource’s for the generation of phosphorus compounds with strong flame-retarding properties.
Introduction
Materials from agricultural sources have long been utilized as precursors for commercially-important chemical agents. [1] This has become increasingly important as concerns about the toxicity and environmental impact of traditional flame retardants and plasticizers have risen.[2-6], [7] In particular, the development of environmentally-friendly, relatively nontoxic flame retardants is receiving great interest. [2-4] Traditional organohalogen compounds have been low-cost, effective flame retardants. However, these materials exhibit several negative properties that are now limiting their use. In a fire, these compounds may be converted to toxic, volatile dioxins [8]. Moreover, they migrate from polymer matrices into which they have been incorporated and enter the environment in several ways–from house dust to food packaging. Perhaps, most importantly, these compounds leach from items discarded in a landfill. [9] They are stable in the environment, tend to bioaccumulate, and enter the human food chain. Human exposure to these materials can lead to a variety of disease states most arising from endocrine disruption. [10, 11] While the toxicity of organophosphorus compounds varies over a broad range–from nerve agents to food additives–as a class they display much lower toxicity then do organohalogen counterparts. [12] In particular, simple phosphate esters, unless halogenated, exhibit negligible toxicity and are widely distributed in the environment. Organophosphorus compounds may be readily prepared from crop-derived alcohols and phenols to generate environmentallyfriendly flame retardants.
Results and Discussion
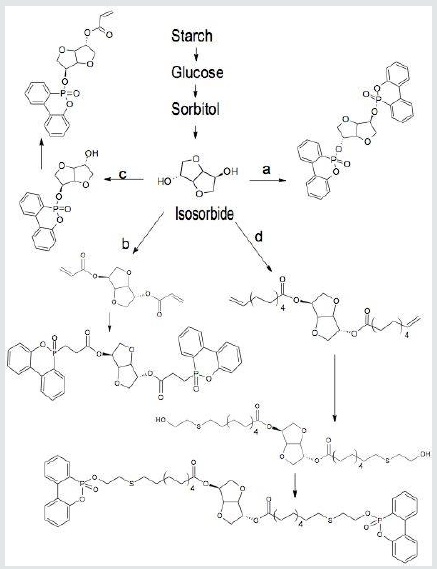
Crop products represent an abundantly-available, annuallyrenewable source of precursors for the generation of nontoxic organophosphorus flame retardants. Starch is a glucose polymer produced by a variety of seed grains, perhaps, most prominently corn. [13] Starch may be hydrolyzed to generate glucose which undergoes hydrogenation to form sorbitol. Double dehydration of sorbitol affords isosorbide, a diether diol, which may serve as a base for the preparation of several types of organophosphorus flame retardants (Scheme 1). [14,15] Isosorbide may also be used for the preparation of polymeric flame retardants. [16-18] In the simplest case, isosorbide may be directly converted to a variety of phosphorus esters. [19, 20] This depicted in [Scheme 1a] for the DOPO (9, 10-dihydro-9-oxa-10-phosphaphenanthrene- 10-oxide) phosphonate. Alternatively, isosorbide may first be converted to the diacrylate (Scheme 1b) and then treated with phosphites (Michael addition) to generate a series of phosphorus derivatives containing phosphorus-carbon bonds. [21] Treatment of isosorbide with 10-undecanoic acid, a biomaterial derived from castor oil, affords a bis-ester containing terminal double bonds (Scheme 1d). This material undergoes smooth thiol-ene reaction with 2-hydroxyethanethiol to provide esters with terminal hydroxyl groups. These esters may be converted to flame-retarding phosphorus esters. [22, 23] All of these phosphorus derivatives of isosorbide function as additive-type flame retardants. Conversion of isosorbide to mono-phosphorus esters followed by esterification with acryloyl chloride (Scheme 1c) generates reactive vinyl monomers which may be covalently incorporated into polymeric materials by copolymerization. [24-26] In all cases, incorporation of these materials into a polymer matrix at a level to provide 1-2% phosphorus provides excellent flame retardance.
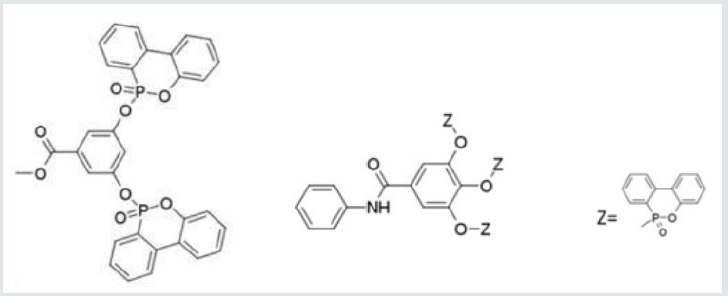
Crop-derived phenols also function as valuable precursors to effective phosphorus flame retardants. Both 3,5-dihydroxbenzoic acid and 3,4,5-trihydroxybenzoic acid (gallic acid) have been utilized for the preparation of phosphorus esters with flameretarding properties. [27, 28] Gallic acid is produced by a wide variety of plants. [29, 30] It contains three hydroxyls and a carboxylic acid group. It was converted to the corresponding anilide and the hydroxyl groups were utilized for the generation of a variety of phosphorus esteres. [27] 3,5-Dihydroxybenzoic acid is also produced by a number of plant crops, most prominently buckwheat. [30] It was converted to the corresponding methyl ester and the ester was used as a base for the preparation of a range of phosphorus compounds. [27, 28] Structures for representative compounds are shown in (Figure 1). Both sets of compounds impart good flame retardancy to a polymeric matrix in which they have been incorporated at a level to provide 1-2% phosphorus. [27,28]
Figure 1: Structures for Representative Phosphorus Flame Retardants Derived from 3, 5-Dihydroxybenzoic Acid and 3,4,5-Trihydroxybenzoic Acid.
Conclusion
Cereal crops and related plants represent valuable sources of biobased alcohols and phenols Which may serve as precursors to environmentally friendly phosphorus flame retardants? These materials are annually renewable, widely dispersed in nature, and generally nontoxic. Starch may be obtained from a variety of cereal grains. It serves as a source of isosorbide, a diether diol, which forms a base for the development of several families of phosphorus flame retardants. 3,5-Dihydroxybenzoic acid, a buckwheat product, and gallic acid, produced by several plants, are plant phenolics from which effective phosphorus flame retardants may be generated.
References
- Princen LH (1982) Alternate Industrial Feedstocks from Agriculture. Economic Botany 36: 302-312.
- Ngo T (2020) Development of Sustainable Flame-retardant Materials. Green Materials 8: 101-122.
- Sonnier R, Taguet A, Ferry L, Lopez-Cuosta JM (2018) Towards Biobased Flame Retardant Polymers, Springer International Publishing AG, Cham, Switzerland
- Sag J, Goeddere D, Kubla P, Griener L, Schonberger F, et al. (2019) Phosphorus-containing Flame Retardants from Biobased Chemicals and their Application in Polyesters and Epoxy Resins. Molecules 24(20): 3746.
- Howell BA, Lazar ST (2018) Biobased Plasticizers from Carbohydrate-derived 2, 5-bis-(Hydroxymethyl)furan. Ind Eng Chem Res 58: 1222-1228.
- Howell BA, Alrubayyi A, Ostrander EA (2019) Thermal Properties of Charring Plasticizers from the Biobased Alcohols, Pentaerythritol and 3,5-Dihydroxybenzoic Acid. J Therm Anal Calorim 138: 2661-2268.
- Howell BA, Daniel YG, Ostrander EA (2018) Flame Retardants from Renewable Sources: Food Waste, Plant Oils and Starch,” in H. N. Cheng and P. B. Smith, Eds., Green Polymer Chemistry: New Products, Processes and Applications (ACS Symposium Series 1920), American Chemical Society, Washington, D. CCh. 25: 405-421.
- Zhang M, Buekens A, Li X (2016) Brominated Flame Retardants and the Formation of Dioxins and Furans in Fires. J. Hazard. Mater 304: 26-39.
- Cristale J, A Bele TG, Lacorte S, de Marchi MRR (2019) Occurence of Flame Retardants in Landfills: A Case Study in Brazil. Envir Res 168: 420-427.
- Darnerud PO (2008) Brominated Flame Retardants as Possible Endocrine Disruptors. Int J Androl 31(2): 152-160.
- Darnerud PO (2003) Toxic Effects of Brominated Flame Retardants in Man and in Wildlife. Environ Int 29(6): 841-853.
- Hoelting L, Krebs A, Nyffeler J, Pape R, Burkle A, et al. (2017) Multiparameter Toxicity Assessment of Novel DOPO-derived Organophosphorus Flame Retardants. Arch. Toxicol 91(1): 407-425.
- Miller D (2018) The Bioharvest is Here. The Progressive Farmer 22-25.
- Yuam D, Li L , Li F, Wang Y, Wong F,et al. (2019) Solvent-free Production of Isosorbide from Sorbitol Catalyzed by a Polymeric Acid. Chem Sus Chem 12(22): 4986-4995.
- Howell BA, Daniel YG (2020) Isosorbide as a Platform for the Generation of New Biobased Organophosphorus Flame Retardants,” Insi. Chem. Biochem 1(2).
- Bauer KN, Tee HT, Velencoso MM, Wurm FR (2017) Main-chain Poly(phosphoester)s: History, Synthesis, Degradation, Bio- and Flame-retardant Applications. Prog. Polym Sci 73: 61-122.
- Hu C, Bourbigot S, Delaunay T, Collinet M, Marcille S, et al. (2019) Synthesis of Isosorbide Based Flame Retardants: Application for Polybutylene Succinate. Polym Degrad. Stab 164: 9-17.
- Mauldin TC, Zammarano M,. Gilman JW, Shields JR, Boday DJ, et al. (2014) Synthesis and Characterization of Isosorbide-based Polyphosphates as Biobased Flame-Retardants Polym Chem 5: 5139-5146.
- Daniel YG, Howell BA (2017) Synthesis and Characterization of Isosorbide bis-Phosphorus Esters,” Heteroat Chem 28(3):
- Daniel YG, Howell BA (2017) Flame Retardant Properties of Isosorbide bis-Phosphorus Esters,” Polym. Degrad. Stab 140: 25-31.
- YG Daniel, BA Howell (2018) Phosphorus Flame Retardants from Isosorbide bis-Acrylate. Polym. Degrad. Stab 156: 14-21.
- Howell BA, Daniel YG (2015) Phosphorus Flame Retardants from Esters of Isosorbide and 10-Undecenoic Acid” in H. N. Cheng, R. A. Gross and P.B. Smith, Eds., Green Polymer
Chemistry: Biobased Materials and Biocatalysis (ACS Symposium Series 1192), American Chemical Society, Washington D. C 21: 339-367.
- Howell BA, Daniel YG (2015) Thermal Degradation of Phosphorus Esters Derived from Isosorbide and 10-Undecenoic Acid. J Therm Anal Calorim 121: 411-419.
- Howell BA, Daniel YG (2020) Synthesis and Characterization of Isomeric Diphenylphosphatoisosorbide Acrylates. Phosphorus, Sulfur, and Silicon and the Related Elements 195: 638-643.
- Howell BA, Daniel YG (2019) “Incorporation of Comonomer exo-5-(Diphenylphosphats)isosorbide-2-endo-acrylate to Generate Flame Retardant Poly(styrene),” Polymers 11(12):
- A. Howell and Y. G. Daniel, “Reactive Flame Retardants from Starch-derived Isosorbide,” in H. N. Cheng and R. A. Gross, Eds., Sustainability and Green Polymer Chemistry. Volume 1: Green Products and Processes (ACS Symposium Series 1372), American Chemical Society, Washington, D. C., 2020, Ch. 12, pp. 209-219.
- BA Howell, KL Oberdorfer, EA Ostrander (2018) Phosphorus Flame Retardants for Polymeric Materials from Gallic Acid and Other Naturally Occurring Multihydroxybeuzoic Acids. Int J Polym Sci 18(3): 1-12.
- BA Howell, EA Ostrander KL Oberdorfer (2020) “Phosphorus Flame Retardants from Crop Plant Phenolic Acids,” in HN Cheng RA Gross, Eds Sustainability and Green Polymer Chemistry. Volume 1: Green Products and Processes (ACS Symposium Series 1372), American Chemical Society, Washington, D. 1: 1-11 pp. 199-208.
- Dewick PM, Haslam E (1969) Phenol Biosynthesis in Higher Plants: Gallic Acid. Biochem J 1969, 113(3): 537-542.
- Ahmed A, Khalid N, Ahmad A, Abbasi NA, MSZ Letif, et al. (2014) Phytochemicals and Biofunctional Properties of Buckwheat: A Review. J Agric Sci 152(3): 349-369.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...