Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6636
Research Article(ISSN: 2637-6636) 
A Modern Standard of Dental Sprout in Children from 13 to 36 Months Against the Established Canon Volume 7 - Issue 5
Yaniris Figueroa Cespedes* and Leslie Imara de Armas Gallegos
- Faculty of Stomatology, Raul Gonzalez Sanchez, Cuba
Received: July 19, 2022; Published: August 02, 2022
*Corresponding author: Yaniris Figueroa Cespedes, master’s in emergencies dental care, First Degree Specialist in Comprehensive General Stomatology, Third Year Resident of Orthodontics, Faculty of Stomatology, Raul Gonzalez Sanchez, Havana, Cuba
DOI: 10.32474/IPDOAJ.2022.07.000275
Abstract
Introduction: Traditionally, ruble professionals have used the Mayoral table as an “established canon” for the study of dental buds. Without taking into account that this pattern of dental emergence should be used as another reference, not as the ideal to be achieved, since each population has its own specificities, determined by the context in which it will develop.
Objective: To establish a modern standard of dental eruption in children between 13 to 36 months of age against the “established canon” Methods an observational, descriptive correlational, retrospective and cross-sectional study was developed. The universe was made up of children enrolled in the second and third year of life of three Children’s Circles. The sample was those who attended during November and December 2020, reviewed by the same examiner. We developed a form created and validated for this purpose. To estimate the mean age of each tooth, the Kärber [1] method was used.
Results: Comparing with Mayoral [2], there is no variation in the order of appearance, by groups of teeth, but there is between types of teeth. Showing a more marked advancement of the first molars, especially the lower ones. Although the second phase outbreak began, due to the female sex, it cannot be argued that the total time of outbreak was somewhat faster in one or the other sex.
Conclusions: The population studied presents a modern pattern of outbreak in children from 13 to 36 months of age not fully consistent with the “established Canon”
Keywords: Dental eruption; dental eruption; dental emergence; eruption chronology; eruptive pattern
Introduction
Traditionally, ruble professionals have used the Mayoral table as an “established canon” for the study of tooth buds. Without taking into account that this pattern of dental emergence should be used as another reference, not as the ideal to be achieved, since each population has its own specificities, determined by the context in which it develops [3,4]. So much so, that clinical and academic experience has shown that the Cuban population presents outbreak patterns that are not in accordance with established standards, with respect to North American and European populations. Rather, said table does not coincide with the periods of dental outbreak of Cuban Children, in whom, from this perspective, it seems that with the passage of time, there is an apparent advance in dental emergence, as well as some differences in the order of appearance of each type of tooth [5,6]. This situation forces us to create a modern standard of order and outbreak chronology that responds to the reality of the Cuban child population. Something that generates many questions in industry professionals, because in Cuba their own guidelines have not been instituted. However, some countries already have them established [5,7]. This lack of epidemiological data on the development of the primary dentition in Cuba forces the repeated use of the standards of Mayoral et al. [2] dating from 1984, but it is not known with certainty how and where they obtained their results. What is clear is that its elaboration was carried out through studies in populations of very different geographic, socioeconomic, cultural, environmental and even ethnic origin from those that make up our country [3,6,8,9].
These notable differences, which do not conform to the established canon, must be considered when applying dental eruption chronology standards from another region, even from the same country. For all these reasons, foreign standards have drawbacks when extrapolating their values to use them in our population. Thus asserting that it is preferable not to adopt references from other countries as a standard for our population [10,11]. Recent publications [12,13] are oriented to the exploration around establishing National Standards for the order of eruption in various regions of the country. However, today they are not of considerable magnitude, being restricted to very specific samples and locations. The achievement of this major objective requires the successive development of research in the health areas. Reason why the results cannot be extrapolated to the Havana or Cuban population. However, it constitutes scientific evidence and a useful reference, given the scarcity of publications on the subject that has already been commented on [14-19]. This situation shows that what has been done so far is insufficient. Therefore, by being able to have up-to-date information about the pattern of in Cuba, we have in our hands a very useful resource in the dental clinical context, by establishing prevention and care strategies in accordance with the real conditions of our population. In addition, it allows us to apply corrective measures for the treatment of malocclusions and pathologies that are generated as a result of the influence of the alteration of various factors. In this way, monitor oral, dental, maxillofacial health in harmony with postnatal growth and development, with the consequent objective of guiding and establishing guidelines to achieve correct occlusion [14-23].
But why did we decide to focus our efforts on conducting our study in this age range and not another?
This age range was adopted taking into account that we study children from day care centers, and it is precisely between these limits when decisive changes occur at the level of craniofacial growth and the development of favorable occlusion. Another element that draws our attention is that the eruption sequence of the posterior teeth is not uniform as in the anterior region. The points of coincidence have shown that there is greater variability in the eruption ranges of primary canines and molars with respect to other types of teeth. Tooth eruption in this area is very complicated and is influenced by several factors that can act positively or negatively in establishing a correct occlusion [8,24]. From this moment on, within the framework of this research, for educational purposes, we will call the period of time in which the first tooth of the posterior sector erupts until the last one of the same sector emerges. Constituting the first phase of the shoot, the period during which the incisors sprout. In the same way, we will limit ourselves to using the terms outbreak and emergency interchangeably, but we will respect the citations that use it as “eruption”, as they are terms that are not mutually exclusive, but complement each other.
Everything expressed conditions the fact that we are facing an investigation that is very necessary and, above all, timely. It is tacitly recognized that each individual and each population have their own characteristics, which makes it clear that it is not possible to give precise dates for the chronology of dental emergence, since a great variability of factors is normal. This makes it clear that it does not occur exactly, but it is useful to keep in mind the average age for diagnostic purposes, to determine noticeable advances or delays in dentition. Turning out to be an instrument for the work of dentists who work with children [13,19,25,26]. This research is different, taking into account that it is ideal to establish a modern outbreak standard in accordance with our country. So, we intend that this difference is the contribution of this study. This prevents distortion in the analysis and results of the sociodemographic data. Therefore, our efforts focused on establishing a modern standard for dental eruption in children between 13 and 36 months of age compared to the “established canon”. To fulfill this major objective, the following specific objectives were developed:
a) Determine the bud order of the possible teeth to erupt in each hemiarch by sex.
b) Determine the bud chronology of the possible teeth to erupt in each hemiarch by sex.
Methods
Between March 2020 and September 2021, a study was developed that, due to its characteristics, is classified as observational, descriptive, correlational, retrospective, and crosssectional. The universe of work was made up of all the children enrolled in the second and third year of life of three Children’s Circles belonging to the Vedado Teaching Polyclinic in the Plaza de la Revolution Municipality. The sample consisted of all the children who attended during the month of November and December 2020. As a prerequisite for inclusion in this research, only children between 13 and 36 months of age, with Cuban nationality, as well as both parents, were considered eligible. Other criteria that excluded certain children were also taken into account, such as: the possibility that a parent would deny participation and present other conditions (avulsion and/or extraction of temporary teeth, syndromes or pathologies that affected eruption, congenital defects). In compliance with what was agreed in the second “Declaration of Helsinki, we include ethical aspects, as it is a study of direct action on the human being, with psychological and social repercussions. Together with this, authorization was formally obtained from the directors of the Children’s Circles and with the Informed Consent signed by the parents/guardians. With the idea of avoiding bias, the oral examination was carried out by the same examiner in a single stage, once. As a strategy for collecting information, we developed a form created and validated for this purpose. Dental eruption was described as erupted tooth or non-sprouted tooth. Depending on whether or not the integrity of the gingival tissue has been broken, any part of its anatomy is visible in the oral cavity, regardless of the degree of eruption.
The chronological age of each of the children, expressed in months, was calculated by subtracting the date of birth from the date of examination. The categorical variable (presence of each tooth) is expressed as a percentage. For its part, the quantitative variable (age) is described by means of the mean and standard deviation of each distribution.
The data on the chronology of dental eruption were presented in the form of mean age with standard deviation/error, as in most of the studies reviewed, which facilitates the interpretation of the results of the analyses. Thus, the weighted averages of age of eruption in which each tooth is present in the mouth are estimated. We analyze each homologous pair separately, depending on whether it is in the maxilla or mandible and based on the percentage, differentiating between boys and girls who present it at a certain age. Speaking of mean age as a statistical term would imply collecting the chronological age of eruption of each tooth in each child and adding and dividing arithmetically by the number of children examined. This was calculated using the Kärber [1] method. One of the most widely explained and accepted methods for obtaining the mean, standard deviation, and standard error of tooth eruption. Based on the statements of other authors L. Hayes and N. Mantel (based on the dose/response curves in comparison and their estimates related to the figures obtained by direct interpolation on the data), in 1958 they describe a procedure for the evaluation dental eruption statistics. This method is based on the statistical principle that the distribution of eruption ages is satisfactorily described, since it follows the pattern of a normal probability curve and through it the median and standard deviation of a normal distribution are obtained.
The conditions to be used are to have the data that cover from 0 to 100% of the eruption range of the tooth under study with an equidistant distribution in time of said range, which in the case of eruption is facilitated by having the date of birth in months and years and separating data by age groups spaced in three months, etc. Both conditions are met by our study, which was designed to be able to apply this method.
Thus, to estimate the average age of eruption of the possible primary teeth to erupt in this age range: canines and molars, it was ordered from smallest to largest. By means of the Cumulative Distribution Functions method, which basically treats that we have an age or lower bound in which no individual has emerged the tooth under study, and an age or upper bound in which it has emerged in all. [27] Between that age of 0% and 100%, as age increases, the percentage of individuals who have the tooth in their mouth will increase. By means of a graph in which we cumulatively mark the percentages of cases with a positive response, the age that corresponds to 50% will be the median age. In this way, it was possible to establish the intervals of ages of eruption of each one of the teeth. Essentially what is obtained is the estimated probability that the event has occurred at a certain age.
The equation to use would be the following: Where the median will be:
M = d (Xs – Si + 1/2)
M will be the median, d = space of time considered, represents the unit of measurement or age strata (3 months), Xs = Age in months at which the range of 100% of the eruption is reached (in this case, 36 months).) + 0.5...Si = 100% cumulative percentage of tooth eruption in each age group.
To obtain the 95% confidence interval of each estimate of the mean age of eruption, another formula derived from the Karber1 method is used for the calculation
EE (m) = d*√Σ[p(1-p)] n-1
where “p” is the proportion of erupted teeth and “n” the corresponding total number of subjects in each age group.
Using the formula: – 95% CI = m+ 1.96* SE(m)
The lower and upper limits of each 95% confidence interval of the mean eruption age are calculated, assuming that the eruption ages are approximately normally distributed. Estimates of the mean eruption ages are compared using the corresponding 95% confidence intervals. It is assumed that the differences observed between two mean ages are statistically significant when the confidence interval of one of the two means being compared does not include the other. All the hypothesis contrasts have been proposed bilaterally, accepting a statistical significance level of 5%. To analyze whether there were differences on one side or between the sexes, the Student’s T test was applied with a significance level of 5%. The statistical software SPSS version 8.0 for Windows was used in the processing stage of the information collected. At the same time, Vancouver Bibliographic Style was used, helping us with the Zotero software for document management of bibliographic references in multiple formats and the categorical organization of documents. The final report was made through Microsoft Office Word 2010.
Results
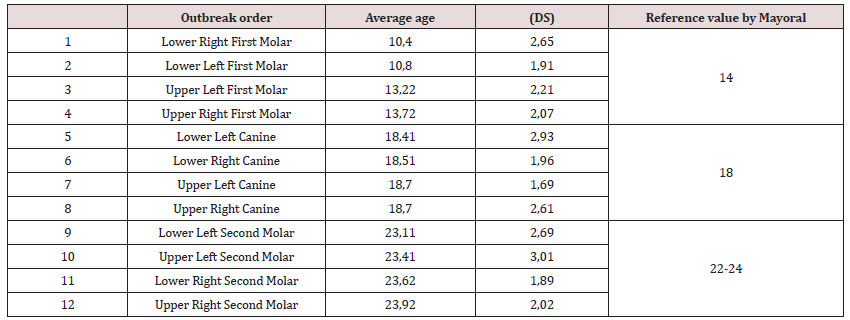
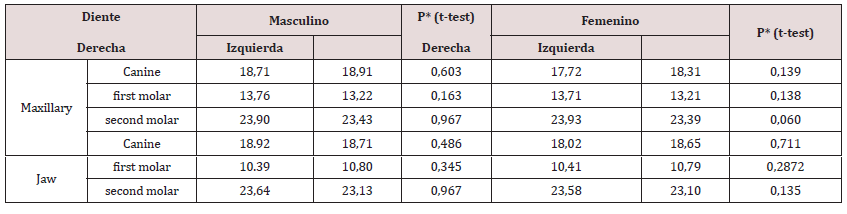
Table 1 reveals the outbreak sequence found for each type of tooth and its reference pattern. It begins with the lower first molars followed by the upper ones. Similarly, lower canines and then the upper ones. Finally in the second molar, with a somewhat typical pattern following the following pattern: first the Lower Left, followed by the Upper Left, then the Lower Right Molar and finally the Upper Right second Molar. Regarding polymorphism, Table 2 shows the outbreak sequence and the estimate of the mean ages of eruption with their standard deviation, for both maxillae; linked to the reference values of Mayoral [2]. Likewise, no differences were found in the order of emergence between the female group with respect to the male group. Based on the established median age of eruption, the natural order of appearance of posterior teeth is proposed, both in boys and girls: starting with the Lower Right and Left First Molar, Upper Left and Right First Molar, then the sequence continues with Upper and Lower Right Canine and Upper and Lower Left Canine, respectively. To finish with a third group, in the following order, Second Lower and Upper Left Molar, followed by Second Lower and Upper Right Molar. When analyzing the chronology of the average ages of the primary dentition eruption (Table 3) by sex, it was observed that although the second phase eruption began, for the female sex (10.39 months with the upper right first molar), it cannot be argued that the total outbreak time was somewhat faster in either sex. Even more so, when no significant differences were observed (p<0.05).
Table 2: Order and chronology of the outbreak of the second phase of the temporary dentition according to sex.
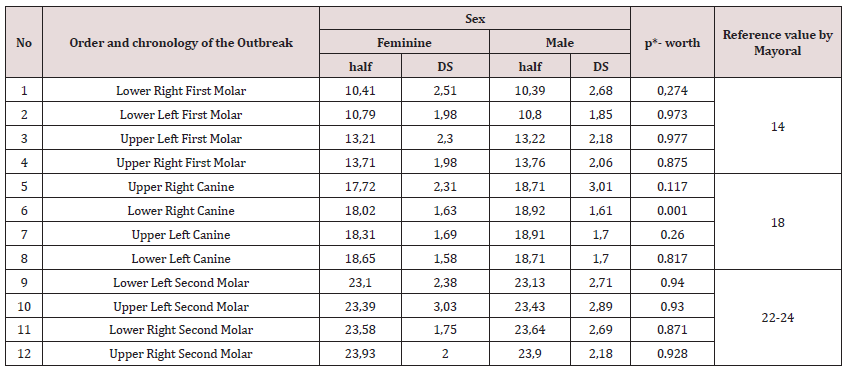
Table 3: Estimation of the bud age (in months) of the second phase of the temporary hemiarch dentition and sex.
Discussion
The intention of establishing a modern standard of dental eruption in children between 13 and 36 months compared to the “established canon”, motivated the present work, which showed that the average age of eruption was similar to that proposed by Mayoral et al. [2], but with a tendency to an earlier emergence in the first molars, especially the lower ones, at least in this territory. In this sense, Morin [28] asserts that the sequence is less variable than the chronology, while Freitas [29] citing Guerrero alleges that the order may be different from the one stated as favorable, and this is completely normal. The second phase outbreak lasted an average of 13 months (time from when the first tooth of the posterior sector erupts until the last tooth of the same sector emerges) (Table 1). Throughout this period, the appearance of the teeth in the mouth is produced by dental groups: at first, the First Lower Molars erupted practically at the same time, between 10.4 and 10.8 months. After a silent period, where the upper ones erupted (between 13.22 and 13.72 months). Subsequently, there was a window period of almost five months (time between the eruption of one group of teeth and another), where the canines appeared in unison (around 18 months, in general). After a final window period of approximately 5 months, Second Molars with similar emergence ages (23 months) emerged, in this case regardless of which hemiarch and maxilla erupted first
In addition, it was found that the age of appearance of the first posterior tooth was around 10.4 ± 2.65 months, and the eruption of the last tooth was 23.92 ± 2.02 months. The comparison with the values given by Mayoral [2] does not show variation in the order of appearance, if it is about tooth groups. However, if we refer to the type of tooth, we find that in the case of Lower Left Second Molar and Upper Right Second Molar, this sequence is altered. In the same way, there is evidence of a tendency to an earlier emergence in the mandible, with the exception of the Lower Right Second Molar, since in our reference pattern in the case of the second molars, the lower ones erupt first and then the upper ones. This sequence is related to what was found by foreign authors, such as Çoban [30], Folayan [31], Guna [32], Al-Batayneh [33], Ghabani [34], who report that they obtained an earlier emergence in the lower canines and first and second molars relative to their upper counterparts. When comparing it with our study, we found that the sequence was maintained, but what is not completely consistent is in the order of the second molars. We have a similar situation when we contrast it with previous studies of the same project, such as those carried out by Busutil [35] and Freitas [29], always with a somewhat different behavior when it comes to second molars. However, Rodríguez [36] describes an earlier emergence in the jaws.
On the other hand, for Burgueño [37] in Spain, and Moataz [27] in Egypt, in the so-called second phase of the deciduous dentition, from the eruption of the first molar, the teeth of the upper arch erupted before those of the lower one. , showing a linear relationship between vertical symmetry and tooth bud. For his part, Carreño16 in his study carried out in a group of Caucasoid mestizos from Cali (Colombia), stated that regardless of whether the teeth emerge first in the maxilla or in the mandible, there are no significant differences between the teeth of both arches.
A curious fact is that Badruddin [38] published a study carried out on children from the Beji subdistrict, suggesting that the first primary molars erupted after the canine. It must be taken into account that the compared studies do not always use the same age estimation methodologies, so it cannot be ruled out that the differences observed are a consequence of this. Which is why such analyzes could also be biased. At this point, we consider that there is no single applicable sequence for all populations, but it is clear that, in the second phase of the deciduous dentition (from the eruption of the first molar), the sequence in terms of dental groups is quite stable, the greatest differences appear when an interarch analysis is done. On the other hand, the study also aimed to determine the order and chronology of the eruption of the possible teeth to erupt in each hemiarch by sex. The dental eruption sequence presented similarity between both sexes. In this sense, Folayan [31] found differences in the outbreak sequence between both sexes, producing small variations. Thus, in the case of girls, the upper second molar erupts before the lower one, and in the case of boys, the lower first molar precedes the upper one.
The comparison with estimated values for the Mayoral [2] reference median behaves similarly for both canines and second molars. However, it showed a more marked advancement in the eruption of the first molars, especially the lower ones. We can also say that in none of the cases was their evidence of a delay with respect to the pattern we referenced. This situation still holds when we contrast it with the standard deviations. At the same time, authors such as Morgado [39] and Ferrreira [40] obtain mean age values similar to ours. Unlike Rodríguez Estévez [41] in an area of San Miguel del Padrón, Freixas [29] in an area of Boyero, Al Graham [12] in an area of Arroyo Naranjo, and Busutil [35] in an area of Old Havana, which marked significant delays in the second phase of dentition decidua (from the bud of the first molar), more evident in the emergence of canines, which could be conditioned by dietary factors associated with a specific population, as referred to by Kohli [42]. Morgado [24] in a study carried out in Ciego de Ávila describes an advancement of the entire dentition, showing a stronger tendency to early eruption than in the current study. Bearing in mind that this was only demonstrated in the case of the first molars.
It is evident that the chronology of emergence differs slightly between the sexes, with a slight advance in females compared to males, except in the case of the Lower Right First Molar. Despite this, in this tooth it does not reach a statistically significant association. Similar results are reported by De Armas [43] in a study carried out in the Cerro Municipality. In the same way, we must bear in mind that, from a clinical point of view, these differences will be almost imperceptible. If added to this, we observe that a statistically significant association was only found in the lower right canine, and even so it does not achieve that orthodontic importance. They show that in our population, it is not possible to define a precise pattern marked by sex. However, it can be seen that in girls the teeth erupt earlier than in boys, with the variation of the Lower Right First Molar. Regarding sexual differences, it is suggested that dental eruption is earlier for all teeth in females, which is associated with hormonal factors [11]. These findings are consistent with the studies by Vejdani et al. [44], who found that boys lagged behind girls except for the first molars. Similarly, Moataz Elkhatib [27], in his cross-sectional study conducted in the Middle East (Egypt), stated that tooth eruption in primary teeth is preceded by females, except in the upper and lower central incisors.
The study by Vejdani et al. [44] reveals that the boys were behind the girls except for the first molars. However, statistically significant differences were found only in girls in the upper second molar (p<0.05).
the other hand, Torres et al. [45] investigated the primary dental eruption in 1250 healthy Spanish children, and obtained, that the last one was the second upper left molar at 33.24 months. The review of the systematics on the subject that concerns us shows a great variability in terms of the data on the chronology of emergence itself, and the factors that may be involved in this phenomenon. Most studies provide eruption dates for a specific tooth or the total number of teeth present in the oral cavity at a certain age. Differences between the findings can lead to bias when comparing between studies. Also, for each pair of contralateral teeth, the probability of dental emergence in the same individual has been contrasted, and whether it is the same or not in both sexes (Table 3). These findings differ from those found by De Armas [43] who posits a chronological advance in girls compared to boys, that is, the total outbreak time was somewhat faster. But it does coincide, in the fact that in both investigations no statistically significant differences were detected in any teeth and in both sexes (p<0.05).
Vinod [15] when comparing the maxillary and mandibular I suggest that there is a tendency to earlier mandibular eruption of second molars in both sexes. In a study carried out in Spain in children, the maxillary teeth erupted before the mandibular ones, the opposite happening in girls. In addition, a more advanced emergence was observed in males, who instead presented a longer eruptive process. [37] The data obtained do not agree with those of other eruption studies carried out by de Choi and Yang [46] in 2001 with Korean babies, as they showed that primary teeth erupt earlier in boys than in girls. Gunashekhar M, et al. [32] conducted a longitudinal study of the age and eruption order of primary teeth in Indian children in Hyderabad and found that the children showed a general trend towards earlier eruption for all teeth except the maxillary second molars and mandibular first molars that erupted earlier in girls. Similar results were shown by Kariya [47], although the difference was significant only for the canine and second molar of both jaws (P ≤.05). Mysore [48] research joins this trend, where maxillary canines and mandibular molars are the exception. Zadzinska et al. [49] and Oziegbo et al. [50] reported that the eruption of the first deciduous tooth was earlier in children Sex appears to play an important role in tooth eruption in Saudi Arabian boys relative to girls being earlier in the jaws [51,30].
Conclusions
a) The population studied presents a modern pattern of outbreaks in children between 13 and 36 months of age that are not entirely consistent with the “established canon” (Mayoral tables).
b) The sequence of the outbreak of the second phase of the primary dentition in this population began with the first molar, followed by the canines, ending with the second molar in correspondence with Mayoral, without difference by sex.
c) The mandibular teeth erupt before the maxillary teeth, as in the Mayoral reference values, except for the second molar, which has a typical sequence, the same for both sexes.
d) The mean age of emergence and the width of its ranges were similar to the reference values, but where a marked advance was noted was in the first molars, especially the lower ones, regardless of sex.
e) There is bilateral symmetry in the time and sequence of the eruption pattern for both maxillae and both sexes.
References
- Henandez M, Boj JR, Sentis J (2002) Usefulness of the Kärber method to obtain the mean ages of tooth eruption. Pediatric Dentistry 10(1): 14.
- Mayoral J, Mayoral G, Mayoral P (1984) Orthodontics. Fundamental principles and practice. 3rd Havana, Editorial Science and Technology.
- Penagos Valdez GR, Sánchez Acuña G, Pinales (2014) Mean age of dental eruption in a school population analyzed by two methods. Bol Méd Hosp Infant Mexico 71(6): 352-35.
- Adriano Anaya MP, Caudillo Joya T, Caudillo Adriano PA (2015) Age of Permanent Eruption in a Child Population of Mexico City. Int J Odontostomat 9(2): 255-262.
- Taboada Aranza MO, Medina Garcia JL (2005) Chronology of tooth eruption in schoolchildren from an indigenous population of the State of Mexico 62(3): 94-100.
- Romo Pinales R, Pérez Rivera ST, De Jesús Herrera MI (2002) Chronology of dental eruption in Vertientes School Population. Specialized Magazine in Health Science 5(1-2): 43-8.
- San Miguel A, Veliz OL, Escudero RZ (2017) Dental eruption, is everything said?. Medical Act of the Center 11(1): 72-5.
- Ayala Pérez Y, Zaldívar C, Caridad L de la, Ayala L, Rosario B del (2018) Tooth eruption and its influencing factors. Medical Scientific Mail 22(4): 681-694.
- García Izquierdo F, López Benito MM, Nuño Mateo F (2003) Importance of temporary teeth. Its eruption chronology. Rev Pediatr Primary Care 5: 439-45.
- De la Tejera Chillón A, Peña Gómez I, Bravo Barrera G, Solano Quinzán Y, Rodríguez Junco A (2017) Chronology and eruption sequence of the first permanent molars. MEDISAN 21(1): 12-8.
- Colomé Ruiz GE, Kú Santana YG, Pérez Traconis LB (2014) Chronology of dental eruption in a population of southeastern Mexico. ADM magazine 71(3): 130-5.
- AL Ganhnam I (2015) Order and chronology of eruption of primary dentition. Grimau Polyclinic. Arroyo Naranjo Municipality.
- San Miguel PentónI A, Veliz Concepción OL, Escudero Alemán RZ, Calcines Ferrer ME (2011) Emergence chronology of the permanent dentition in children from the municipality of Santa Clara: Part I. Rev Cuba Estomatol 48(3): 208.
- Šindelářová R, Žáková L, Broukal Z (2017) Standards for permanent tooth emergence in Czech children. BMC Oral Health 17(140): 8.
- Vinod, Singh R, Suryavanshi R, Ashok Kumar, Singh RK, Ranjan R (2016) Eruption chronology of Primary Teeth in Garhwa district, Jharkhand, India. IAIM 3(5): 81-4.
- Carreño B, Cruz S de la, Piedrahita A, Sepulveda W, Moreno F, et al. (2017) Chronology of dental eruption in a group of Caucasoid mestizos from Cali (Colombia). Rev Estomatol 25(1): 16-22.
- De Souza N, Manju R, Hegde AM (2018) Development and evaluation of new clinical methods of age estimation in children based on the eruption status of primary teeth. J Indian Soc Pedod Prev Dent 36(2): 185-190.
- Galician Arms LI, Rodriguez Gonzalez S, Batista Gonzalez NM, Fernández Pérez E (2019) Update on the order and chronology of the outbreak of the temporary dentition.
- Morgado Serafín D (2013) The science-technology-society view of tooth eruption behavior according to chronology and risk factors. Medical 19(2): 2-11.
- Alata AM (2019) Dental development and eruption Academic work to opt for the Title of Second Specialty in Pediatric Dentistry. Inca Garcilaso from Vega University. Faculty of Stomatology. Lima Peru.
- Lam CU, Hsu C-YS, Yee R, Koh D, Lee YS, et al. (2016) Influence of metabolic-linked early life factors on the eruption timing of the first primary tooth. Clinical Oral Investigations 20(8): 1871-1879.
- Navarro J, Cobas N, Pardo MI (2018) Risk factors for temporary tooth eruption. Convention and International Fair Cuba Health 2018 Palace of Conventions, Havana, Cuba.
- Álvarez Serna, MC, Anaya Daza, MI, Daza Plata, AM, et al. (2019) Chronology and sequence of canine and premolar eruption in children aged 9 to 15 years in two educational institutions in the city of Bucaramanga. Santo Tomás University, Bucaramanga, Colombia.
- Orellana TO, Marengo Castillo HA, Mendoza Zapata JB (2013) Sequence of dental eruption of lower canines and premolars in a sample of Peruvian children. Odontol Sanmarquina 16(1): 4.
- Garcia M, Alvarez I, Hernandez NM (2016) Chronology and bud order of the permanent dentition. Medical Act of the Center 10(2): 59-61.
- De Armas Gallegos LI, Figueroa-Céspedes Y, De la Torre Molina Y (2021) What Has Changed in the Order and Chronology of Outbreak in Temporary Dentition? Inter Ped Dent Open Acc J 5(4): 449-452.
- Moataz Elkhatib, Norhan El Dokky, Rania Nasr (2021) The eruption sequence of primary and permanent teeth in a group of children A cross-sectional study. EDJ 67(41:54): 14.
- Morón BA, Santana Y, Pirona M, Rivera L, Rincón MC, et al. (2006) Chronology and eruption sequence of permanent teeth in Wayúu school children. Idelfonso Vasquez Parish. Maracaibo Municipality Zulia State. Dental Act Venez 44(1): 31-37.
- Freixas Piñeiro Y (2016) Order and chronology of the primary dentition bud. Salvador Allende Polyclinic. Boyeros Municipality. 2015. Thesis to opt for the Title of Firs Degree Specialist in Orthodontics. University of Medical Sciences of Havana. Faculty of Stomatology. Havana, Cuba.
- Çoban B, Kansu L, Dolgun A (2018) Timing and sequence of eruption of primary teeth in southern Turkish children. Acta Medica Alanya 2(3): 199-205.
- Folayan M, Owotade FA Lawal B, KN (2007) The timing of eruption of the primary dentition in Nigerian children. Am J Phys Anthropol 134(4): 443-8.
- Guna Shekhar M, Tenny J (2010) Longitudinal study of age and order of eruption of primary teeth in Indian children 2(3): 113-6.
- Ola B. Al Batayneha, Ashraf Shaweesh (2018) Clinical duration of eruption of deciduous teeth in Jordanian children: A cross-sectional study. Arch Oral Biol 90(1): 86-90.
- Khaled Ghabani M (2017) The influence of weight and height on the eruption of primary dentition University of Valencia.
- Busutil Corbo (2018) Order and Chronology of Outbreak of the temporary dentition. Tomas Romay Polyclinic. Old Havana. 2016-2018. Thesis to opt for the Title of First-Degree Specialist in Orthodontics. Faculty of Stomatology "Raul Gonzalez Sanchez", Havana, Cuba.
- Rodríguez González S (2020) Order and chronology of primary tooth eruption. Stomatological Clinic «Salvador Allende». Closed. 2017-2020.” Thesis to opt for the Title of First-Degree Specialist in Orthodontics. University of Medical Sciences of Havana Faculty of Stomatology “Raúl González Sánchez”, Havana.
- Burgueño Torres L, López Gallardo G, Martínez Mourelle RM (2011) Chronology and sequence of eruption of primary teeth in a child sample from the Community of Madrid. Scientific Dent Ed Impr 8(2): 31-38.
- Badruddin IA, Putri MR, Rahardjo A (2017) Factors Associated with Primary Teeth Eruption Pattern in Children Under Three Years Old in Beji Depok, West Java. J Int Dent Med Res 10(Special Issue): 564-568.
- Morgado Serafín D, García Herrera A (2011) Chronology and variability of tooth eruption. Medical 17(Suppl 2): 7.
- Ferreira L, Neto E, Oliveira A, Zandonate E (2015) Chronology of Deciduous Teeth Eruption: Agreement between Classic Authors. Brazilian Research in Pediatric Dentistry and Integrated Clinic 15(1): 361-370.
- Rodríguez Estévez M (2015) Order and chronology of the primary dentition bud. Luis A. Carbó Polyclinic, 2014. [Thesis to opt for the Title of First Degree Specialist in Orthodontics. Raúl González Sánchez School of Stomatology. Havana, Cuba.
- Kohli MV, Patil GB, Kulkarni NB, Bagalkot K, Purohit Z, Dave N, et al (2014) A Changing Trend in Eruption Age and Pattern of First Deciduous Tooth: Correlation to Feeding Pattern. J Clin Diagn Res 8(3): 199-201.
- De Armas Gallegos LI, Batista González NM, Fernández Pérez E (2021) Order and chronology of primary tooth eruption. Medical and Surgical Sciences International Journal of 2 8(2): 10.
- Vejdani J, Naemi V (2011) Relationship between birth weight and eruption time of first deciduous tooth. J Res Dent Sci 7(4): 34-40.
- Torres LB, Martínez MRM, Pérez MD, García JM de N (2018) Sexual dimorphism of primary dentition in Spanish children. Acta Odontol Scand 76(8): 545-552.
- Nk C, Kh Y (2021) A study on the eruption timing of primary teeth in Korean children. ASDC J Dent Child 68(4): 244-249.
- Kariya P, Tandon S, Singh S, Tewari N (2017) Polymorphism in emergence of deciduous dentition: A cross-sectional study of Indian children. J Invest Clin Dent 9(1): 5.
- Mysore Devraj I, Nandlal Bhojraj, Narayanappa D (2018) A cross sectional study on eruption timing of primary teeth in children of Mysore, Karnataka. Indian J Dent Res 29(6): 726.
- Zadzinska E, Sitek A, Rosset, I (2016) Relationship between pre-natal factors, the perinatal environment, motor development in the first year of life and the timing of first deciduous tooth emergence. Annals of Human Biology 43(1): 25-33.
- OziegbeEo, Adekoya SofoworaC, Esan TA, Owotade FJ (2008) Eruption chronology of primary teeth in Nigerian children. J Clin Pediatr Dent 32(4): 341-345.
- Alnemer K, Pani Sc, Althubaiti AM, Bawazeer M (2007) Impact of birth characteristics, breast feeding and vital statistics on the eruption of primary teeth among healthy infants in Saudi Arabia, an observational study. BMJ Open 7(12): 18621.
Editorial Manager:
Email:
pediatricdentistry@lupinepublishers.com

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...