Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6636
Case ReportOpen Access
An Unusual and Aggressive Ewing Sarcoma Involving Parapharangeal Space: A Case Report and Review of Literature Volume 1 - Issue 3
Mimansha Patel*
- Department of Oral Pathology, Triveni Institute of Dental Science Hospital and Research Centre, India
Received: April 14, 2018; Published: April 24, 2018
*Corresponding author: Mimansha Patel, Department of Oral Pathology, Triveni Institute of Dental Science Hospital and Research Centre, India
DOI: 10.32474/IPDOAJ.2018.01.000112
Abstract
Ewing’s sarcoma (ES) is a malignancy primarily affecting bone tissue that is commonly diagnosed in adolescents and young adults. Its occurrence in the head and neck region is unusual and generally involves the mandible and maxilla. We report here a case of Extraskeletal Ewing’s sarcoma (EES) of the Parapharangeal space in a 20 year-old female who presented with blurred vision, dysphasia. CT examination showed a large, seemingly well-defined soft tissue mass in the left side of face involving parapharyngeal space with intracranial extension. The patient undergone chemotherapy and radiotherapy. A review of the literature revealed only one previous reports of EES occurring in the parapharyngeal space.
Keywords: Ewing’s Sarcoma; Mandible; Parapharyangeal space; Diagnosis
Introduction
Extraskeletal Ewing’s sarcoma (EES) is a rare, rapidly growing, round-cell, malignant tumour that can develop in the soft tissues at any location. It was first described by Tefft [1] in four patients who presented with paravertebral soft tissue tumours with histological appearance resembling Ewing’s sarcoma. In a clinicopathological study of 39 cases of EES reported by Angervall et al. [2] the paravertebral region was the most frequent site of involvement, followed by the lower extremity and chest wall. The head and neck region is an unusual primary site for this tumour and, according to Chao [3], there were only five out of 118 cases of EES located in the head and neck region. Herein, we report a case of EES occurring in the left side of face involving parapharyngeal space.
Case Report
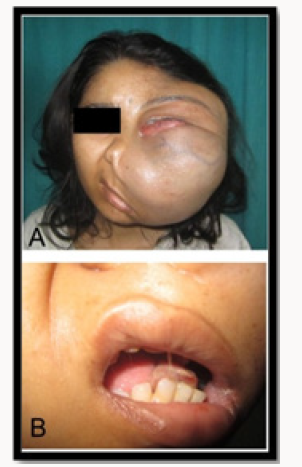
A twenty two year-old female presented swelling in left side of face in June 2013 which had been present for 7 months (Figure 1). She also complained of loss of vision from left eye, history of thick consistency of saliva, difficulty in mastication. Five years earlier she had history of previous hospitalization at Government Medical Hospital Nagpur where FNAC was done it was suggestive of Small round cell tumor. It was operated for Round Cell Tumor of mandible of left side (Hemimandi bulectomy was done ). Patient has undergone 6 cycle of chemotherapy.
Physical examination showed a large, tender, smooth bulging submucosal mass located in the left side of face involving parapharyngeal region. Extraoral Examination: Facial asymmetry due to swelling present in left side of face which causes nose to deviate to right side and swelling was also blocking the nostril. Prominent vascular marking was also seen. The swelling was extending, A/P – From left nostril to the posterior auricular region and S/I – From hairline of forehead extending beyond the lower border of mandible. It size was 20 x 18 cm approx, roughly oval in shape, smooth surface and diffused borders (Figure 1).
On Intraoral Examination: Mouth opening of two fingers. Anterior lower teeth drifted towards left side and tongue displaced towards right side. Proliferative growth present over the floor of mouth and buccal mucosa region of left side its borders could not be accesed due to the inaccessibility (Figure 1). Provisional Diagnosis was given as connective tissue malignancy. In connective tissue malignancy it can be Small cell osteosarcoma, Fibrosarcoma, Chondrosarcoma, and in Small round cell tumor it can be Ewing’s Sarcoma/PNET, Neuroblastoma, Medulloblastoma, Rhabdomyosarcoma, Wilm’s Tumor and Small Cell Lymphoma/ Non Hodgkin’s Lymphoma.
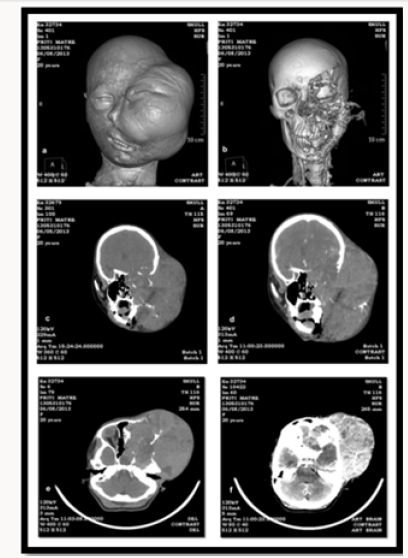
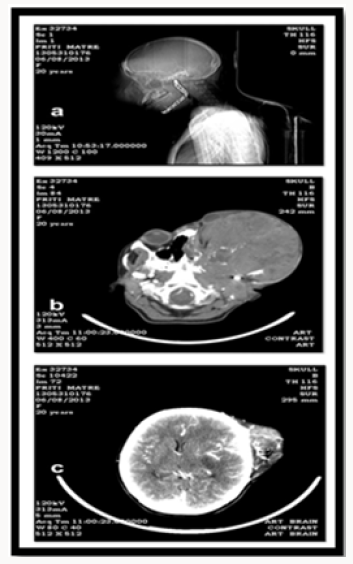
Investigation was carried out including radiographs which include volume rendered imaging, CT Scan Tomogram, Coronal and Axial CT Scan was carried out. The volume rendered imaging shows a large lobulated hetrogenous lesion of density 64 HU enhancing up to 70 HU. The lesion is causing extensive destruction fronto temporo parietal bone, sphenoid bone, zygomatic, ethmoid, maxilla on the left side and extending superiorly into the left orbit and displacing the eyeball anteriorly. With intracranial extension in temporo-parietofrontal region. Medially the lesion is extending to the left sphenoid ,ethmoid sinus and nasal cavity, Inferiorly the lesion has destoryed the left maxillary sinus, maxilla, alveolar ridge, hard palate. Lateral tomogram film shows large opacity on the left side of face and arch bar because of previous treatment. Coronal CT Scan pre contrast shows large mass lesion on left side of face which is isodense to brain with coarse calcific foci seen within the lesion. The lesion is causing extensive destruction of bones of face and its post contrast shows lesion enhacement. While comparing the pre and post contrast of Axial CT Scan it was interpreted that the lesion is causing extensive destruction of the bone and showing intense contrast enhancement. This all features like extensive bone destruction and intense contrast enhancement are suggestive of Aggressive nature of lesion. It was seen in the Post Contrast Axial CT Scan that the lesion is invading the brain parenchyma and the lesion is causing mass effect in the form of midline shift by 7.2 mm to the right side. Hematological examination were in normal limits (Figures 2 & 3).
Figure 4: a) Grossing details. b&c)Histopathology shows tumours cells with prominent nuclei but scanty cytoplasm in 4x,10x, and 40x. d)Immunohistochemical study for CD99 strong membranous immunoreactivity. e) PAS positivity seen.
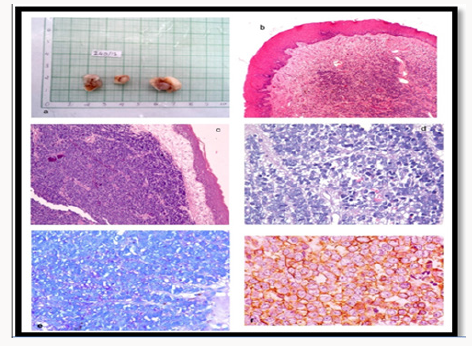
As in earlier FNAC smears shows homogenous population of cell in sheets as well as singly with increased nucleo cytoplasmic ratio, nucleus are round with homogenous chromatin pattern, suggestive of ROUND CELL TUMOR. The tissue was send for complete processing. From this, the patient underwent an incisional biopsy and the material was sent to the pathologist for histopathological analysis. Microscopically, the specimens showed solid sheets of small-round cell population scattered in a fibrovascular stroma interposed by fibrous septa. Most of the individual cells exhibited scant cytoplasm, round and oval hypercromatic nuclei and inconspicuous nucleoli (Figure 4). Neither mitotic figures nor necrotic areas were seen. Intracytoplasmic glycogen was demonstrated by PAS stain after diastase pretreatment. An immunohistochemical study using a standard avidin-biotinperoxidase complex technique (DAKO; Carpinteria, CA, USA) was performed and revealed positivity for CD99. Histopathological and immunohistochemical findings supported the diagnosis of ES. The patient started the treatment with multiagent chemotherapy including ifosfamide, carboplatin, and etoposide as well as vincristine, cyclophosfamide and doxorrubycin (ICE/VAC regimen). The patient than discontinued the treatment.
Discussion
EES is a rare malignant neoplasm of uncharacterized mesenchymal cell origin. This tumour is equally prevalent in males and females. Young adolescents and adults between the ages of 10 years and 30 years are predominantly affected, however, cases have been documented in patients between 14 months and 63 years of age [2,4]. Thus, the advanced age of our patient is not unprecedented. EES usually arises in the soft tissues of the trunk, paravertebral region, intercostal area, lower extremities and pelvis [2,4]. EES in the head and neck regions is uncommon, in which the nose, eyelid, nasopharynx, parotid gland, scalp and cervical region have been described [3,5-12]. However, no case originating from the parapharyngeal space has been reported to date. Clinically, patients with extremity EES usually present with a painless, rapidly growing mass but tumours arising elsewhere may be painful. Our patient with parapharyngeal EES, however, presented with a large, painless tumour and multiple cranial nerves dysfunction. Although EES tends to spread locally, it usually has a pseudo capsule on gross specimen and thus appears well circumscribed on CT or MR images. Most tumours are of low attenuation or contain areas of lower attenuation than the adjacent muscle on CT and appear hypo echoic on ultrasound scans, reflecting the frequent microscopic findings of tumour necrosis [10,13]. On MR images, these tumours are generally of low to intermediate signal intensity on T1 weighted images, of high signal intensity on T2 weighted images, and exhibit heterogeneous contrast enhancement [8, 10,12]. In accordance with the previous description, the tumour from our patient appeared to be well circumscribed, inhomogeneous and showed no calcification on CT. Anterior displacement of the ICA and perineural spread along the cranial nerve V and IX to XII complex were well demonstrated. It is worth noting that CT also demonstrated arterial encasement and irregular bone destruction, suggesting a highly aggressive malignant tumour. The differential diagnosis in this case, mainly included salivary gland tumours, neurogenic tumours and paragangliomas.
Tumors of the Ewing sarcoma family of tumors (ESFT) are characterized by morphologically similar roundcell neoplasms as well as by the presence of common chromosomal translocation. In 1918, Stout [1] reported a case of an ulnar nerve tumor composed of undifferentiated round cells that formed rosettes; this lesion was subsequently defined as primitive neuroectodermal tumor (PNET) of soft tissue. In 1921, Ewing [2] reported a case of round-cell tumor in the radius of a 14-year-old girl as a “diffuse endothelioma of bone,” proposing an endothelial derivation (Ewing sarcoma [ES]). Angervall and Enzinger [3] reported the first case of an ES arising in soft tissue (i.e, extraskeletal ES) in 1975. In 1979 [4] reported a “malignant small-cell tumor of the thoracopulmonary region” (ie, Askin tumor) with histologic features similar to those in PNET. In 1984 [5] described a neuroectodermal tumor of bone, calling it a PNET of bone. Initially, ESFT were believed to be biologically distinct. However, based on their wide spectrum of neural differentiation (PNET being the most differentiated); their immunohistochemical, cytogenetic, and molecular uniformity; and their identical response to Ewing-based chemotherapy regimens, it was determined that these sarcomas are related and that they originate from unique mesenchymal stem cells capable of multilineage differentiation [6]. A common chromosomal translocation that results in EWS-ETS fusion (between the EWS gene on chromosome 22 and an ETS-type gene, most commonly the FLI1 gene on chromosome 11) has been implicated in 80% to 95% of cases of ES, PNET, and Askin tumor [6,7] (Figure 4). Thus, these lesions have been grouped as the same entity, called ESFT. Treatment principles are the same regardless of anatomic location. Herein we have chosen to focus on ES of the bone.
Epidemiology
Currently, it is believed that ES most likely can be ascribed to spontaneous genetic translocations rather than to exposure to environmental factors or drugs, mendelian inheritance, disease, or traumatic events [6,8]. The incidence of secondary ES after radiotherapy (RT) has been reported to be <3%.9 The histogenesis of these tumors remains unknown, and debate continues regarding the neuroectodermal or mesenchymal lineage. Although rare, ES is the third most frequent primary sarcoma of bone, after osteosarcoma and chondrosarcoma [10]. It is the second most common bone tumor (after osteosarcoma) occurring in children and adolescents, accounting for approximately 3% of all pediatric malignancies and approximately 10% of all primary malignant bone tumors [10,11]. ES is reported in all age groups, from infancy to advanced age, but the condition is most commonly diagnosed in persons in the second decade of life [12,17].
Clinical Features
Site
ES occurs most often in bone. Soft tissue involvement is rare. Almost any bone can be affected, and the sites of origin are roughly split anatomically between the central and peripheral skeleton [8,15].
Signs and Symptoms
The most common presenting symptoms of ES are pain, swelling, and a mass, especially in an extremity.
Imaging Features
Plain Radiograph Unlike osteosarcoma, which usually originates in the metaphysis, ES of the long bones tends to arise from the diaphysis or metadiaphysis. Of the 206 patients in the Intergroup Ewing’sSarcomaStudy7299group,121 (58.7%) presented with lesions located in the metadiaphysis73(35.4%) with lesions in the diaphysis, 11 (5.3%) with lesions in the metaphysis, and only 1 with a lesion in the epiphysis (0.5%).25 Typically, ES appears as an ill-defined, permeative, mottled, or focally motheaten, destructive intramedullary lesion. Periosteal reaction, with a laminated or “onion skin” appearance (a prominent multilayered reaction), is common in ES, but it is not pathognomonic. Periosteal reactions may be seen, such as a sunburst or spiculated pattern (the latter displaying as a perpendicular reaction), as well as the Codman triangle (ie, triangular lifting of the periosteum from the bone at the site of detachment).
Advanced Imaging Studies
Initial plain radiographs, particularly of the pelvis, may appear to be normal, and advanced imaging studies are needed for tumor localization. MRI provides the most precise definition of the local extent of bone tumor, including the degree of expansion into the intramedullary region, extent of soft-tissue involvement, and the relationship of the lesion to adjacent neurovascular structures. MRI may also be used to assess responses to neoadjuvant chemotherapy and irradiation. CT is useful for staging the disease and detecting macrometastasis. As an adjunct to MRI, CT may also be helpful in target planning for radiation. A technetium Tc-99m whole-body bone scan is important in detecting skip or distant metastases.
Staging
According to the Enneking staging system adopted by the Musculoskeletal Tumor Society, almost all ESFT neoplasms are classified as stage IIB or III
Pathology
Grossly, ESFT presents as a gray white tumor with a variable amount of necrosis, hemorrhage, or cyst formation. Morphologically, ESFT are composed of sheets of small round cells with a high nuclear-to-cytoplasmic ratio. They are often classified into a group of small, blue, round-cell tumors that include neuroblastoma, alveolar rhabdomyosarcoma, and lymphoblastic lymphoma. The cells typically have scant, weakly eosinophilic cytoplasm that usually contains glycogen (making them positive on periodic acid- Schiff [PAS] stain), diastase degradable granules, and round nuclei with evenly distributed chromatin and little mitotic activity.
Treatment
Most patients with apparently localized disease at diagnosis have subclinical micrometastatic (ie, systemic) disease; thus, systemic therapy using multidrug chemotherapy as well as local disease control via surgery and/or RT is indicated in the treatment of all patients. Regardless of tumor extent, most patients receive four to eight cycles of neoadjuvant chemotherapy [6].
Metastatic Ewing Sarcoma
ES has a strong potential to metastasize. In the SEER study, 26% to 28% of patients presented with distant macrometastasis [17]. More than 10% of patients present with multiple bone metastases at initial diagnosis. Although metastases in the lungs, bone, bone marrow, or a combination therefore are detectable in approximately 25% of patients, metastases to lymph nodes, the liver, and the brain are rare [17].
Summary
ESFT is a rare malignancy of unknown origin that most often affects young children and adolescents. Because most patients with apparently localized disease at diagnosis have occult metastatic (systemic) disease, multidrug chemotherapy as well as local disease control with surgery and/or RT is currently indicated for all patients. Despite marked improvement in survival during the past 40 years for patients with localized disease, improvements have been relatively lesser in patients with metastatic or recurrent disease. An improved understanding of the complex biology of ESFT may lead to the successful development of biologically targeted therapies. As our understanding of the regulatory pathway responsible for ESFT transformation, growth, and metastasis becomes more refined, the number of potential therapeutic targets will expand in parallel [18-20].
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Acknowledgment
The authors would like to thank Dr. Minal Chaudhary, (Professor, Vice Dean); Dr. Alka Hande (Reader, Department of Oral Pathology), Dr. Nitin Bhola HOD. Department of Oral Surgery), Dr.Madhuri Gawande (HOD, Department of Oral Pathology);Dr. Swati Patil (Professor, Department of oral pathology), Dr. Gaurav Khemka Lecturer, Department of Oral Surgery).
References
- Stout AP (1918) A tumor of the ulnar nerve. Proc NY Pathol Soc 18: 2-13.
- Ewing J (1921) Diffuse endothelioma of bone. Proc NY Pathol Soc 21: 17-24.
- Angervall L, Enzinger FM (1975) Extraskeletal neoplasm resembling Ewing’s sarcoma Cancer. 36(1): 240-251.
- Askin FB, Rosai J, Sibley RK, Dehner LP, McAlister WH (1979) Malignant small cell tumor of the thoracopulmonary region in childhood: A distinctive clinicopathologic entity of uncertainhistogenesis. Cancer 43(6): 2438-2451.
- Jaffe R, Santamaria M, Yunis EJ (1984) The neuroectodermal tumor of bone. Am J Surg Pathol 8(12): 885-898.
- Ludwig JA, (2008) Ewing sarcoma: Historical perspectives current state of the art and opportunities for targeted therapy in the future. Curr Opin Oncol 20(4): 412-418.
- Delattre O, Zucman J, Melot T (1994) The Ewing family of tumors: A subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med 331(5): 294-299.
- Grier HE (1997) The Ewing family of tumors: Ewing’s sarcoma and primitive neuroectodermal tumors. Pediatr Clin North Am 44(4): 991- 1004.
- Tucker MA, D Angio GJ, Boice JD (1987) Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med 317(10): 588- 593.
- Gibbs CP Jr, Weber K, Scarborough MT (2002) Malignant bone tumors. Instr Course Lect 51: 413-428.
- Herzog CE (2005) Overview of sarcomas in the adolescent and young adult population. J Pediatr Hematol Oncol Medline 27(4): 215- 218.
- Rodríguez Galindo C, Navid F, Liu T, Billups CA, Rao BN (2008) Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol 19(4): 814-820.
- Rodriguez-Galindo C, Billups CA, Kun LE (2002) Survival after recurrence of Ewing tumors The St. Jude Children’s Research Hospital experience 94(2): 561-569.
- Rodríguez-Galindo C, Liu T, Krasin MJ (2007) Analysis of prognostic factors in ewing sarcoma family of tumors. Review of St. Jude Children’s Research Hospital studies Cancer 110(2): 375-384.
- Cotterill SJ, Ahrens S, Paulussen M (2000) Prognostic factors in Ewing’s tumor of bone Analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol 18(17): 3108- 3114.
- Gurney JG, Swensen AR, Bulterys M Ries LA, Smith MA, et al. (1999) Malignant bone tumors, Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975-1995. Bethesda, MD, National Institutes of Health pp. 99-110.
- Esiashvili N, Goodman M, Marcus RB (2008) Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance epidemiology and end results data. J Pediatr Hematol Oncol 30(6): 425- 430.
- Dunst J, Jürgens H, Sauer R (1995) Radiation therapy in Ewing’s sarcoma: An update of the CESS 86 trial. Int J Radiat Oncol Biol Phys 32(4): 919- 930.
- Dunst J, Schuck A (2004) Role of radiotherapy in Ewing tumors. Pediatr Blood Cancer 42(5): 465-470.
- Ozaki T, Hillmann A, Hoffmann C (1996) Significance of surgical margin on the prognosis of patients with Ewing’s sarcoma: A report from the Cooperative Ewing’s Sarcoma Study Cancer 78(4): 892-900.
Editorial Manager:
Email:
pediatricdentistry@lupinepublishers.com

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...