
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6679
Review Article(ISSN: 2637-6679) 
Immunogeneicity of Recombinant Therapeutic Interferon Alpha Volume 1 - Issue 3
Hafiza Rida Farooq Chudhary1*, Ramisha Khan1, Sami Ullah1, Muhammad Bilal1 and Hamid Bashir1
- 1Center for Applied Molecular Biology, University of the Punjab, Pakistan
Received: March 05, 2018; Published: March 09, 2018
*Corresponding author: Hafiza Rida Farooq Chudhary, Centre for applied Molecular Biology, 87-West Canal Bank Road, University of the Punjab, Lahore-53700, Pakistan
DOI: 10.32474/RRHOAJ.2018.01.000112
Abstract
Therapeutic recombinant interferons and cytokines are being used to treat different diseases but these proteins can start immunogenic reactions. These reactions neutralize the effect of therapeutic proteins and make them useless. There are many factors responsible for causing and effecting immunogenicity including dosage, route of administration, genetic status, and polymorphism and Allergic responses. The body's first immune response against recombinant therapeutic cytokines is mediated by innate system, subsequently activating the adaptive immune system. The key factors in immunogenicity are the glycosylation and aggregated structure of therapeutic protein that distinguishes the protein/cytokine from self-proteins. There are many ways to reduce immunogenicity like to decrease the number of epitopes for T-cells or by screening the history of allergies. IFNs are basically proteins in nature many of which are related both in 3D structure and amino acid sequences. Recombinant Interferons are widely used these days against viral infections and cancers. In this review the antiproliferative activity and the effects of various recombinant human interferons on the cytotoxic and cytostatic activity of natural killer cells and monocytes are discussed in detail.
Keywords: Interferons; Neutralizing antibodies; Immunotherapy
Introduction
Many diseases are being treated with drugs having protein nature. These exogenous proteins having therapeutic role are used as a replacement therapy for self-proteins. These Protein therapeutics are checked and analyzed to maintain biosafety measures and toxicity, viral and bacterial contaminations are removed. Beyond checking these measure there is immune response to a protein drug that is called "immunogenicity" can neutralize the effect of therapeutic cytokine (Barandun and others [1]; Dasgupta and others [2]). The concept was that 'self' derived protein therapeutics like recombinant human cytokines IFNβi-3 IFNα4,5 GM-CSF6 and human anti-TNFa7,8 antibodies will not cause immunogenicity. But there are many examples of recombinant proteins that cause host immune responses directed against the therapeutic (Baker and others [3]; Casadevall [4]; Cassinotti and others [5]). Mostly immunogenic reaction involves anaphylaxis, cytokine release syndrome, and production of antibodies that can cross-react with recombinant cytokine and cause neutralization (Jullien and others [6]; Moussa and others [7]).Third type of Immunogenicity involves the generation of anti-therapeutic antibodies and activation of both adaptive (involved high affinity, highly specific antibodies and 'memory' cells) and non-adaptive (initiated by innate receptors not long-lasting). These responses can be neutralizing and non-neutralizing and involved mostly polyclonal antibodies of multiple isotypes (IgM, IgG and IgE). Antibodies that are produced during these responses have variable region. Beacuse protein therapeutics are derived from endogenous proteins by molecular biology techniques like recombinant human erythropoietin that's why any immunogenic response towards these proteins can cross can cross-react with the endogenous protein. In this way it results in morbidity and mortality (Baker and others [3]; Casadevall [4]). There are many factors that affect immunogenicity of therapeutic protein products involving patient-related and product-related factors. These factors determine thee immunogenicity risk assessment (Jullien and others [6]) Figure 1.
Factors playing a role in causing Immunogenicity
There are many factors that are responsible for causing immunogenicity like a deficiency of T cell and B cell tolerance that is resulted due to the absence of the endogenous protein. But immunogenicity can be observed in some patients who already have endogenous proteins. It means other than T and B cell tolerance there are some factors are involved.
Figure 1: Immunogenicity of therapeutic cytokines (Moussa and others [7]).
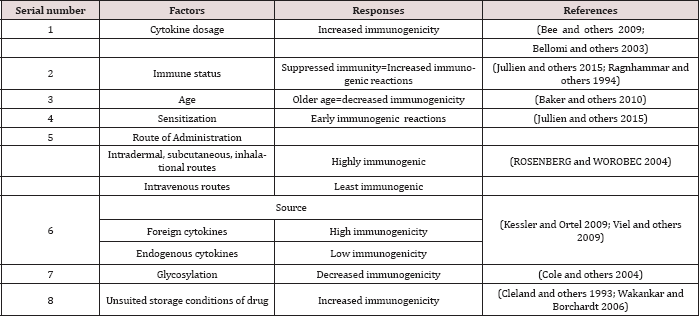
a. Dose of the therapeutic recombinant cytokine or protein because every protein or drug should be administered in an appropriate amount otherwise high or low amount can cause immunogenicity (Bee and others [8]; Bellomi and others [9]).
b. Immunologic conditions status of the Patient like if a pa- tienfs immune system is suppressed then there will be less chance of immunogenicity as compared to healthy individual (Jullien and others [6]; Ragnhammar and others [10])
c. Age of the Patient matters a lot like older individual's immune system is not as activated as an adult so there will be less chance of immunogenicity (Baker and others [3]).
d. Prior allergy or sensitization to that therapeutic product or related product results in antibodies production that's why when these products are administered results in early immunogenic reactions (Jullien and others [6]).
e. Route of administration also effects immunogenicity like intradermal, subcutaneous, and inhalational routes are considered highly immunogenic as compared to intramuscular and intravenous (IV) routes. The intravenous route is least responsible for immune response (Rosenberg and Worobec [11]).
f. Genetic condition like HLA of the patient can play a major role in causing immunogenicity (Hoffmann and others [11]).
g. Recombinant therapeutic protein is human or non-human determine immunogenicity like Foreign proteins have more chances of causing immunogenicity as compared to proteins derived from endogenous proteins (Kessler and Ortel [13]).But due to polymorphism these proteins can also immunogenicity (Viel and others [14]).
h. Glycosylation reduces protein immunogenicity by covering its immunogenic epitopes and reduces protein aggregation (Cole and others [15]; Viel and others [14]).
i. Storing conditions of the drug like if stored in abnormal conditions then oxidation, reduction, methylation or related damage to the protein can result inimmunogenicity (Cle- land and others [16]; Morris and others [17]; Wakankar and Borchardt [18]). Potential cytokines, their immunogenic factor and subsequent responses are tabulated in Table 1.
Table 1:
Response of body's immunity against protein therapeutics
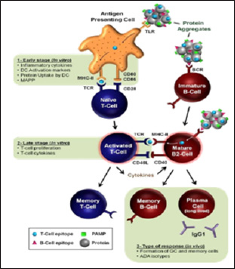
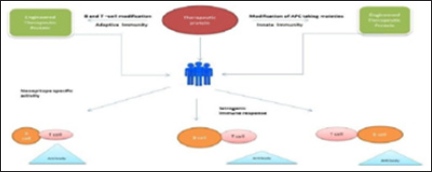
The first line of defense against protein therapeutics mostly recombinant cytokines involve innate receptor activation with subsequent activation of APCs (antigen presenting cells) e.g. B-cells and dendritic cells (Diebold [19]; Vos and others [20]; Yarovinsky and others [21]). These innate receptors present on APCs will in turn activate the adaptive immune response (Adams and others [22]; Medzhitov and Janeway [23]). It has been shown that various functional groups present on drug therapeutics could stimulate the immune system for providing immunogenicity e.g. glycosylation of drugs (Hou and others [24]). This glycosylation make a drug therapeutic unlike 'self' and as a result immune system gets activated against it (De Groot and Scott [25]). The following figure illustrates the activation of adaptive immunity; It has been shown that the protein therapeutics/ cytokines that are aggregated or multimeric in nature shows enhanced immunogenicity as they can enhance humoral immunity more efficiently. It has also been shown that therapeutic recombinant cytokine IFN-a could aggregate in transgenic mice and could raise specific antibodies (Bachmann and others [26]; Braun and others [27]; Rosenberg [28]).
Sometimes, antibodies could preexist in the body derived from the endogenous parts of a therapeutic protein i.e. recombinant cytokine. These occur when the auto reactivity of T/B cells is not screened against the self-proteins. Also, the difference in the protein sequence (cytokine) of natural and recombinant proteins could impart the immunogenicity differently (De Groot and Scott [25]; Marshall and others [29]; Reding and others [30]; Reding and others [31]; Rosenberg [28]). The route of administration influences the immunogenicity of a therapeutic cytokine. Intracellular ad-ministrations elicit enhanced immunogenic response as compared to sub cuntaneous (Schellekens [32]). In case of sub-cutaneous administration, dendritic cells in epidermis also called as Langerhans cells acts as APC and phagocytize the protein and after that process of antigen presentation pathway takes place (De Groot and Scott [25]; De Smedt and others [33]; Pritchard and Smith [34]; Sparwas- ser and others [35]).
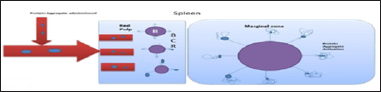
In case of administered cytokines (mostly intracellular), the first line of defense is MZ (marginal zone) B cells as they are strategically placed in the marginal zone of lymph nodes and spleen (Sauerborn and others [36]). The figure below illustrates the mechanism of immune response activation against recombinant therapeutic cytokines. Apart from these general mechanisms, there are many underlying factors that influences the immunogenicity against recombinant therapeutic cytokines, that remains largely unknown (Hermeling and others [37]).
Ways to reduce immunogenicity
There are many ways to reduce immunogenicity of the therapeutic cytokine or protein is to decrease the number of epitopes for T-cells. This is done by deimmunization and by doing this procedure immunogenicity can be decreased to a great extent (Baker and others [3]). The other way to reduce immunogenicity is to screen the history of allergies of the patient because if he is allergic to that therapeutic protein or related one then immunogenic- ity will enhance. To select the appropriate rote of administration and change it during treatment according to the result will prove effective. Third way is to already check the number or quantity of antibodies in the patient for that therapeutic cytokine. Evaluation of the polymorphism for that protein can be proved very effective as it mismatches or similar to therapeutic recombinant cytokine. It is important to remove all the normal cell related impurities. Protein should be glycosylated as it decreases immunogenicity and storage conditions of the drug should be very maintained and appropriate so that denaturation can't occur (Galsky and others [38]; Pandit-Taskar and others [39]; Vallabhajosula and others [40]).
Immunogenicity of therapeutic recombinant Interfer-ons
Interferons were first discovered as powerful antiviral agents some 59 years ago. The recombinant IFN- α2a and IFN-α2b first got approval for the treatment of hairy cell leukemia some 30 years ago. Currently these IFNs are used widely for the treatment of viral infections and various types of cancers. IFNs are basically proteins in nature many of which are related both in 3D structure and amino acid sequences. IFNs are categorized into two types; Type I which usually consist of IFN α, β and ω. While as Type II includes INF-Y which are basically produced by activated T-cells and Natural killer cells. On the other hand, IFN- α and ω are produced by leukocytes while IFN -β are the products of fibroblast (Pfeffer and others [41]) Figure 2.
Figure 2: Activation of adaptive immunity (De Groot and Scott [25])
Recombinant Interferons are widely used these days against viral infections. In one such study recombinant human interferon -consensus (IFN-Conl) showed greater antiproliferative activity and enhanced natural killer cell killing of target cells than that of IFN- α2a and IFN-α2b, but the antiviral activities of IFN-Conl were similar to that of IFN- α2a and IFN-α2b. So IFN-Conl would be significant in low protein concentration than other IFNs for clinical applications (OZES and others [42]). Another study shows that IFN-Conl as compared to IFN-α is a lower inducer of IL-lβ (Blatt and others [43]).
When three recombinant human interferons naming IFN- α2a, IFN-β and INF-ϒ were compared in human cultured fibroblast cells against cardiotrophic enterovirus. After few days when a persistent infection was developed IFNs were added. As a result IFN-β and INF-ϒ showed higher activity and reduced viral load significantly on the contrary IFN- α2a was found to be less effective than the other two interferons (HEIM and others [44]). In another research natural IFN-a reduced same amount of viral load at lower concentration as compared to recombinant IFN-α2a (Heim and others [45]).
When the effect of recombinant human leukocyte interferon (IFLrA) on the cytotoxic and cytostatic activity of natural killer cells and monocytes was studied it was found that a very small amount of interferon resulted in high natural killing and monocyte-mediated activity (Herberman and others [46]). In a study on cancer patients when a single dose of IFN-arA was injected into cancer patients the cytotoxicity of natural killer cells of all patients declined significantly (Lotzova and others [47]).
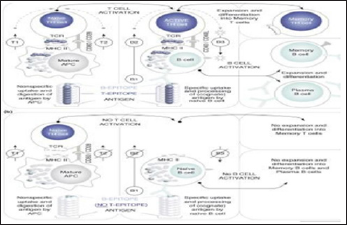
In a study two human recombinant IFNs (A&D) along with five of their hybrids containing various portions of A and D were tested along with a fibroblast recombinant IFNs for the modulation of cytolytic activity of NK cells in humans. It was observed in mouse that these interferons caused correlation between antiviral activity and augmentation of mouse NK activity. On the other hand such correlation was not observed in humans Figure 3.
Figure 3: Immune activation by recombinant therapeutic proteins
In a research it was found that there is a selective antiproliferative effect of recombinant human interferons on malignant human myeloid progenitor cells as compared to normal cells (Grant and others [48]; Ortaldo and others [49]). In another such study it was revealed that, in addition to antiviral activity the recombinant human leukocyte interferons produced in E.coli also exhibit antiproliferative activities (Defrance and others [50]; Evinger and others [51]). In a study it was illustrated that recombinant human interferon-gamma act as a B-cell growth factor (BCGF) but this proliferation of B-cell is short lasting as compared to the proliferation induced by recombinant IL-(Defrance and others [50]; Romagnani and others [52]). PCSK6 aid in the cell proliferation, migration, and inflammation in rheumatoid arthritis fibroblast like synoviocytes (RAFS) by activating NF-kB, STAT3 and ERK1/2 signaling pathways (Jiang and others [53]).
As the focus of this article, interferon should be taken into consideration as they are promising for the future. Currently, interferons are being used in viral defense as they generate antiviral state especially against HBV and HCV infections but many studies indicated its adverse effects. For example interferon could induce the proliferation of pro-inflammatory cytokines like TNF and interluk- ins which could alter the mechanism and actions of many important metabolic precursors including tryptophan, serotonin etc. The immunogenicity and inflammation also links with depression as serotonin levels are disturbed, many patients have been shown to develop depression (Fried [54]).
It has been observed that aggregated or partially denatured repeated injections of recombinant IFNs , causes decrease in the immune tolerance to self-antigens leads to the to the production of antidrug antibodies (De Santi and others [55]). ADAs cause neutralization of the drug, immune complex disease, allergic reactions and severe autoimmune reactions. IFNs can cause damage by promoting tumor growth because it has observed that IFNg causes resistance to NK cells that results in enhanced metastatic ability and up -regulation of non classical MHC class I molecules that cause inhibition of NK and T cell killing so the assessment of immunogenicity before giving interferon is very necessary [56] Figure 4.
Figure 4: Ways to reduce immunogenicity.
Future perspective
a. Numerous cytokines like TGF-β, CCN2, IFN-α and IFN-β had a great potential for the treatment of connective tissue diseases like Systemic sclerosis (SSc).
b. Recombinant IL-7 has potential role in Tumor-direct Im-munotherapy and as an Immunorestorative.
c. TGF-β is predicted to have a great role in engineered or-ganogenesis.
d. Recombinant cytokines will be the key components of future HIV therapies.
e. As interferon shows to be producing adverse effects in patients usually inflammation so restricting the dose of interferon or using interferon in a combination therapy may prevent inflammatory problems associated with the cytokines.
f. Assessment of immunogenicity before giving interferon is very necessary
References
- Barandun S, Kistler P, Jeunet F, Isliker H (1962) Intravenous Administration of Human ϒ-Globulin. Vox Sang 7(2): 157-174.
- Dasgupta S, Bayry J, André S, Dimitrov JD, Kaveri SV, et al. (2008) Auditing protein therapeutics management by professional APCs: toward prevention of immune responses against therapeutic proteins. J Immunol 181(3): 1609-1615.
- Baker M, Reynolds HM, Lumicisi B, Bryson CJ (2010) Immunogenicity of protein therapeutics: The key causes, consequences and challenges. Self/nonself 1(4): 314-322.
- Casadevall N (2003) Pure red cell aplasia and anti-erythropoietin antibodies in patients treated with epoetin. Nephrol Dial Transplant 18(suppl_8): viii37-viii41.
- Cassinotti A, Ardizzone S, Porro GB (2008) Adalimumab for the treatment of Crohn's disease. Biologics 2(4): 763-777.
- Jullien D, Prinz JC, Nestle FO (2015) Immunogenicity of biotherapy used in psoriasis: the science behind the scenes. The Journal of Investigative Dermatology 135(1): 31-38.
- Moussa EM, Panchal JP, Moorthy BS, Blum JS, Joubert MK (2016) Immunogenicity of therapeutic protein aggregates. Journal of pharmaceutical sciences 105(2): 417-430.
- Bee JS, Nelson SA, Freund E, Carpenter JF, Randolph TW (2009) Precipitation of a monoclonal antibody by soluble tungsten. J Pharm Sci 98(9): 3290-3301.
- Bellomi F, Scagnolari C, Tomassini V, Gasperini C, Paolillo A, et al. (2003) Fate of neutralizing and binding antibodies to IFN beta in MS patients treated with IFN beta for 6 years. J Neurol Sci 215(1-2): 3-8.
- Ragnhammar P, Friesen HJ, Frodin JE, Lefvert AK, Hassan M, et al. (1994) Induction of anti-recombinant human granulocyte-macrophage colony- stimulating factor (Escherichia coli-derived) antibodies and clinical effects in nonimmunocompromised patients. Blood 84(12): 4078-4087.
- Rosenberg AS, Worobec A (2004) A risk-based approach to immunogenicity concerns of therapeutic protein products, part I. Biopharm international 17(11): 22-26.
- Hoffmann S, Cepok S, Grummel V, Lehmann Horn K, Hackermueller J, et al. (2008) HLA-DRB1* 0401 and HLA-DRB 0408 Are Strongly Associated with the Development of Antibodies against Interferon-p Therapy in Multiple Sclerosis. The American Journal of Human Genetics 83(2): 219227.
- Kessler CM, Ortel TL (2009) Recent developments in topical thrombins. Thrombosis and Haemostasis 102(1): 15-24.
- Viel KR, Ameri A, Abshire TC, Iyer RV, Watts RG, et al. (2009) Inhibitors of factor VIII in black patients with hemophilia. New England Journal of Medicine 360(16): 1618-1627.
- Cole KS, Steckbeck JD, Rowles JL, Desrosiers RC, Montelaro RC (2004) Removal of N-linked glycosylation sites in the V1 region of simian immunodeficiency virus gp120 results in redirection of B-cell responses to V3. J Virol 78(3): 1525-1539.
- Cleland JL, Powell MF, Shire SJ (1993) The development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation. Crit Rev Ther Drug Carrier Syst 10(4): 307-377.
- Morris MJ, Divgi CR, Pandit Taskar N, Batraki M, Warren N, et al. (2005) Pilot trial of unlabeled and indium-111-labeled anti-prostate-specific membrane antigen antibody J591 for castrate metastatic prostate cancer. Clinical cancer research 11(20): 7454-7461.
- Wakankar AA, Borchardt RT (2006) Formulation considerations for proteins susceptible to asparagine deamidation and aspartate isomerization. Journal of pharmaceutical sciences 95(11): 2321-2336.
- Diebold SS (2009) Activation of dendritic cells by toll-like receptors and C-type lectins. Handb Exp Pharmacol (188): 3-30.
- Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ (2000) B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunological reviews 176: 154-170.
- Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, et al. (2005) TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308(5728): 1626-1629.
- Adams EW, Ratner DM, Seeberger PH, Hacohen N (2008) Carbohydrate- Mediated Targeting of Antigen to Dendritic Cells Leads to Enhanced Presentation of Antigen to T Cells. Chembiochem 9(2): 294-303.
- Medzhitov R, Janeway CA (1997) Innate immunity: impact on the adaptive immune response. Current opinion in immunology 9(1): 4-9.
- Hou B, Reizis B, DeFranco AL (2008) Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and-extrinsic mechanisms. Immunity 29(2): 272-282.
- De Groot AS, Scott DW (2007) Immunogenicity of protein therapeutics. Trends Immunol 28(11): 482-490.
- Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, et al. (1993) The influence of antigen organization on B cell responsiveness. Science 262(5138): 1448-1451.
- Braun A, Kwee L, Labow MA, Alsenz J (1997) Protein aggregates seem to play a key role among the parameters influencing the antigenicity of interferon alpha (IFN-a) in normal and transgenic mice. Pharm Res 14(10): 1472-1478.
- Rosenberg AS (2006) Effects of protein aggregates: an immunologic perspective. The AAPS journal 8(3): E501-E507.
- Marshall SA, Lazar GA, Chirino AJ, Desjarlais JR (2003) Rational design and engineering of therapeutic proteins. Drug discovery today 8(5): 212-221.
- Reding M, Wu H, Krampf M, Okita D, Diethelm Okita B, et al. (1999) CD4+ T cell response to factor VIII in hemophilia A, acquired hemophilia, and healthy subjects. Thrombosis and haemostasis 82(2): 509-515.
- Reding MT, Wu H, Krampf M, Okita DK, Diethelm Okita BM, et al. (2000) Sensitization of CD4+ T cells to coagulation factor VIII: response in congenital and acquired hemophilia patients and in healthy subjects. Thrombosis and haemostasis 84(4): 643-652.
- Schellekens H (2005) Factors influencing the immunogenicity of therapeutic proteins. Nephrology Dialysis Transplantation 20(suppl 6): vi3-vi9.
- De Smedt T, Pajak B, Muraille E, Lespagnard L, Heinen E, et al. (1996) Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J Exp Med 184(4): 1413-1424.
- Pritchard NR, Smith KG (2003) B cell inhibitory receptors and autoimmunity. Immunology 108(3): 263-273.
- Sparwasser T, Koch ES, Vabulas RM, Heeg K, Lipford GB, et al. (1998) Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. European journal of immunology 28(6): 2045-2054.
- Sauerborn M, Brinks V, Jiskoot W, Schellekens H (2010) Immunological mechanism underlying the immune response to recombinant human protein therapeutics. Trends in pharmacological sciences 31(2): 53-59.
- Hermeling S, Crommelin DJ, Schellekens H, Jiskoot W (2004) Structure- immunogenicity relationships of therapeutic proteins. Pharmaceutical research 21(6): 897-903.
- Galsky MD, Eisenberger M, Moore Cooper S, Kelly WK, Slovin SF, et al. (2008) Phase I Trial of the Prostate-Specific Membrane Antigen- Directed Immunoconjugate MLN2704 in Patients With Progressive Metastatic Castration-Resistant Prostate Cancer. Journal of clinical oncology 26(13): 2147-2154.
- Pandit Taskar N, O Donoghue JA, Morris MJ, Wills EA, Schwartz LH, et al. (2008) Antibody mass escalation study in patients with castration- resistant prostate cancer using 111In-J591: lesion detectability and dosimetric projections for 90Y radioimmunotherapy. Journal of Nuclear Medicine 49(7): 1066-1074.
- Vallabhajosula S, Goldsmith SJ, Kostakoglu L, Milowsky MI, Nanus DM, et al. (2005) Radioimmunotherapy of prostate cancer using 90Y-and 177Lu-labeled J591 monoclonal antibodies: effect of multiple treatments on myelotoxicity. Clinical cancer research 11(19): 7195s-7200s.
- Pfeffer LM, Dinarello CA, Herberman RB, Williams BR, Borden EC, et al. (1998) Biological properties of recombinant a-interferons: 40th anniversary of the discovery of interferons. Cancer research 58(12): 2489-2499.
- Ozes On, Reiter Z, Klein S, Blatt Lm, Taylor Mw (1992) A comparison of interferon-Con1 with natural recombinant interferons-a: antiviral, antiproliferative, and natural killer-inducing activities. Journal of interferon research 12(1): 55-59.
- Blatt LM, Davis JM, Klein SB, Taylor MW (1996) The biologic activity and molecular characterization of a novel synthetic interferon-alpha species, consensus interferon. J Interferon Cytokine Res 16(7): 489-499.
- Heim A, Stille Siegener M, Pring Akerblom P, Grumbach I, Brehm C, et al. (1996) Recombinant interferons p and y have a higher antiviral activity than interferon-a in coxsackievirus B3-infected carrier state cultures of human myocardial fibroblasts. Journal of interferon & cytokine research 16(4): 283-287.
- Heim A, Brehm C, Stille Siegener M, Muller G, Hake S, et al. (1995) Cultured human myocardial fibroblasts of pediatric origin: Natural human interferon-a is more effective than recombinant interferon-a 2a in carrier-state coxsackievirus B3 replication. Journal of molecular and cellular cardiology 27(10): 2199-2208.
- Herberman RB, Ortaldo JR, Mantovani A, Hobbs DS, Kung Hf, et al. (1982) Effect of human recombinant interferon on cytotoxic activity of natural killer (NK) cells and monocytes. Cellular immunology 67(1): 160-167.
- Lotzová E, Savary CA, Quesada JR, Gutterman JU, Hersh EM (1983) Analysis of Natural Killer Cell Cytotoxicity of Cancer Patients Treated With Recombinant lnterferon. Journal of the National Cancer Institute 71(5): 903-910.
- Grant S, Bhalla K, Weinstein IB, Pestka S, Fisher PB (1982) Differential effect of recombinant human leukocyte interferon on human leukemic and normal myeloid progenitor cells. Biochemical and biophysical research communications 108(3): 1048-1055.?
- Ortaldo J, Mason A, Rehberg E, Moschera J, Kelder B, et al. (1983) Effects of recombinant and hybrid recombinant human leukocyte interferons on cytotoxic activity of natural killer cells. Journal of Biological Chemistry 258(24): 15011-15015.
- Defrance T, Aubry J, Vanbervliet B, Banchereau J (1986) Human interferon-gamma acts as a B cell growth factor in the anti-IgM antibody co-stimulatory assay but has no direct B cell differentiation activity. J Immunol 137(12): 3861-3867.
- Evinger M, Maeda S, Pestka S (1981) Recombinant human leukocyte interferon produced in bacteria has antiproliferative activity. J Biol Chem 256(5): 2113-2114.
- Romagnani S, Giudizi M, Biagiotti R, Almerigogna F, Mingari C (1986) B cell growth factor activity of interferon-y. Recombinant human interferon-y promotes proliferation of anti-^-activated human B lymphocytes. Journal of Immunology 136(10): 3513-3516.
- Jiang H, Wang L, Wang F, Pan J (2017) Proprotein convertase subtilisin/ kexin type 6 promotes in vitro proliferation, migration and inflammatory cytokine secretion of synovial fibroblast like cells from rheumatoid arthritis via nuclear kB, signal transducer and activator of transcription 3 and extracellular signal regulated 1/2 pathways. Molecular Medicine Reports 16(6): 8477-8484.
- Fried MW (2002) Side effects of therapy of hepatitis C and their management. Hepatology 36(5 Suppl 1): S237-44.
- De Santi L, Costantini M, Annunziata P (2005) Long time interval between multiple sclerosis onset and occurrence of primary Sjogren's syndrome in a woman treated with interferon-beta. Acta Neurol Scand 112(3): 194-196.
- Reding M, Wu H, Krampf M, Okita D, Diethelm Okita B, et al. (1999) CD4+ T cell response to factor VIII in hemophilia A, acquired hemophilia, and healthy subjects. Thrombosis and haemostasis 82(2): 509-515.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...

.png)











