
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6652
Research Article(ISSN: 2637-6652) 
UV Protective Pigments and Growth Abnormalities during Light and Heat Stress of Maldivian Corals Volume 2 - Issue 4
Pierre Madl1* , Wolfgang Loch2 and Karen Loch2
- 1Department Physics and Biophysics, University of Salzburg, Austria
- 2Department of Natural History-Zoology, Darmstadt, Germany
Received: March 18, 2019; Published: March 25, 2019
Corresponding author: Pierre Madl, Department Physics and Biophysics,University of Salzburg, Hellbrunnerstr. 34, A-5020 Salzburg, Austria
DOI: 10.32474/MAOPS.2019.02.000145
Abstract
During one of the recent visits in Dec. 2015 on Angaga (Ari Atoll, Maldives) the authors found a badly damaged reef devastated by bleaching-events as well as starfish coral predation. Nonetheless, some huge mono-specific stands of live branching Acropora abrolhosensis were still present in isolated patches. A very striking and common feature of these colonies concerned their bluish terminal branches with large longitudinal extensions that we associate with photo-protective fluorescent pigments (FPs) that seem to play a crucial role in this morphological adaptation. The streamlined tips were of slender appearance and regularly associated by a complete absence of radial corallites. Pigmentation in these stripped areas from endosymbionts did only occur around the scarcely present corallite calices and towards the very distal parts close to the apex. On a later visit in May 2016 it was found that most of these colonies with their peculiar appearance had died. Only few specimens still revealed the extensive bluish hue of their slender tips, which after sloughing off their coenosarc revealed its radiant white skeleton.
Keywords: Photo-protection; Heat stress; Growth abnormalities; A. Abrolhosensis; TB; SDR
Introduction
Abiotically induced Tissue Bleaching (TBL) is commonly associated with stagnant water movement accompanied by elevated Sea Surface Temperatures (SSTs) that last for extended periods of time and thereby exceeding the upper tolerance levels of most corals. TBL removes the autotrophic supply as endosymbionts are expelled as a result of a protective anti-toxic reaction [1]. Species most susceptible to TBL include species with branching morphologies, so that within a month of peak temperatures, these are the first ones to die [2]. In many cases, mortality rates in affected areas may rise to as much as 80-100 %. A visit to the Angaga reef (Ari Atoll, Maldives, northern Indian Ocean) in April 2005 - almost seven years after the severe 1998 bleaching event, which resulted in a massive die off of most scleractinian coral species - revealed a partially regenerated reef of acroporid table corals up to 1 m across consisting mostly of A. cytherea, A. hyacinthus and A. clathrata. Back then, live coral coverage across four 20 m transects was found to be at an average of 46 % [3].
Revisiting the same site 10 years later, in December 2015 and following the atypical weather patterns during summer of that year, which affected most sections of the Ari Atoll, revealed a slowly recovering reef yet again subject to extensive TBL that extended from the reef flat all the way down to the fore reef section some 15 m deep [4]. Based on remote sensing data [5] the duration of SSTs exceeding 30°C lasted for four weeks.
While it was possible to still spot some larger aggregates of unaffected corals on Angaga reef in December 2015, a follow-up visit in May 2016 presented an even more devastating picture: only approx. 1-2 % live coral cover left, with about 10 % of scleractinia showing signs of recent bleaching, and the reminder already overgrown with dense algal strata. Again, the NOAA database confirmed that over a period starting with June 2015 to January 2016, the average SST was 1°C higher than the long-term average. By the end of March till the end of May 2016, SST exceeded the 30°C level, with a two-week peak reaching even 3°C [5].
Material and Methods
Status of Maldivian reefs on all visits have been performed either underwater using SCUBA-gear on fore-reef areas or simply snorkelling over them for reef flats and the lagoon area. All visited sites have been referenced using GPS-coordinates. Yet the most extensive census was performed at Angaga Island reef (Ari- Atoll, Maldives) with the corresponding coordinates: N 03°49.278‘; E 72°39.144‘. During the visits in December 2015 and May 2016 the census regarded visual inspections of the entire reef with a particular focus on acroporid species. Abnormal growth morphologies have been documented using an 14 MPxls underwater still camera and land-based macro-imaging (Olympus µTough-8010).
Results
Growth anomalies in the apical region of branching Acropora:
During the visit to the lagoon of Angaga Reef in Dec. 2015, intact mono-specific stands of Acropora abrolhosensis covered an area of roughly 1000 m2 . Yet striking morphological changes could be observed that concerned their branches - especially the elongated apical tips (more than 20cm long), shaded in a light bluish hue along with an extremely slender growth form (Figure 1). It is well known that in calm areas such as lagoons and the backreef regions of fringing reefs, morphological compression is less pronounced promoting slender growth morphology of branching colonies [6]. The observed abnormalities however, exceeded the well-known morphotypes with regard to this particular species.Figure 1: Single stand of Acropora abrolhosensis with elongated apical tips and slender growth form shaded in a bluish hue (Angaga Resort reef, Ari Atoll, Dec. 2015).

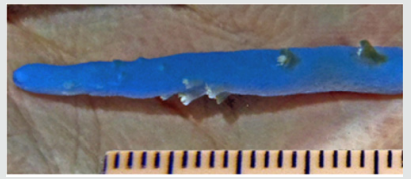
On top of that, some colonies showed not just a slender growth form of the branching tips, but also a “streamlining” phenomenon, with a concomitant loss of radial corallites (Figures 2 & 3). A closer investigation revealed a transition zone whereby the primarily endosymbiont-pigmented coenosarc around the polyps of the distal section gave way towards a azooxanthellate proximal ringlet separating the smooth and slender bluish tips devoid of any corallites. Only in few cases have the tips been fully bleached thereby exposing the underlying skeleton.
Figure 2: Slender tips of A. abrolhosensis with partial loss of radial corallites and intense incorporation of FPs at the apical region.

Figure 3: Detail of a single terminal branch revealing the almost complete loss of radial corallites (scale bar in mm).
Some five months later (May 2016) these peculiar looking colonies that clustered in huge mono-specific aggregates had largely died; i.e., around 98 % overgrown with algae and the reminder having either lost their polyps or shed their coenosarc (Figure 4). There have been few sightings however, in which the entire colony still revealed this light bluish hue (Figure 5a).
Figure 4: A recently bleached and partly dead colony of Acropora abrolhosensis (Angaga reef, Ari Atoll, May 2016). Only the tips still reveal a bluish hue; polyps are no longer present.

Figure 5a: Slender tips of A. abrolhosensis (Angaga reef, Ari Atoll, May 2016) devoid of radial corallites.

Figure 5b: Detail of recently dead A. abrolhosensis colony (Angaga reef, May 2016). Corallivores (genera Oxymonacanthus and Chaetodon) fed on the coenosarc (not shown), tissue partially eroded, possibly triggering SDR (the magnified insert shows the sloughed off tissue in more details).

Figure 6a: Elongated tip of A. muricata (Madoogali reef , Ari Atoll, May 2014), revealing reduced radial corallites morphology and blotches of endosymbiont loss.

Evidence from Past Visits: Referring to archive material obtained during a May 2014 visit from Madoogali reef (Ari Atoll, GPS: N 4° 5.689’; E 72° 45.127’), it was possible to identify similar tipanomalies on A. muricata (formerly A. formosa). These colonies too featured prolonged, slender terminal branches along with extended axial and reduced radial corallites (Figures 6a & 6b) but lack the bluish hue as observed on later visits on A. abrolhosensis. The partly denuded areas (loss of corallites and endosymbionts) were limited to the tips and the coenosarc around radial corallites (Figure 6a). However, growth abnormalities were far less pronounced than in the case of A. abrolhosensis.
Figure 6b: Same sample devoid of coenosarc. The poorly developed radial corallites near the apex become visible.
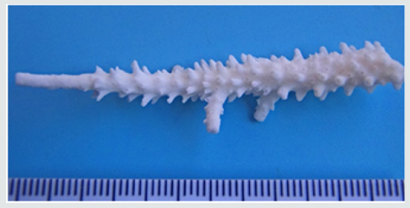
Figure 7: Bleached Acropora nasuta (Angaga reef-flat, Ari Atoll, May 2016). The branch tips reveal the characteristic “slendering” just below the apex along with the accumulation of photo-protective FPs.

Figure 8: Bleached Acropora humilis (Angaga reef-flat, Ari Atoll, May 2016). The apical branches reveal the characteristic “slendering”.

Similar growth abnormalities have also been noticed on Angaga reef (May 2016) among other acropoids with digitate growth forms that have been subjected to bleaching, i.e. A. austera, A. digitifera, A. divaricata, A. gemmifera, A. globiceps, A. humilis, A. nasuta, A. tenuis and A. robusta. The slender morphology of the branching tips of these colonies was limited up to 2 cm below the apex (Figures 7 & 8). Similar symptoms of abiotic stress have also been observed among species of A. muricata. With regard to the phenotypic differences due to various growth forms (digitate versus columnar, as seen in Isopora palifera, (Figure 9) such anomalies vary only slightly.
Figure 9: Noticeable changes in a large Isopora palifera (deeper lagoon of Gangehi reef, Ari Atoll, May 2015). The apical growth region is markedly reduced in thickness, bleached sections can be spotted.

Another striking observation was made during sampling. Affected colonies revealed a significantly reduced tolerance to mechanical stress - apart from the reduced thickness - rendering them extremely fragile. This was especially evident during sampling of A. abrolhosensis, where apparently branches with normallooking morphology showed no resistance to even the slightest mechanical stress; i.e. both normally pigmented sections as well as bluish coloured branches with a thickness of 15-20 mm could easily be broken off the colony. This observation can also be noted when referring to Figure 1 & 5a – the former colony shows the apical tips still in place, whereas in the latter these tips are almost entirely missing. A visible inspection of the fractured cross-sectional area confirmed the compromised mechanical statics via deep-reaching skeletal defects.
General Status of Maldivian Reefs: According to personal accounts provided by hydroplane pilots of the Maldivian airline (personal communication, May 2016), they witnessed bright and bleached hotspot on their flights over all atolls, as far south as to the Gan atoll (i.e. few degrees south of the equator, thus covering a north-south extension of some 900 km). An indication that almost the entire Maldives are affected by this massive die off.
Discussion
Although, both the eye-catching bluish pigmentation as well as the growth anomalies in A. abrolhosensis are most likely attributable to repetitive exposure to elevated SST, the bluish hue of their tips however seem to be associated to excess UV-exposure. This is in line with previous findings that identified UV-absorbing amino acids in reef building corals [7]. Other experts attribute the phenomenon of colour morphs likewise to the elevated concentration of photoprotective FPs [8]. Lacking the natural pigmentation (lower concentration of endosymbionts per unit surface area) a broader spectrum of light penetrates all the way to the underlying skeletal matrix. Less absorption also implies that more light is reflected back into the coenosarc by the skeleton itself, which further boosts production of photo-protective FPs [9].
Corals produce large amounts of FPs – they are ubiquitous in shallow reef-building corals and can make up a significant portion of the total soluble protein in a coral [10]. FPs absorb highenergy photons and re-emit them at lower wavelengths [8]. This concords with studies that investigated the increase in resistance to bleaching in the euphotic zone during periods of heat stress, which is attributed to FPs thus acting photo-protectively [11]. FPs are regularly found in regions of abnormal growth [9]. Indeed, the closeup in Figure 3 shows the slender apical region of A. abrolhosensis with that atypical bluish hue. The pigments responsible for this blue colour have been identified in the past as a pocilloporinlike compounds - also in acroporid species - with an extinction coefficient in the UV and in the visible region at 560 to 590nm [12]. These pigments were found not only to act as a photo-protectant or as accessory photosynthetic pigments but serve moreover in the coral’s immunological and chemical defence systems [13]. Apart from innate immune response and camouflage purposes, additional hypotheses regarding FPs include an antioxidant capacity and the regulation of dinoflagellate population density [10]. However, the precise ecological function of FPs in corals still remains a mystery, nonetheless the extraordinary modulating effect on their expression rate in response to environmental factors such as light and heat are already well documented [14-16]. Observations made in the recent past assign corals -at least to a certain extent – the ability to adapt to such periods of stress. Although such kind of hardening has been observed among corals thriving in variable environments subjected to a wide range of temperature fluctuations [17], given the current situation across the Maldives this seem not to be the case for corals under prolonged stress exposure.
The large mono-specific aggregations of A. abrolhosensis within the extensive lagoon of Angaga reef, protected against excessive mechanical disturbance (wave action), implied a reduced exchange of water masses with the outer area of the lagoon, thus the average lagoon temperature generally tended to be somewhat higher than the surrounding open ocean surface water. Furthermore, the shallow depth and coral sand constituting the albedo of the lagoon sediment pose additional light-stress as more of it is reflected back towards the corals. Additionally, the slender elongated tips with an already low concentration of endosymbiotic algae, must likewise lead to an increase in the production of protective FPs as light flux through the coenosarc is tendentially higher as more light is reflected by the underlying aragonite skeleton.
Initially, A. abrolhosensis seemed to have coped quite well with excessive light and did so by shifting its proteome towards an increased FP-density [8] with a concomitant lower concentration of endosymbionts per unit area of coenosarc. Probably it is this higher photon flux density that enabled this species to maintain its autotrophic requirements even though its endosymbiont density was obviously suppressed. Hence, the observed phenotypic abnormalities most likely are also attributed to these influences. However, higher metabolic activity of endosymbionts can be upregulated only to a certain extent. It was even reported that the photosynthetic rate in corals among symbiotic dinoflagellates in culture can withstand exposure to heat-stress for several hours up to temperatures of 31 °C [18]. Once this threshold is exceeded, photosynthetic yield drastically declines and in some cases ceases altogether.
Figure 10a-d: Bleached acroporid species revealing photo-protective FPs accumulation (Angaga reef, May 2016).

In general, excessive production of photo-protective FPs, as well as multiple morphological modifications among many acroporid species underline the collective efforts to cope with adverse environmental conditions, observations that has been made on several occasions when abiotic conditions where unfavourable (Figure 10a-d). Yet prolonged and most importantly, in combination with repetitive sub-lethal exposure (just below the bleaching threshold) eventually does induce abitotic TBL [3,19], which ultimately leads to the widespread collapse of Maldivian reefs [4].
Referring to archive material made during May 2014 from Madoogali reef (Ari Atoll), it was possible to identify similar branching and tip anomalies on A muricata (Figure 6a & 6b), yet these peculiar morphological manifestations were less pronounced than in A. abrolhosensis. Back then, the authors assumed that the production of photo-protective FPs occurred only to a minor extent. While partly bleached areas with growth abnormalities were far less striking than in the case of A. abrolhosensis, such mild adaptations that not necessarily attract attention if not looking intentionally for them, have been already observed over past years. This suggests that such peculiar growth forms are not at all unique phenomena assigned to selected species, but are rather a common occurrence on Maldivian atolls.
Numerous observations made during past visits already put the focus on the branching tips as these where found to be more prone to sub-lethal TBL. The stress-related morphic changes observed on other digitate acroporid colonies (Figures 7 & 8) across Angaga reef (May 2016) were similar to those seen among A. abrolhosensis and A. muricata. There are differences however, in that the latter revealed no reduction in size of the radial corallites compared to A. abrolhosensis. This observation is in agreement with [18] in so far as the accelerated growth dynamics of the tips along with an increased photosynthetic rate was found to coincide with elevated but sub-bleaching temperature stress. In such situations both radial growth dynamics (thickness of branches) among A. abrolhosensis and A. muricata along with the formation of radial corallites are clearly reduced. Other digitate acroporid species seem to do only slightly better, in that their radial corallites are at least rudimentarily expressed. Even among the relatively massive, columnar growing Isopora palifera (Figure 9) was it possible to detect similar growth anomalies among the apical region [4].
Continuos sub-lethal events of SSTs over the last years unavoidably boosted metabolic activity; a phenomenon that can be also accounted for in acroporids accelerated growth rates. Particularly the apical branches of A. abrolhosensis revealed a greatly reduced aragonite skeletal density. As mentioned above, the fragility of these colonies was striking, rendering them subject to excessive wave activity or mechanical stress exerted by vertebrates. Indeed, upon closer inspection, deep-reaching skeletal defects have been noted indicating that the precipitation dynamics and it’s subsequent crystallisation kinetics of the carbonate matrix seem to have been disturbed as the boosted deposition rate of “fresh” argonite matrix on previous layers have not yet properly crystallised, thereby severely compromising their mechanical resistivity.
Additional Abiotic Factors: During the severe bleaching event of 1997/98, weather conditions over the Maldives were characterized by a persisting high-pressure system over the northwestern region of the Indo-Pacific with top temperature episodes prevailing during April / May 1997 and again at the beginning of 1998. Production of Di-Methyl-Sulfide (DMS) on behalf of coral species may have been abundant but subsequent formation of cloud condensation nuclei [20] that would have led to increased cloud formation most likely was blocked for two reasons: (1) due to persisting high pressure conditions that usually come along with an already retarded aerosol condensation dynamics [21,22,24] and (2) as a result of the aerosol-laden brown haze originating from the Indian sub-continent [25], especially during the months of February and March, when these aerosols shift the balance from a cloud-forming dynamics to one where haze dominates [23]. Topped with stagnant wind conditions – with no mixing of the uppermost water layers with deeper cooler water – provided the basis for the caloric accumulating of solar radiation over most of the day. Back then, recorded SST over 6-8 weeks soared to a staggering 32 - 33 °C.
Additional Intrinsic Properties of Coral Host and Symbiont: The El-Nino induced events and the resulting bleaching episodes during 2015 and 2016 however were not attributable to such stagnant weather conditions (contrary to the 1998 heat- and lightstress induced TBL event above max. tolerance levels of corals). At the beginning of 2016 the Maldives experienced high-temperature episodes that came along with low-pressure systems, windy conditions and a substantial amount of rain and turbid mixing of the upper water layers. Thus, bleaching threshold temperature levels have hardly been exceeded; nonetheless was TBL observed over many areas among most atolls. An explanation can be found in the coping strategies employed by corals repeatedly exposed to sub-lethal abiotic conditions as highlighted above. It is well known that stressed organisms undergo enormous changes both in terms of their immuno-chemical as well as epigenomic responses. Sublethal stress capable to induce TBL as observed over the periods May 2015 till May 2016 with slightly higher than average values in SST seem to have induced such epigenetic effects that prompted these coral species to express a more pronounced and thus a chronic stress phenotype with a substantial reduction in overall resilience [26]. As a result, protracted bleaching took place further trimming the already compromised regulatory adaptation possibilities of acropoids, which resulted in a state of shock and ultimately in massive die off.
While short-term stress episodes do have rather limited effects on the epigenome, chronic stress is known to induce changes in the epigenome. Particularly, genes associated with extreme temperatures and ocean acidification among Acropora species tend to lack methylation, enabling these corals under normal conditions to rapidly respond when environmental conditions change [27]. Likewise do clades A and B of Symbiodinium reveal unexpectedly high numbers of Trx-domain encoded genes that play key roles in the oxidative stress response that have been shown to be differentially expressed in response to high temperature, salinity, and ultraviolet exposure in corals [28]. Yet with transcritptional plasticity of both host and symbiont maintained at such a high level, the question remains how can a system further adapt if genes associated to stress-related coping are already running at high gear? In fact, one gets the impression that Maldivian corals have already reached the upper regulatory limits - a behavior usually attributed to a group of moderately variable (MV) corals that are less fit to cope with chronically altered abiotic conditions. It was even shown that the ratio of up- to down-regulated genes in response to thermal stress make a difference as the transcriptome of thermally sensitive MV-group corals are indeed biased towards the maximal range of response [29]. This is of particular relevance as Symbiodinium clade C - which is more sensitive to thermal stress than clade D - is the predominant endosymbiont among Maldivian corals [30].
SDR-Like Symptoms, a Peculiar Coral Pathology: One particularly striking feature can be seen in Figure 5b and regards resemblances to a specific coral disease termed SDR, shut down reaction, [31,32] also known as stress-related necrosis among species kept in captivity [34]. SDR is known to occur frequently in acroporids when injured or abraded, where apoptosis seems to be the underlying phenomenon [35]. In this particular case as depicted in Fig. 5b, it is very likely that the triggering stress was predation by corallivorous fish (personal observation). Since SDR is taking place at an extremely rapid rate - approx. at 10cm per hour along a moving front – it could in theory also be observed in this case. Unfortunately, back then such a record-taking effort was not made. Since SDR is usually associated with colonies under stress [36] we are very inclined to attribute the documented manifestation to this particular pathology. However, and to our knowledge no detailed further investigations regarding the true nature of SDR has been carried out to date. Thus we can only speculate about this phenotypic coincidence during repetitive stress-events.
Outlook
In the aftermath of the massive 1998 bleaching event till 2010, corals across the Maldives have been able to partly regenerate to previous status [3,4,33,37]. However, and as a result of repetitive events of abiotically induced TBL over the last couple of years, Maldivian corals being already tuned to a very stenobiotic window of survivability, suffered a severe blow during 2015/2016, which culminated in a dramatically low live coral coverage. Even species such as Porites rus, P. cylindrica, Pavona varians and species of the genus Favites that usually proved to be more stress tolerant during the massive bleaching event of 1998 [19] were now affected. In essence, it was observed that no scleractinian coral species within the Ari Atoll showed signs of long-lasting coping strategies that would suggest some kind of adaptation to increasing SSTs. A census carried out by the end of May 2016 on Angaga reef revealed a rate of 1-2 % and was constituted mainly by massive Porites species, while the rather rare recruits spotted along the reefs were often found to be bleached. As a result, we expect for the decade to come that Maldivian reefs are currently in a bistable state and with the very likely trend that a shift from a coral dominated biocoenosis into an algal one will occur in the near future [38]. Once this chain of events attains an irreversible dynamic in which corals no longer are the dominant species, it will be very difficult for many atolls to withstand bioerosive forces. Indeed, there is no doubt that the disastrous collapse of remnant live coral stands on Maldivian atolls that are already affected by both sea-level rise and an ever increasingly unstable reef-statics opens the final chapter of the widespread collapse of Maldivian atolls (Figure 11 & 12). Yet, with fewer reef builders present or eventually even absent, Maldivian atolls face the fate of so-called give-up reefs. A truly bitter trend for those who currently populate these atolls.
References
- Lesser MP (2004) Experimental biology of coral reef ecosystems. J Exp Mar Biol Ecol 300(1-2): 217-252.
- McClanahan T (2002) Coral Bleaching, Diseases and Mortality in the Western Indian Ocean. Coral Health and Disease pp. 157-176.
- Loch W, Loch K (2015) Korallenbedeckung und Artendiversitaet. Hessisches Landesmuseum Darmstadt. Kaupia 20: 57-92.
- Loch W, Loch K (2015) Korallenbleiche auf den Malediven. Hessisches Landesmuseum Darmstadt. Kaupia 20: 47-56.
- NOAA (2017) Coral Reef Watch, Satellite and Information Service.
- Veron JEN (2000) Corals of the World. Vol 3. Australian Institute of Marine Science, Townsville, p 426.
- Dunlap WC, Shick JM (1998) Ultraviolet Radiation-absorbing mycosporine-like Amino Acids in Coral Reef Organisms: A Biochemical and Environmental Perspective J Phycol 34(3): 418-430.
- Gittins JR, D’Angelo C, Oswald F, Edwards RJ, Wiedenmann J (2015)
- Gittins JR, D’Angelo C, Oswald F, Edwards RJ, Wiedenmann J (2015) Fluorescent protein-mediated colour polymorphism in reef corals: Multicopy genes extend the adaptation/ acclimatization potential to variable light environments. Mol Ecol 24(2): 453-465.
- D’Angelo C, Smith EG, Oswald F, Burt J, Tchernov D, et al. (2012) Locally accelerated growth is part of the innate immune response and repair mechanisms in reef-building corals as detected by green fluorescent protein (GFP)-like pigments. Coral Reefs 31(4): 1045-1056.
- Roth MS. Deheyn DD (2013) Effects of cold stress and heat stress on coral fluorescence in reef-building corals. Sci Rep 3:1421.
- Salih A, Larkum A, Cox G, Kuehl M, Hoegh-Guldberg O (2000) Fluorescent pigments in corals are photoprotective; Nature 408(6814): 850-853.
- Shibata K (1969) Pigments and UV-absorbing substance in corals and a blue-green alga living in the Great Barrier Reef. Pl Cell Physiol 10(2): 325-335.
- Dove SG, Takabayashi M, Hoegh-Guldberg O (1995) Isolation and Partial Characterization of the Pink and Blue Pigments of Pocilloporid and Acroporid Corals. Biol Bull 189(3): 288-297.
- Bay LK, Ultrup KE, Nielsen HB, Jarmer H, Goffard N, et al. (2009) Microarray analysis reveals transcriptional plasticity in the reef building coral Acropora millepora. Mol Ecol 18(14): 3062-3075.
- Rodriguez Lanetty M, Harii S, Hoegh Guldberg O (2009) Early molecular responses of coral larvae to hyperthermal stress. Mol Ecol 18(24): 5101- 5114.
- Desalvo MK, Voolstra CR, Sunagawa S, Schwarz JA, Stillman JH, et al. (2008) Differential gene expression during thermal stress and bleaching in the Caribbean coral Montastraea faveolata. Mol Ecol 17(17): 3952- 3971.
- Carilli J, Donner SD, Hartmann AC (2012) Historical Temperature Variability Affects Coral Response to Heat Stress. PLoS ONE 7(3): 34418.
- Roth MS, Goericke R, Deheyn DD (2012) Cold induces acute stress but heat is ultimately more deleterious for the reef-building coral Acropora yongei. Sci Rep 2: 240.
- Loch K, Loch W, Schuhmacher H, See WR (2002) Coral Recruitment and Regeneration on a Maldivian Reef 21 Months after the Coral Bleaching Event of 1998. Mar Ecol 23(3): 219-236.
- Jones GB, Curran M, Broadbent A, King S, Fischer E, et al. (2007) Factors affecting the cycling of dimethylsulfide and dimethylsulfoniopropionate in coral reef waters of the Great Barrier Reef. Environ Chem 4(5): 310- 322.
- Jones GB, Trevena AJ (2005) The influence of coral reefs on atmospheric dimethylsulphide over the Great Barrier Reef, Coral Sea, Gulf of Papua and Solomon and Bismarck Seas. Mar Fresh Res 56(1): 85-93
- Jones G (2013) Marine biology: Coral animals combat stress with sulphur. Nature 502(7473): 634-645.
- Satheesh SK, Ramanathan V (2000) Large differences in tropical aerosol forcing at the top of the atmosphere and Earth‘s surface. Nature 405(6782): 60-63.
- Deschaseaux E, Jones G, Miljevic B, Ristovski Z, Swan H, et al. (2012) Can corals form aerosol particles through volatile sulphur compounds?. Proceedings of the 12th Intl. Coral Reef Symposium, Cairns.
- Ramanathan B, Crutzen PJ, Kiehl JT, Rosenfeld D (2001). Aerosols, Climate, and the Hydrological Cycle. Science 294(5549): 2119-2124.
- Larcher W (1995) Physiological Plant Ecology. (3rd edn).; Springer.
- Diamond JL, Roberts SB (2015) Germline DNA methylation in reef corals: patterns and potential roles in response to environmental change. Mol Ecol 25(8): 1895-1904.
- Bayer T, Aranda M., Sunagawa S, Yum LK, DeSalvo MK, et al. (2012) Symbiodinium Transcriptomes: Genome Insights into the Dinoflagellate Symbionts of Reef-Building Corals. PLOS ONE 7(4): 35269.
- Barshis DJ, Ladner JT, Oliver TA, Seneca FO, Traylor-Knowles N, et al. (2013) Genomic basis for coral resilience to climate change. PNAS 110(4): 1387-1392.
- Baker A (2004) Symbiont Diversity on Coral Reefs and Its Relationship to Bleaching Resistance and Resilience. pp. 177-194.
- Antonius A (1977) Coral mortality in reefs: A problem for science and management. Miami 2: 618-623.
- Sutherland KP, Porter JW, Torres C (2004) Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar Ecol Prog Ser 266: 273-302.
- Mc Clanahan TR, Muthinga NA (2014) Community change and evidence for variavle warm-water temperature adaptation of corals in Northern Male Atoll, Maldives. Mar Pollut Bull 80(1-2): 107-113.
- Bornemann E, Wellington G (2006) Apoptosis in Diseased Reef Corals. Coral Reef Society and Itnl. Soc. for Reef Studies, Okinawa 1: 142-148.
- Bruckner WA (2016) White Syndromes of Western Atlantic Reef-Building Corals. Wiley & Sons, Hoboken 22: 316-332.
- Antonius A (1981) Coral reef pathology: A review. Coral Reef Symp, Manila 2: 3-6.
- Lasagna R, Albertelli G, Giovannetti E, Grondona M, Dilani A, et al. (2008) Status of Maldivian reefs eight years after the 1998 coral mass mortality. Chem Ecol 24(1): 67-72.
- Rossi C, Madl P, Foletti A, Mocenni C (2015) Energy flow and dissipative structures. Signpost Research, Kerala 4: 71-94.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




