Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6679
Research Article(ISSN: 2637-6679) 
Antibiotic Resistance Pattern of Nesseria Gonorrhoea at the Genitourinary Medicine Clinic, Hospital Kuala Lumpur, Malaysia Volume 1 - Issue 5
Meena Nithianandan and Azura Mohd Affandi*
- Department of Dermatology, Hospital Kuala Lumpur, Malaysia
Received: May 07, 2018; Published: May 14, 2018
Corresponding author: Azura Mohd Affandi, Department of Dermatology, Hospital Kuala Lumpur, Malaysia
DOI: 10.32474/RRHOAJ.2018.01.000124
Abstract
Background: In the era of super bugs, there is a need to monitor antibiotic resistance patterns. Due to the emergence of antimicrobial resistance worldwide, local antibiotic resistance patterns should be monitored periodically to alert early intervention. This audit was conducted to analyse the antibiotic resistance patterns among the gonococcal urethritis cases that presented to the Genitourinary Medicine (GUM) Clinic, Hospital Kuala Lumpur (HKL), Malaysia.
Methodology: This is a retrospective study on the antibiotic resistance patterns based on 370 culture positive gonorrhoea obtained from urethral swab samples sent between 2011 and 2015. Antimicrobial susceptibility testing by standard disc diffusion method was performed to detect sensitivity to penicillin, tetracycline, ciprofloxacin, cefuroxime, azithromycin and ceftriaxone. All data was obtained from microbiology report and patient records.
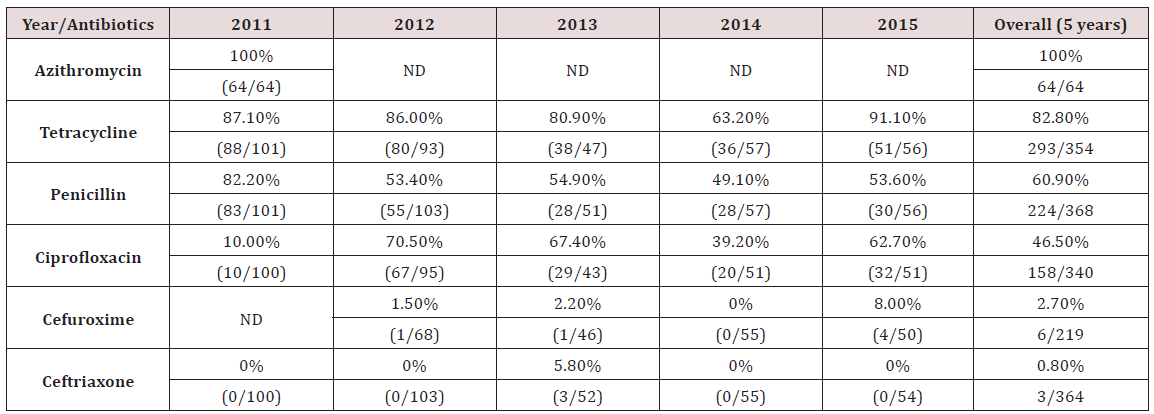
Results: A total of 370 positive culture isolates of N.gonorrhoeae (new and recurrent cases) from 2011 to 2015 were reviewed. Highest level of resistance detected was to azithromycin (100%, 64/64) followed by tetracycline (82.8%, 293/354). Resistance to penicillin was noted in 60.9% (224/368) of all isolates. Both penicillin and tetracycline showed a decreasing resistance trend from 2011-2015. The fourth commonest antibiotic resistance was to ciprofloxacin at 46.5% (158/340). Cephalosporins tested were cefuroxime and ceftriaxone, which showed resistance rates of 2.7% (6/219) and 0.8% (3/364), respectively.
Conclusion: The complete resistance to azithromycin is alarming since it is a common antibiotic used to treat urethral discharge using the syndromic approach. Penicillin and tetracycline resistance remain high in Malaysia and other Western Pacific countries. The current first line antibiotic for treating gonorrhoea in GUM Clinic, HKL is ceftriaxone. Clinicians should be aware of the newly discovered increase in resistance observed to ceftriaxone.
Keywords: Neisseria gonorrhoeae; Gonorrhoea; Antibiotic Resistance
Introduction
The last decade has seen Neisseria gonorrhoeae emerging as a true superbug, bringing.com closer to a time of untreatable gonorrhoea. This diplococcal microbe is able to recombine its genes and invade the immune system through antigenic variation. It is also naturally competent to acquire new deoxyribonucleic acid (DNA), enabling N. gonorrhoeae to spread new genes, disguise itself with different surface proteins, and prevent the development of immunological memory an ability that has led to antibiotic resistance and has made vaccine development difficult. Gonorrhea is a debilitating disease, which was responsible for an estimated 445,000 years lived with disability in 2015, according to a systemic analysis for the Global Burden of Disease Study [1]. Patients infected with N.gonorrhoeae are known to present with urethral discharge, malaise and symptoms that may suggest a urinary tract infection. Nevertheless, urogenital gonorrhea may be asymptomatic in 40% of men and often manifests as urethritis [2,3].
Unfortunately, it is also asymptomatic in more than half of women [4]. In men, untreated urethral infection can lead to epididymitis, reduced fertility, and cause urethral strictures. In women, if present, symptoms are non specific and include abnormal vaginal discharge, dysuria, lower abdominal discomfort, and dyspareunia. The lack of discernible symptoms results in unrecognized and untreated infections, which can lead to serious complications [5]. Overall, 10%-20% of female patients develop pelvic inflammatory disease (PID) and, consequently, are at risk for infertility [6]. Pregnancy complications associated with gonorrhea include chorioamnionitis, premature rupture of membranes, preterm birth, ectopic pregnancies, and spontaneous abortions [5,7,8]. Infants of mothers with gonococcal infection can be infected at delivery, resulting in neonatal conjunctivitis (ophthalmia neonatorum). Such untreated conjunctivitis may lead to scarring and blindness.
Extragenital infections are common in both sexes and frequently occur in the absence of urogenital infection [9,10]. Rectal infections are usually asymptomatic but can manifest as rectal and anal pain or discharge. Pharyngeal infections are mostly asymptomatic, but mild sore throat and pharyngitis may occur. Although bacterial concentrations are generally lower than in other infection sites, the pharynx is thought to be a favourable site for resistance emergence due to acquisition of resistance traits from commensal Neisseria spp [11]. Disseminated gonococcal infections with gonococcal arthritis also occur. Because they are frequently asymptomatic, extragenital infections often remain untreated, despite their key role in disease transmission. Co-infection with other major Sexually Transmitted Infections (STIs) HIV, Herpes simplex virus, Chlamydia trachomatis, Mycoplasma genitalium, and Treponema pallidum are common and may result in synergistic effects on transmission and disease severity. Attempts to treat and control gonorrhoea are compromised by the emergence and spread of antibiotic resistant N.gonorrhoeae. Antibiotic resistance pattern vary between different geographical areas. It is therefore important to know the local antibiotic resistance pattern, so that appropriate treatment can be instituted. In Malaysia, Kanamycin was used as the first line antibiotic to treat gonorrhoea during the early 1970’s and 80’s, which was subsequently changed to Spectinomycin, followed by Ceftriaxone since the early 1990’s [12] There are many surveillance programmes on antibiotic resistance patterns of N.gonorrhoeae such as GRASP (Gonococcal Resistance to Antimicrobial Surveilance Programme), that is based in London, UK, and WHO (World Health Organization) Antimicrobial Surveilance Programme [13,14].
Materials and Methods
All patients with positive culture for gonorrhoea, who attended the GUM clinic in HKL between 2011-2015, were included in this study. Antimicrobial susceptibility testing by standard disc diffusion method was performed to detect sensitivity to Penicillin, Tetracycline, Ciprofloxacin, Cefuroxime, Azithromycin and Ceftriaxone. Data was obtained from patient records and formal microbiology laboratory results.
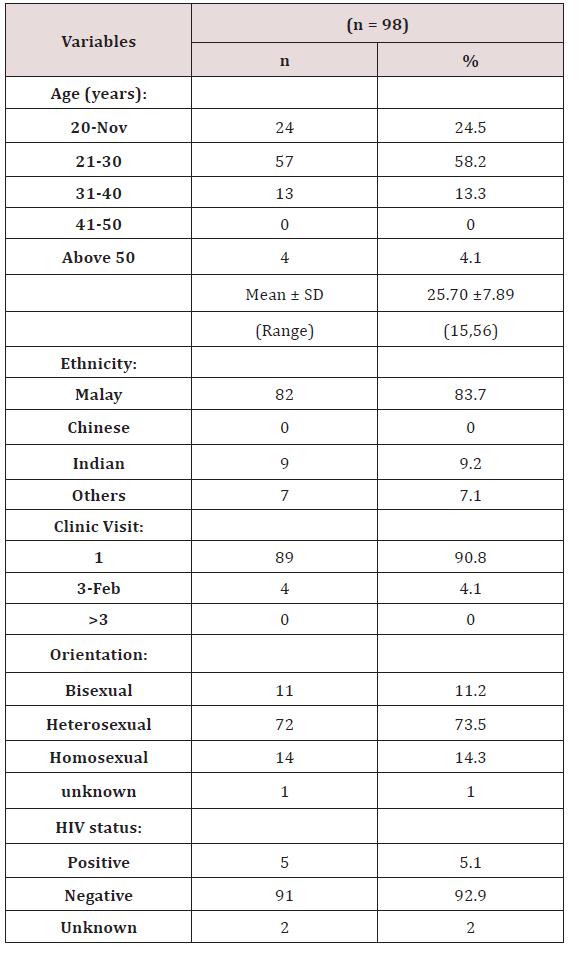
Results
370 positive culture isolates of N.gonorrhoeae from patients seen in 2011-2015 were included in this study. Most of the data were obtained from the microbiology laboratory results. Demographic data was available for 98 patients only. More than half of the patients (58.2%) were between 21-30 years old. Most patients were Malay (83.7%), followed by Indian (9.2%). Overall, the heterosexually orientated patients represented about 73% of gonococcal urethritis cases. Majority of cases (92%) tested negative for HIV (Tables 1 & 2). The highest level of resistance detected was to azithromycin (100%, 64/64), followed by tetracycline (82.8%, 293/354). Resistance to penicillin was noted in 60.9% (224/368) of all isolates. Both penicillin and tetracycline showed a decreasing resistance trend from 2011-2014, but increased in 2015. The fourth commonest antibiotic resistance was to ciprofloxacin at 46.5% (158/340), followed by cefuroxime 2.7% (6/219). Resistance to ceftriaxone was 0.8% (3/364), although reviews previously in 2001-2005 showed no resistance [12]. The results were compared to data obtained from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) and WHO (World Health Organization) Antimicrobial Surveillance Programme [13,14].
Discussion
Azithromycin
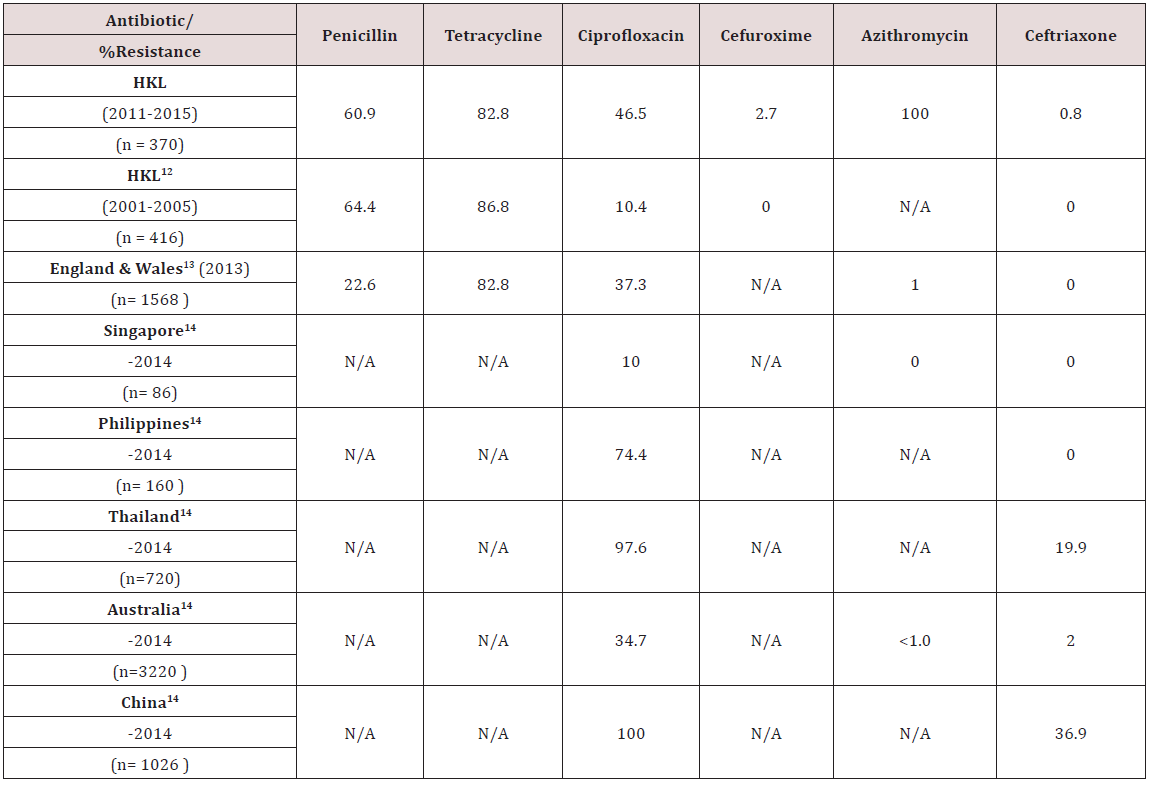
The rate of resistance to Azithromycin in this study was higher than expected. All 64 samples tested for sensitivity to Azithromycin showed resistance. In Singapore, no resistance to Azithromycin has been documented [14]. Similarly in England & Wales and Australia, the rate of resistance is significantly lower, at 1% or less [13,14] (Table 3). Studies have indicated concerns for increasing resistance to Azithromycin, likely due to delay in diagnosis of gonorrhoea and suboptimal dose of Azithromycin used [15].
Tetracycline
The rate of resistance to Tetracycline is high. In our study, 82.8% of N.gonorrhoea isolates were resistant to Tetracycline. This is slightly lower, compared to an earlier analysis done in HKL from 2001-2005, where 86.8% of isolates were resistant [12]. The resistance rates in England & Wales was similar to ours [13] (Table 3). Looking at the trend, there was a reduction in resistance rates from 87.1% in 2011 to 63.2% in 2014. However, the rate increased to 91.1% in 2015 (Table 2). In HKL, Doxycycline is sometimes used to treat non-gonoccocal urethritis but never as primary treatment for gonorrhoea. Tetracycline has never been used for treating gonorrhoea in HKL as the resistance is very high. Nevertheless, the resistance pattern is continuously monitored for epidemiological purposes.
Penicillin
Since the 1940’s, Penicillin was successfully used to treat gonorrhoea, but quickly developed decreased sensitivity and deemed not a suitable treatment after 1970. This can be due to Penicillinase Producing N.gonorrhoeae (PPNG) or Chromosomal Mediated Resistance N.gonorrhoeae (CMRNG) [16]. Our study shows that the rate of N.gonorrhoeae resistance to Penicillin has reduced, from 82.2% in 2011 to 53.6% in 2015 (Table 2). Compared to an earlier study done in HKL in 2001-2005, there was a slight drop in resistance to penicillin in HKL from 64.4% to 60.9% in 2011-2015. However, our resistance rates were much higher compared to the resistance rate reported in England & Wales of 22.6% (Table 3) [13].
Ciprofloxacin
In the early 1990’s, Ciprofloxacin was widely used especially by general practitioners to treat gonorrhoea although studies had already began demonstrating the beginning of reduced sensitivity to quinolones. The resistance to Ciprofloxacin in HKL showed a steady increase from 10.0% in 2011 to 62.7% in 2015 (Table 2). When compared to an earlier review in HKL from the period 2001- 2005, we can see marked increase in resistance to Ciprofloxacin from 10.4% to 46.5% in 2011-2015 (Table 3). The resistance rate reported in HKL from 2011-2015 was similar to England & Wales and Australia, which reported resistance of 37.3% and 34.7% respectively (Table 3) [13,14]. Among the Asian countries, Singapore reported the lowest resistance to Ciprofloxacin (10.0%) [14]. Other Asian countries, like the Phillipines, Thailand and China reported an alarmingly high resistance to Ciprofloxacin, which is between 74.4%-100% (Table 3) [14].
Cephalosporin–Cefuroxime and Ceftriaxone
Although Cefuroxime is not a recommended treatment for gonorrhoea, its resistance pattern is monitored for epidemiological purposes. Our study showed a resistance rate of 2.7% to Cefuroxime in 2011-2015, whereas an earlier study in 2001-2005 showed no resistance to Cefuroxime (Table 3). Susceptibility testing for Ceftriaxone use in the treatment of gonorrhoea in HKL between 2001-2005 indicated no resistance, however, recent data from 2011-2015 showed a resistance rate of 0.8% (Table 3). Ceftriaxone is the first line treatment of gonorrhoea in HKL and clinicians should be aware that we are seeing a small percentage of resistance in some cases. No resistance was noted in Singapore and the Phillipines (Table 3) [14]. Resistance rates to Ceftriaxone in Thailand and China are significantly higher, at 19.9% and 36.9% respectively (Table 3) [14].
Conclusion
Attempts to treat and control gonorrhoea are compromised by the emergence and spread of antibiotic-resistant N.gonorrhoeae. WHO expert committee has recommended that treatment regimen be altered once resistance to a particular antibiotic reaches 5%. High rates of resistance to Penicillin and Tetracycline have been documented in HKL and in the Western Pacific region. Within 15 years, a marked increase in Ciprofloxacin resistance (10% to 46.5%) is evident. Resistance to Cefuroxime and Ceftriaxone was discovered, which was not found in the previous study. Ceftriaxone remains the first line antibiotic in treating gonorrhoea in HKL, and clinicians need to be aware of the small percentage of resistance detected to Ceftriaxone.
Acknowledgement
We would like to thank the Director General, Ministry of Health, Malaysia for permission to publish this study, and the staff from Genitourinary Medicine Clinic, HKL for data collection.
References
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (2016) Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053): 1545-1602.
- Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK (1974) Asymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significance. N Engl J Med 290(3): 117-123
- Harrison WO, Hooper RR, Wiesner PJ, Campbell AF, Karney WW, et al. (1979) A trial of minocycline given after exposure to prevent gonorrhea. N Engl J Med 300(19): 1074-1078.
- Van Der Pol B (2014) Sexually transmitted infections in women. Scand J Clin Lab Invest Suppl 244: 68-74.
- Maxwell GL, Watson WJ (1992) Preterm premature rupture of membranes: results of expectant management in patients with cervical cultures positive for group B streptococcus or Neisseria gonorrhoeae. Am J Obstet Gynecol 166(3): 945-949.
- Tsevat DG, Wiesenfeld HC, Parks C, Peipert JF (2017) Sexually transmitted diseases and infertility. Am J Obstet Gynecol 216(1): 1-9.
- Liu B, Roberts CL, Clarke M, Jorm L, Hunt J, et al. (2013) Chlamydia and gonorrhoea infections and the risk of adverse obstetric outcomes: a retrospective cohort study. Sex Transm Infect 89(8): 672-678.
- Donders GG, Desmyter J, De Wet DH, Van Assche FA (1993) The association of gonorrhoea and syphilis with premature birth and low birthweight. Genitourin Med 69(2): 98-101.
- Koedijk FD, van Bergen JE, Dukers Muijrers NH, van Leeuwen AP, Hoebe CJ, et al. (2012) The value of testing multiple anatomic sites for gonorrhoea and Chlamydia in sexually transmitted infection centres in the Netherlands, 2006-2010. Int J STD AIDS 23(9): 626-631.
- Van Liere GA, Hoebe CJ, Dukers Muijrers NH (2014) Evaluation of the anatomical site distribution of Chlamydia and gonorrhoea in men who have sex with men and in high risk women by routine testing: crosssectional study revealing missed opportunities for treatment strategies. Sex Transm Infect 90(1): 58-60.
- Unemo M, Nicholas RA (2012) Emergence of multidrug resistant, extensively drug resistant and untreatable gonorrhea. Future Microbiol 7(12): 1401-1422.
- Mohd Affandi A, Gangaram HB, Hussein SH (2007) Antibiotic Resistance Pattern of Neisseria gonorrhoeae in Hospital Kuala Lumpur, Malaysia (2001-2005). Malaysian Journal of Dermatology 19: 35-40.
- Public Health England (2012) Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) 2012 Report. PHE publications gateway, pp. 2014442
- WHO(2012) World Health Organization. Baseline report on global sexually transmitted infection surveillance. Geneva.
- Chisholm SA, Neal TJ, Alawattegama AB, Birley HD, Howe RA (2009) Emergence of high-level azithromycin resistance in Neisseria gonorrhoeae in England and Wales. J Antimicrob Chemother 64(2): 353- 358.
- Unemo M, Shafer WM (2011) Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Ann N Y Acad Sci 1230(1): E19-E28.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...

.png)