
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6636
Case Report(ISSN: 2637-6636) 
Biphasic Variant of Clear Cell Odontogenic Carcinoma - A Case Report Volume 1 - Issue 1
Sufyan Ahmed1, Syeda Hala Raza1* and Haider Ali2
- 1Department of Oral and Maxillofacial Surgery Abbasi Shaheed Hospital, Karachi Medical and Dental College, Pakistan
- 2Department of Plastic Surgery, Civil Hospital, Dow University of Health Sciences Karachi, Pakistan
Received: January 18, 2018; Published: February 01, 2018
*Corresponding author: Syeda Hala Raza, Department of Oral and Maxillofacial Surgery Abbasi Shaheed Hospital, Karachi Medical and Dental College, Pakistan
DOI: 10.32474/IPDOAJ.2018.01.000102
Abstract
Clear cell odontogenic carcinoma (CCOC) is a rare neoplasm of the jaw. This report outlines the clinical, histopathological and immuno-histochemical findings of CCOC and describes management strategy according to experience of authors and review of literature. We present a case of CCOC in a 70 years old female at posterior region of mandible. Microscopically, this tumor consisted of nests of cells with eosinophillic cytoplasm, small oval nuclei and a few clear cell areas. Treatment comprised of wide local excision and modified radical neck dissection with no recurrence.
Keywords: Clear cell odontogenic carcinoma; Radical neck dissection; Wide local excision
Introduction
Clear cell odontogenic carcinoma (CCOC) is a rare intraosseous carcinoma of the jaw described in 1985 for the first time by Hansen [1]. In 1992, WHO classified CCOC as benign odontogenic tumor however, in the WHO classification of 2005 it was considered to be a malignant tumor of odontogenic origin due to its aggressive nature, predilection to local recurrence, loco-regional lymph node metastasis and distant metastasis [2]. Only 82 cases have been reported until now excluding the present case [3]. In this study, we report an additional rare case of CCOC extending from the left premolar area to the left ascending ramus of mandible and discuss the previous literatures.
Case Report
Figure 1: Intra-Oral Clinical Photograph.
Clinical photograph of the patient showing intra-oral findings i.e. moderate oral hygiene, attrition of occlusal surface of all teeth, an expansile swelling at left mandibular buccal sulcus extending from premolar area to ascending ramus, overlying mucosa fixed with the swelling. No ulceration and color changes can be seen. Clinically this is the typical but inconclusive presentation of Clear cell odontogenic carcinoma.
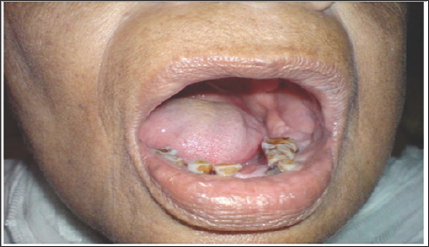
A 70 year old female reported to the department of Oral and Maxillofacial Surgery at Abbasi Shaheed Hospital Karachi, Pakistan with a complain of pain and swelling in lower left molar area along with mobility in left mandibular first molar. On clinical examination, there was facial asymmetry with extra oral swelling at left angel of the jaw measuring 3 x 3cm. On palpation, the swelling was bony hard and non tender with no anesthesia or paresthesia of overlying skin and mucosa. Neck examination revealed firm lymph node at level I measuring up to 1cm in diameter and not fixed with overlying skin or underlying structures. Intraoral, swelling in the left buccal sulcus extending from first mandibular premolar to the ascending ramus (Figure 1). There was 3rd degree mobility in left mandibular first molar. Orthopantomogram revealed a lytic lesion in the left posterior region of the lower jaw (Figure 2). On Computed tomography, erosion and destruction of mandibular body and ramus was seen. The soft tissue mass involved buccinators, orbicular is oris and medial pterygoid muscles. Multiple enlarged lymph nodes seen bilaterally, one ipsilateral node measuring 1.3 x 1 cm was present at level one. An incision biopsy was performed and specimen submitted for histopathological evaluation. According to histopathology there was fibrous tissue exhibiting an infiltrating lesion composed of masses and small aggregates of polygonal cells.
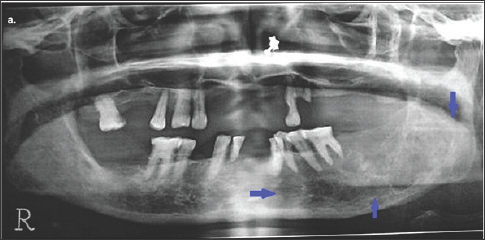
Figure 2: Orthopantomogram of the patient showing a multilocular osteolytic lesion in the left posterior region of lower jaw.
Orthopantomogram of the patient showing radiographic findings i.e. a multilocular osteolytic lesion in the left posterior region of lower jaw (as pointed out with blue arrows). There are ill defined area of bone erosion .Lesion mainly involves the alveolar ridge and is extended up to the inferior border of mandible. Teeth in left quadrant of lower jaw appear floating in the lesion.
The cells showed moderate to abundant granular pale eosinophillic cytoplasm. Nuclei were vesicular with open chromatin pattern and occasional mitotic filling. Histological diagnosis was established as "Clear Cell Odontogenic Carcinoma". Therefore, the patient underwent wide local resection with hemi-mandibulectomy and modified radical neck dissection under general anesthesia. The resected specimen was sent for histopathological and immuno- histochemical evaluation with demarcation of surgical margins. The report showed the lesion measuring up to 4 x 3cm with tumor free resection margins. Three out of the 15 neck nodes revealed metastases, with the largest lymph node measuring 0.4 x0.3cm. Microscopically, tumor cells infiltrating the bony trabeculae were arranged in form of nests having moderate amount of eosinophillic cytoplasm, with small round to oval nuclei (Figure 3). In few areas of cytoplasm neoplastic cells appear clear. No extra-capsular extension or perineural invasion was seen. Immune histochemistry revealed focal positivity for intra cytoplasm glycogen on special stain (PAS with Diastase) and diffuses positivity for cytokeratin (AE1/AE3 immuno-histochemical stain). Patient recovery was uneventful and the patient is under regular follow up.
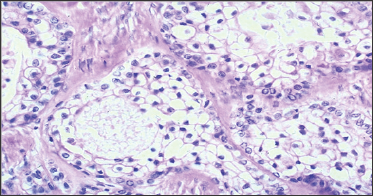
Figure 3: Histopathology, Microscopic Image.
This microscopic slide shows fibrous tissue exhibiting an infiltrating lesion composed of masses and small aggregates of polygonal cells with moderate to abundant granular pale eosinophillic cytoplasm. Nuclei are vesicular with occasional mitotic filling.
Discussion
In 1985 Hansen et al. for the first time presented three cases and coined the term clear cell odontogenic tumor [1]. Bang et al. in 1989 reported similar cases showing aggressive behavior, recurrence and metastatic spread and designated them as clear cell odontogenic carcinoma [4]. CCOC mostly occurs in the fifth to seventh decades of life with a female predilection (1.62: 1). The most common presenting sign was as a painless swelling of the jaw in anterior region, often with mobility of the related teeth and few cases showed palpable lymph nodes [5]. The etiology of this neoplasm is not known clearly and the diagnosis of CCOC is more of an exclusion criteria. CCOC must be differentiated from clear cell carcinoma of salivary glands and other benign tumors having clear cells and metastatic tumors such as renal clear cell carcinoma [6]. Perineural or vascular invasion is a classical feature of malignancy and can be used to identify CCOCs. There are three histological subtypes of CCOC the biphasic, monophonic and ameloblastomatous. In biphasic pattern clear cell nests are intermixed with polygonal cells having eosinophilic cytoplasm's while the monophonic pattern consists of cell nests containing exclusively clear cells. In ameloblastomatous pattern there are palisaded ameloblastomatous cells at the periphery of clear cell nests. The case that we report was characteristic of the biphasic pattern.
Immuno cytochemically, CCOC have diastase-digestible, PAS- positive granules, indicating the presence of glycogen within the cytoplasm [7]. These tumor cells also show positive staining for cytokeratin, CK-19 and epithelial membrane antigen [8]. The treatment protocol preferred by most authors as reported in the literature is wide local excision of tumor [3]. The extent of surgery is defined by factors such as size of the lesion, soft tissue involvement, lymph node metastasis and the presence or absence of positive surgical margins. In addition, elective neck dissection and adjuvant radiotherapy is suggested. Due to the recurrences and metastasis as reported, long term follow-up of patients with CCOC is very important.
Conclusion
Clear cell odontogenic carcinoma is an unusual low grade malignancy and differentiating it from other clear cell neoplasm is important in order to devise an appropriate treatment plan. Management should include wide local resection with neck dissection and long-term follow-up.
References
- Didilescu AC, Skaug N, Marica C, Didilescu C (2005) Respiratory pathogens in dental plaque of Hospitalized patients with chronic lung diseases. Clinical Oral Investigations 9(3): 141-147.
- Scannapieco FA (1999) Role of oral bacteria in respiratory infection. Journal of Periodontology 70(7): 793-802.
- Deo V, Bhongade ML, Ansari S, Chavan RS (2009) Periodontitis as a potential risk factor for chronic obstructive pulmonary disease: a retrospective study. Indian Journal of Dental Research 20(4): 466-470.
- Sharma N, Shamsuddin H (2011) Association between respiratory disease in hospitalized patients and periodontal disease: a crosssectional study. Journal of Periodontology 82(8): 1155-1160.
- Scannapieco FA, Ho AW (2002) Potential associations between chronic obstructive pulmonary disease and periodontal disease: analysis of National Health and Nutrition Examination Survey III. Journal of Periodontology 72(1): 50-56.
- Wang Z, Zhou X, Zhang J, Zhang L, Song Y, et al. (2009) Periodontal health, oral health behaviours, and chronic obstructive pulmonary disease. Journal of Clinical Periodontology 36(9): 750-755.
- Hayes C, Sparrow D, Cohen M, Vokonas PS, Garcia RI (1998) The association between alveolar Bone loss and pulmonary function: the VA Dental Longitudinal Study. Annals of Periodontology 3(1): 257-61.
- Garcia RI, Nunn ME, Vokonas PS (2001) Epidemiologic associations between periodontal disease and chronic obstructive pulmonary disease. Annals of Periodontology 6(1): 71-77.
- Hyman JJ, Reid BC (2004) Cigarette smoking, periodontal disease, and chronic obstructive pulmonary disease. Journal of Periodontology 75(1): 9-15.
- Katancil JA, Kritchevsky S, Weyant RJ, Corby P, BretzW, et al. (2005) Periodontitis and airway obstruction. Journal of Periodontology 76(11): 2161-2167.
- Azarpazhooh A, Leake JL (2006) Systematic review of the association between respiratory diseases and oral health. Journal of Periodontology 77(9): 1465-1482.
- Alberg AJ, Samet JM (2003) Epidemiology of Lung Cancer. Chest 123: 21S-49S.
- Thun MJ, Henley SJ, Burns D, Jemal A, Shanks TG, et al. (2006) Lung cancer death rates in lifelong non-smokers. Journal of the National Cancer Institute 98(10): 691-699.
- Burcham PC (1998) Genotoxic lipid peroxidation products: Their DNA damaging properties and role in formation of endogenous DNA adducts. Mutagenesis 13(3): 287-305.
- Waldner MJ, Neurath MF (2009) Colitis-associated cancer: the role of T cells in tumor development. Seminars in Immunopathology 31(2): 249256.
- Ezzati M, Lopez AD (2003) Estimates of global mortality attributable to smoking in 2010. Lancet 362(9387): 847-852.
- Mannino DM, Aguayo SM, Petty TL, Redd SC (2003) Low lung function and incident lung cancer in the United States: data from the First National Health and Nutrition Examination Survey follow-up. Archives of Internal Medicine 163(12): 1475-1480.
- Lange P, Nyboe J, Appleyard M, Jensen G, Schnohr P (1990) Ventilatory function and chronic mucus hyper-secretion as predictors of death from lung cancer. The American Review of Respiratory Disease 141(3): 613617.
- Prendergast GC (2008) Inflammatory mediators in cancer etiology and targets for therapy and prevention. Can Rev Onl 9: 17-18.
- Taraseviciene Stewart L, Voelkel NF (2008) Molecular pathogenesis of emphysema. The Journal of Clinical Investigation 118(2): 394-402.
- Tomar SL, Asma S (2000) Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. Journal of Periodontology 71(5): 743-751.
- Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K (2008) Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. The Lancet. Oncology 9(6): 550-558.
- Arora M, Weuve J, Fall K, Pedersen NL, Mucci LA (2010) An exploration of shared genetic risk Factors between periodontal disease and cancers: a prospective co-twin study. American Journal of Epidemiology 171(2): 253-259.
- Chrysanthakopoulos NA (2016) Correlation Between Periodontal Disease Indices and Lung Cancer in Greek Adults: a Case - Control study. Experimental Oncology 38(1): 49-53.
- Amabile N, Susini G, Pettenati-Soubayroux I (2008) Severity of periodontal disease correlates to inflammatory systemic status and independently predicts the presence and angiographic Extent of stable coronary artery disease. Journal of Internal Medicine 263(6): 644-652.
- Moutsopoulos NM, Madianos PN (2006) Low grade inflammation in chronic infectious diseases: paradigm of periodontal infections. Annals of the New York Academy of Sciences 1088: 251-264.
- Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ (2004) C-reactive protein and the risk of incident colorectal cancer. J Am Med Assoc 291(5): 585590.
- Tezal M, Sullivan MA, Hyland A, Marshall JR, Stoler D, et al. (2009) Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer epidemiology, biomarkers and prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 18(9): 2406-2412.
- Fitzpatrick SG, Katz J (2010) The association between periodontal disease and cancer. A review of the literature. Journal of Dentistry 38(2): 83-95.
- Soder B, Jin LJ, Klinge B, Soder PO (2007) Periodontitis and premature death: a 16-year longi- tudinal study in a Swedish urban population. Journal of Periodontal Research 42(4): 361-366.
- Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Taylor PR, et al. (2005) Tooth loss is associated with increased risk of total death from upper gastrointestinal cancer, heart disease, and stroke in a Chinese population-based cohort. International Journal of Epidemiology 34(2): 467-474.
- Zaris S, Bojic B, Jankovic L, Dapcevic B, Popovic B, et al. (2009) Periodontal therapy improves gastric Helicobacter pylori eradication. Journal of Dental Research 88(10): 946-950.
- Chrysanthakopoulos NA, Vlassi C (2013) Reasons for Extraction of Permanent Teeth and Risk Indicators in a General Dental Practice in Greece. Int J Med Dent 3: 315-321.
- Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917): 860-867.
- Marx J (2004) Cancer research. Inflammation and cancer: the link grows stronger. Science 306(5698): 966-968.
- Milasin J, Jakoba NN, Stefanovic D, Sopta J, Pucar A, et al. Periodontal Inflammation as Risk Factor for Pancreatic Diseases: 131-156.
Editorial Manager:
Email:
pediatricdentistry@lupinepublishers.com

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...











