Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1768
Research Article(ISSN: 2641-1768) 
A Review on Nerve Conduction Studies Volume 1 - Issue 3
Mehrnaz Moattari1, Farahnaz Moattari2, Gholamreza Kaka3 and Homa Mohseni Kouchesfahani1*
- 1Department of Animal Biology, Kharazmi University, Iran
- 2Faculty of Agriculture and Natural Resources, Persian Gulf University, Iran
- 3Neuroscience Research Center, Baqiyatallah University of Medical Sciences, Iran
Received: September 14, 2018; Published: September 21, 2018
Corresponding author: Homa Mohseni Kouchesfahani, Department of Animal Biology, Kharazmi University, Iran
DOI: 10.32474/SJPBS.2018.01.000115
Abstract
Electrophysiological measurements are indispensable tool for investigating the functional integrity of peripheral nerves in both clinical and laboratory environments. Nerve conduction study is a property of both the axons and myelin sheath. The nerve conduction velocity is highly dependent on rapid signal propagation enabled by myelination. The compound motor action potential (CMAP) which correlates with the number of functional axons is an indicator for axonal damage when significantly reduced. Because nerve conduction velocity (NCV) is sensitive to both myelin sheath and axonal changes, it is an excellent measure of the function of the peripheral nervous system. Nerve conduction studies may be diagnostically helpful in patients suspected of having almost any peripheral nervous system disorder including disorders of nerve roots, peripheral nerves, muscle and neuromuscular junction. Cranial nerves and spinal cord function may also be assessed. Here, we mentioned the components and properties of nerve conduction study.
Keywords: Amplitude; Compound Motor Action Potential; Late Response; Latency; Nerve Conduction Study; Nerve Conduction Velocity; Sensory Nerve Action Potential
Introduction
Nerve conduction study is a simple and reliable test for evaluation of peripheral nerve function. The test is not invasive. In this technique nerve conduction in the largest and fastest myelinated fibers are recorded and it establishes the type and degree of abnormality. The site of lesion can be identified and localized[1]. This technique can be used for evaluation of conductive velocity in upper extremity, mostly the median and ulnar nerves and in the lower extremity including the peroneal, tibial, and sural nerves. NCS is a test commonly used to evaluate the function of the motor and sensory nerves of the human body. Nerve conduction studies are used mainly for evaluation of paresthesia (numbness, tingling, burning) and/or weakness of the arms and legs [1]. The type of study required is dependent in part, by the symptoms presented. Some indications of nerve conduction studies are:
a.Symptoms indicative of nerve damage as numbness, weakness.
b.Differentiation between local or diffuse disease process (mononeuropathy or polyneuropathy).
c.Get prognostic information on the type and extent of nerve injury [2].
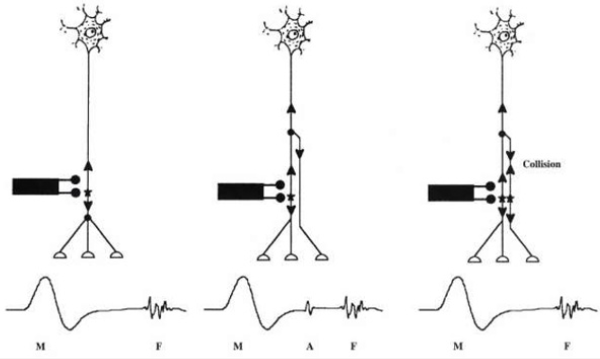
Conduction velocity is the speed at which motor and sensory impulses traverse a given segment of nerve (meter/sec). This technique commonly deals with motor conduction studies (MCS), sensory conduction studies and late responses [3]. Motor conduction studies are technically less demanding than sensory and mixed nerve studies; thus; they usually are performed first [4]. Motor responses typically are in the range of several millivolts (mV), as opposed to sensory and mixed nerve responses, which are in the microvolt (mcV) range. Thus, motor responses are less affected by electrical noise and other technical factors. This technique also, represents the conduction of an impulse along peripheral motor nerve fibers. In motor nerve conduction, the nerve is stimulated supramaximal by means of surface electrode placed over the nerve where it is relatively superficial, with the cathode is closer to recording electrode [5]. Needle stimulating electrode is used in deep nerves as sciatic nerve. Recording from one muscle supplied by this nerve distal to site of stimulation using surface electrodes:
a.Active electrode over muscle belly and
b.Reference electrode over muscle tendon.
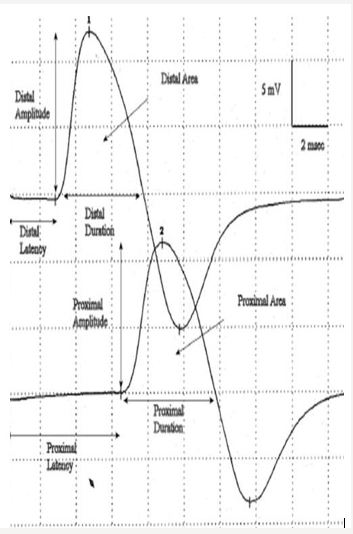
Motor conduction velocity decreased in lesions affecting the axon of peripheral nerve esp. in diseases affecting myelin sheath than those affecting axoplasm. It should be noted that striking reduction occurs in infectious polyneuritis and Charcot Marie- Tooth disease [6]. Motor conduction studies measure compound muscle action potential (CMAP) and motor nerve conduction velocity. Stimulation of any peripheral nerve evokes an electrical and mechanical response in those muscles innervated by the nerve distal to site of stimulation. The electrical response is called CMAP or M- wave. Compound term represents the summation of all underlying individual muscle fiber action potentials and is a biphasic potential with an initial negativity, or upward deflection from the baseline. In fact, the size of the response called the amplitude and measured in millivolts (mv). It corresponds to the integrity of the motor unit but cannot distinguish between pre- and postganglionic lesions because the cell body is located in the spinal cord [6]. Compound muscle action potential (CMAP) comprises of latency, amplitude, duration, and area of the CMAP (Figure 1).
Figure 1: Compound muscle action potential (CMAP) comprises of latency, amplitude, duration, and area of the CMAP.
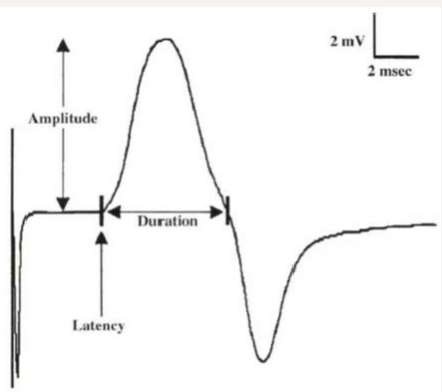
The latency (ms) is defined as the time from the stimulus to the initial CMAP deflection from baseline and measured in milliseconds (ms). Motor latency includes several unmeasurable events.
I.Utilization time (time required to produce rheobasic stim).
II.End plate potential.
These events are eliminated when 2-point stimulation Study is used. In some nerve segments where only one site can be stimulated for reasons of anatomical inaccessibility, latency measurement must replace conductive velocity. Latency varies directly with the distance of stimulating electrode from muscle. Latency represents three separate processes:
a.The nerve conduction time from the stimulus site to the neuromuscular junction (NMJ)
b.The time delay across the NMJ
c.The depolarization time across the muscle [2].
CMAP amplitude is most commonly measured from baseline to the negative peak (base line-to-peak) and less commonly from the first negative peak to the next positive peak (peak-to-peak). Also, amplitude is the height in millivolts from baseline to the peak of negative deflection. Amplitude is normally decreased with proximal stimulation. Amplitude is normally constant in size on repeated stimulation. And is directly proportional to number of muscle fibers depolarized. Low CMAP amplitudes most often result from loss of axons (as in a typical axonal neuropathy), conduction block. Causes of low CMAP are including:
a.Axonal neuropathy
b.Demyelation with conduction block
c.Presynaptic NMJ disorder
d.Advanced myopathy [6].
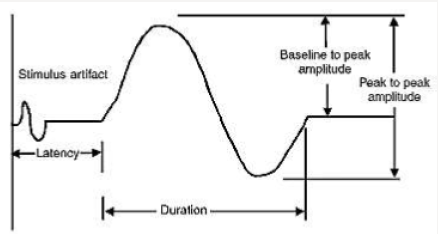
CMAP duration usually is measured from the initial deflection from baseline to the first baseline crossing (i.e., negative peak duration), but it also can be measured from the initial to the terminal deflection back to baseline (Figure 2). The former is preferred as the terminal CMAP returns to baseline very slowly and can be difficult to mark precisely. Duration is primarily a measure of synchrony (i.e., the extent to which each of the individual muscle fibers fire at the same time). In other words, it is the time required for action potential in the fast conducting fibers to reach nerve. For example, when muscle fibers are discharged in near synchrony, it means shorter duration of action potential. If conduction velocities vary widely among different axons. The neurophysiologist concludes that some muscle fibers are activated earlier than others or longer duration of CMAP. Duration increased in demyelinating disease. CMAP area is a function of both the amplitude and duration of the waveform. CMAP area also is conventionally measured between the baseline and the negative peak. Differences in CMAP area between distal and proximal stimulation sites take on special significance in the determination of conduction block from a demyelinating lesion. The normal configuration of CMAP is in two forms.
A.G1 over end plate region of stimulated muscle and it is biphasic, negative and positive.
B.G2 not over end plate region of stimulated muscle and it is triphasic with initial positivity. Normally only minimal changes in configuration at proximal sites of stimulation are seen. If an initial positive deflection exists, it may be due to:
a.Inappropriate placement of the active electrode from the motor point
b.Volume conduction from other muscles or nerves
c.Anomalous innervations [6].
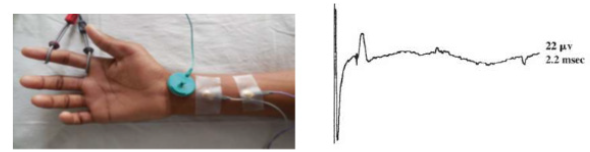
Figure 3: Sensory nerve action potential (SNAP)-sum of all the individual sensory fibers that depolarize.
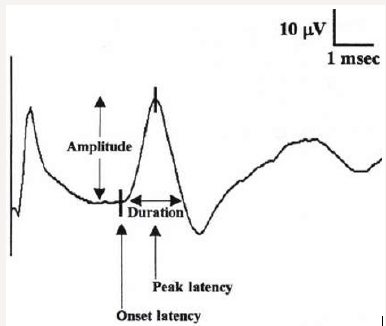
Motor nerve conduction velocity measures the speed of the fastest conducting motor axons. Conduction velocity (m/s) calculated as: distance between the proximal and distal stimulation sites divided by proximal latency - distal latency [7]. Setting for sensory conduction studies are as follows. Sensitivity: 10-20mcv/ division sweep: 20ms, current: 5-30mA (50-300V). Sensory fibers usually have a lower threshold to stimulate than do motor ftbers. As in motor studies, the current is slowly increased from a baseline of 0 mA, usually in 3- to 5-mA increments, until the recorded sensory potential is maximized. Sensory conduction studies comprise of sensory nerve action potential (SNAP) and sensory nerve conduction velocity [7]. Sensory nerve action potential (SNAP), is a compound potential that represents the summation of all the individual sensory fiber action potentials. SNAPs usually are biphasic or triphasic potentials. For SNAP, onset latency, peak latency, duration, and amplitude are measured [8] (Figure 3). Onset latency is the time from the stimulus to the first deflection from baseline and represents nerve conduction time from the stimulus site to the recording electrodes for the largest cutaneous sensory fibers, so used to calculate conduction velocity. This NCS represents the conduction of an impulse along the sensory nerve fibers. It is performed by electrical stimulation of a peripheral nerve and recording from a purely sensory portion of the nerve, such as on a finger. Peak Latency is measured at the midpoint of the first negative peak. In this factor, interobserver variation is less. For sensory conduction studies, a pair of recording electrodes (GI and G2) are placed in line over the nerve at an interelectrode distance of 3 to 4 cm, with the active electrode (G I) placed closest to the stimulator. Recording ring electrodes are conventionally used to test the sensory nerves in the fingers [2,9]. Onset latency is the time required for an electrical stimulus to initiate an evoked potential. Onset latencies reflect conduction along the fastest nerve fibers [10]. Peak latency in SNAP : it represents the latency along the majority of the axons and is measured at the peak of the waveform amplitude (first negative peak) [11]. Both latencies are primarily dependent on the myelination of a nerve. Peak latency can be ascertained in a straightforward manner. Some potentials, especially small ones, it may be difficult to determine the precise point of deflection from baseline. Peak latency cannot be used to calculate a conduction velocity [7]. SNAP amplitude -sum of all the individual sensory fibers that depolarize. Low SNAP amplitudes indicate a definite disorder of peripheral nerve. Conduction velocity-Only one stimulation site is required to calculate a sensory conduction velocity [11]. SNAP duration usually is measured from the onset of the potential to the first baseline crossing (i.e., negative peak duration). Like the motor studies, sensory latencies are on the scale of milliseconds (ms) [11]. The SNAP duration typically is much shorter than the CMAP duration (typically 1.5ms vs 5-6ms). The SNAP amplitude is most commonly measured from baseline to negative peak. Like the motor studies, sensory latencies are on the scale of milliseconds (ms). Low SNAP amplitudes indicate a definite disorder of peripheral nerve. Sensory conduction velocity represents the speed of the fastest, myelinated cutaneous sensory fibers and can be determined with one stimulation, simply by dividing the distance traveled by the onset latency [12]. It can also be useful in localizing a lesion in relation to the dorsal root ganglion (DRG). It can be abnormal with normal SNAPs if the lesion is proximal to the DRG or affecting a purely motor nerve. The DRG is located in the neural foramen and contains the sensory cell body. Lesions proximal to it (root, spinal cord) preserve the SNAP despite clinical sensory abnormalities. This is because axonal transport from the cell body to the axon continues to remain intact [13]. SNAPs are typically considered more sensitive than CMAPs in the detection of an incomplete peripheral nerve injury. The active and reference pickup should not be too close together. If this occurs, similar waveforms are recorded at both sites and rejected, dropping the amplitude of the waveform (Figures 4 & 5).
Figure 5: The active and reference pickup should not be too close together. Effect on the amplitude ofvarying the inter-electrode separation.
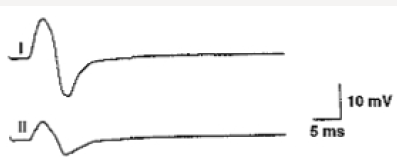
1.Normal.
2.Pickups are too close.
Temporal Dispersion & Phase Cancellation
Figure 6: SNAPs remains normal in lesions proximal to the dorsal root ganglia, including lesions of the nerve roots, spinal cord, and brain.
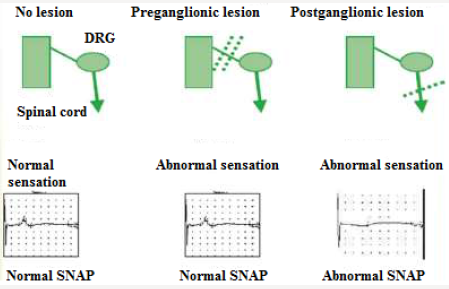
(SNAPs) and (CMAPs) both are compound potentials. They represent summation of individual sensory and muscle fiber action potentials, respectively. With distal stimulation, fast and slow fiber potentials arrive at the recording site at approximately the same time [12]. With proximal stimulation, the slower fibers lag behind the faster fibers. Temporal dispersion & phase cancellation is more prominent with SNAP than CMAP for 2 reasons (Figure 6).
I.The CMAP duration is much longer than the SNAP.
II.The range of fiber conduction velocity is less spread in motor than sensory fibers (12 m/sec vs. 25 m/sec).
For this reason, a drop of 50% is considered normal when recording a proximal SNAP. Drop of 15% is considered normal when recording a proximal CMAP [2].
Antidromic Sensory Study
Antidromic studies are performed by recording potentials directed toward the sensory receptors. The antidromic method has the advantage of a higher-amplitude SNAP but is followed by a large volume-conducted motor potential [13].
Orthodromic Sensory Study
Orthodromic studies are obtained by recording potentials directed away from these receptors. In orthodomic vs antidromic sensory study, latency and conduction velocity remain same except amplitude which is more in antidromic due to proximity of the recording electrode to the underlying nerve [13]. Antidromic studies are easier to record a response than orthodromic studies. It may be more comfortable than orthodromic studies due to less stimulation required, have larger amplitudes due to the nerve being more superficial at the distal recording sites (Figure 7). It is with more chances of volume conducted motor potential [13].
Late Response
When a nerve stimulus applied it travels in to 2 direction, peripheral stimulation (orthodromic) result in M-response while towards anterior horn Cell stimulation(antidromic) result in to late response. In routine only one late response is measured for example, F response. For eliciting the late response, some author changes the direction of stimulator (cathode end proximally) so that maximum number of nerve stimulated. In lab usually, no change seen by doing this [2]. Late response consists of
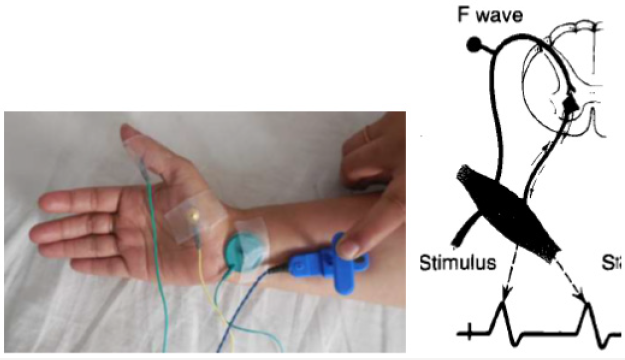
F Reflex: First described by Magaladery and Mc Dougal. F response derives its name from foot because it was first recorded from the intrinsic foot muscles (Figure 8). Stimulation is followed by depolarization which travels in both directions: first directly to the muscle fiber producing the M response and retrograde up to the motor axon and to anterior horn, where it is repropagated back through the axons to produce the delayed F response. F-wave is small late motor response occurring after the CMAP. It consists of approximately 1-5% of the CMAP amplitude. It is pure motor response; it is not representing a true reflex and usually polyphasic& varies with each stimulation. Normally peroneal F waves may be absent or non-persistent F responses may be absent in sleeping or sedated patients. F responses may be absent with low amplitude distal CMAPs [2].
H Reflex: Submaximal stimulation of the afferent sensory fiber (1A) ->orthodromic conduction to the spinal cord->synaptic stimulation of the alpha motor neuron->evoked H response in the muscle. A rudimentary M response is produced when a few motor axons are directly stimulated [2].
A Reflex: A reflex is not a true reflex. It is another late potential that often is recognized during the recording of F responses. It is typically occurring between the F response and the direct motor (M) response. It is an axon reflex is identified as a small motor potential that is identical in latency and configuration with each successive stimulation. Axon reflexes typically are seen in reinnervated nerves, especially when a submaximal stimulus is given.
a.This waveform represents collateral sprouting following nerve damage (Figure 9).
b.Also shows that stimulus is submaximal [2].
Interpretation of Nerve Conductions
The speed of nerve conduction is related to
a.The diameter of the nerve
b.The degree of myelination (a myelin sheath is a type of “insulation “around the nerve).
A normally functioning nerve will transmit a stronger and faster signal than a damaged nerve. In general, the range of normal conduction velocity will be approximately 50 to 60 meters per second. However, the normal conduction velocity may vary from one individual to another and from one nerve to another. The Interpretation of nerve conductions is complex, but in general, different pathological processes result in:
I.Changes in the latencies,
II.Changes in the amplitudes and
III.Slowing of the conduction velocity.
For examples;
A.Slowing of the NCS usually indicates there is damage to myepn.
B.Slowing across the wrist for the motor and sensory latencies of the median nerve indicates focal compression of the median nerve at the wrist, called carpal tunnel syndrome.
C.Slowing of all nerve conductions in more than one limb indicates generalized peripheral neuropathy (eg. in diabetes mellitus) [14].
Physiologic Variation among Different Nerve Segments
More slowly in the legs than in the arms (progressive reduction in axonal diameter, shorter intermodal distances, and lower distal temperatures). Faster in the proximal than in the distal nerve segments.
Effects of Temperature: Lower temperatures slow down impulse propagation while at the same time augmenting the amplitude of nerve and muscle potential (consequence of the temperature coefficients governing voltage-sensitive sodium (Na+) and potassium (K+) conductance). Conduction velocity changes nonlinearly with increase in skin temperature, showing more pronounced effect in the lower temperature range:
i.1.5 ( motor) & 2.5m/s ( sensory ) ↑ with 1°C ↓in Temp.
ii.Target-34°C or above ( lower limit for warming 32°C).
Maturation and Aging: Conduction velocity - half the adult value in full-term infants to the adult range at age 3-5 years ( rate of myelination).
a) In children and adolescents, from age 3 to 19 years, both motor and sensory conduction velocities tend to increase slightly in the upper limb and decrease in the lower limb as a function of age and growth in length.
b) begin to decline after 30–40 years of age, but the values normally change by less than 10 m/s by the sixtieth year.
c) The most distal branches, such as the interdigital nerves, may degenerate earlier.
Height
a.Height shows negative association with sensory, amplitude and positive association with distal latencies.
b.Sural, peroneal and tibial nerve conduction velocities all have inverse correlation with height in normal.
c.Relevant in interpretation of late responses ( F response and H reflex)-impulse travel twice the length of limb [13].
Conclusion
Peripheral nerves contain many nerve fibres of different diameters, degrees of myelination, and afferent or efferent connections. Using the nerve conduction study, neurophysiologists investigate the fastest 20% of these fibres and the aim of the investigation is to document focal or continuous abnormalities in the length of the mixed, motor or sensory nerve.
References
- Weiss LD, Weiss JM, Pobre T, Kalman A (2015) Nerve conduction studies. Easy EMG A Guid to Perform Nerve Conduct Stud Electromyogr 17.
- Preston DC, Shapiro BE (2012) Electromyography and Neuromuscular Disorders. E-Book: Clinical-Electrophysiologic Correlations (Expert Consult-Online). Elsevier Health Sciences.
- Alberts NW (2009) Engaging with Charcot-Marie-Tooth disease: A grounded theory approach.
- Brown PW (1972) Factors influencing the success of the surgical repair of peripheral nerves. Surg Clin North Am 52(5): 1137-1155.
- Kong X, Lesser EA, Gozani SN (2010) Nerve conduction studies: Clinical challenges and engineering solutions. IEEE Eng Med Biol Mag 29(2): 26- 36.
- Kimura J (2001) Electrodiagnosis in diseases of nerve and muscle: principles and practice. Oxford University Press, USA.
- Pugdahl K, Fuglsang Frederiksen A, De Carvalho M, Johnsen B, Fawcett PRW, et al. (2007) Generalised sensory system abnormalities in amyotrophic lateral sclerosis: a European multicentre study. J Neurol Neurosurg Psychiatry 78(7): 746-749.
- Levin KH (2004) Nerve Conduction Studies: Practical Physiology and Patterns of Abnormalities. Acta Neural Belq 106(2): 73-81.
- Lee HJ, De Lisa JA (2005) Manual of nerve conduction study and surface anatomy for needle electromyography. Lippincott Williams & Wilkins.
- Kobayashi M, Pascual Leone A (2003) Transcranial magnetic stimulation in neurology. Lancet Neurol 2(3): 145-156.
- Gooch CL, Weimer LH (2007) The electrodiagnosis of neuropathy: basic principles and common pitfalls. Neurol Clin 25(1): 1-28.
- Cuddon PA (2002) Electrophysiology in neuromuscular disease. Vet Clin Small Anim Pract 32(1): 31-62.
- Mallik A, Weir AI (2005) Nerve conduction studies: essentials and pitfalls in practice. J Neurol Neurosurg Psychiatry 76(suppl 2): 23-31.
- Oh SJ (2003) Clinical electromyography nerve conduction studies, 3rd (Edn.). Eur J Neurol 10(5): 605.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...