Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6652
Research Article (ISSN: 2637-6652) 
Surfactant Mediated Biodegradation of Aromatic Hydrocarbons Using Rhamnolipid Producing Bacterial Isolate Volume 2 - Issue 2
Saroj Yadav, Sadath Mohammed, Sagar Grover, Malla Sudhakar and Sibi G*
- Department of Biotechnology, Indian Academy Degree College-Autonmous, Bangalore, India
Received: October 08, 2018; Published: October 15, 2018
Corresponding author: Sibi G, Head of the Department, Department of Biotechnology, Indian Academy Degree College- Autonomous, Bangalore, India
DOI: 10.32474/MAOPS.2018.02.000135
Abstract
Aromatic hydrocarbons (toluene and xylene) are highly water soluble and their contamination with groundwater is a serious issue. Hydrocarbons are growth substrates for microorganisms which use them as a sole source of carbon and energy. Surfactants increase the solubility and dissolution of hydrocarbons thus making them susceptible to degradation. In this study, biodegradation of toluene and xylene using a soil bacterium was evaluated in the presence of chemical surfactant. The colonies that were tested positive for xylene and toluene degradation in the primary screening were analyzed by 16s rRNA sequencing and identified as Bacillus Cereus. The presence of chemical surfactant enhanced the growth of bacterial isolate and its bioremediation activity indicated the possible role of the tween in bioremediation process. It was observed that pH of the medium has reached near neutral from initial pH of 8.2 at the end of 21 days cultivation period. Addition of hydrocarbons in the mineral salt medium (MSM) has enhanced the bacterial growth as confirmed by increasing optical density throughout the cultivation period. Increase in protein content of bacterial isolate indicated its metabolic activity in the presence of hydrocarbons. Similarly, increase in the rhamnolipid concentration of B. cereus grown in MSM along with hydrocarbons and chemical surfactant indicated the role of rhamnolipids during the biodegradation of xylene and toluene.
Keywords: Aromatic Hydrocarbons; Rhamnolipid; Surfactant; Xylene; Toluene; Bacillus Cereus; Bioremediation
Introduction
Aromatic hydrocarbons are molecules containing one or more aromatic rings in their structure and are the most prevalent pollutants in the environment. These compounds are widely used as fuels and industrial solvents and they enter the environment through oil spillage, fossil fuel combustion and natural petroleum seepage. Bioaccumulation of aromatic hydrocarbons in food chains makes them as potential human health hazards. Biodegradation is the removal and transformation of contaminants using living organisms into harmless substances. Microorganisms can degrade environmental pollutants by transforming them into harmless metabolites [1]. It is noteworthy to mention that aromatic compounds are growth substrates for microorganisms which use them as a sole source of carbon and energy. The breakdown of aromatic hydrocarbons by ring cleavage through microbial activity is an important step in carbon cycle, [2] and the degradation involving microorganisms may be aerobic or anaerobic process. Aerobic degradation involves oxidation of alkyl side chain of the aromatic ring to produce carboxylic acids [3], whereas anaerobic process involves enzymatic degradation [4]. Toluene is an aromatic hydrocarbon and causes various health hazards [5-7]. Xylene is mainly used as solvent in industries and medical technologies. Xylene can enter into soil and water bodies where it remains for months before transforming into other chemicals. Inhalation of xylene vapor causes depression of the nervous system [8]. Both toluene and xylene are highly water soluble and their contamination with groundwater is a serious issue. Surfactants increase the solubility and dissolution of hydrocarbons thus making them susceptible to degradation. Majority of the surfactants are derived from petroleum products which further causes environmental problems. Biosurfactants produced by microorganisms are alternate to synthetic surfactants as they are not only eco-friendly but also functions well at extreme conditions. Rhamnolipids are a class of biosurfactants consisting of one or two rhamnose molecules with a glycosidic linkage [9,10]. When hydrocarbons are used as carbon source, some microorganisms produce rhamnolipids to utilize them [11,12]. In this study, biodegradation of hydrocarbons (toluene and xylene) using a soil bacterium was evaluated in the presence of chemical surfactant. Rhamnolipid synthesis by the bacteria during the degradation process was also evaluated.
Materials and Methods
Isolation of Aromatic Hydrocarbon Degrading Bacteria
The soil which was contaminated with crude oil and petroleum hydrocarbons for over 6 months was selected as the source to isolate the aromatic hydrocarbon degrading bacteria. About 0.1gm of soil sample was suspended in 0.9ml of distilled water and subjected to serial dilution. The tubes 10-9 and 10-10 were then plated onto the mineral salt medium (MSM). The plates were sprayed with toluene (10%) and xylene (10%) and incubated at 25°C for 5-10 days. The organisms that formed clear zones around the colonies were considered as hydrocarbon degraders. The isolates were further screened by overlay technique [13]. 0.1ml of sample aliquots was added to the plates and the inoculum was spread over the agar surface. The plates were then incubated at 37°C for 48 hours and the grown predominant colonies were selected for further studies.
Identification of Aromatic Hydrocarbon Degrading Strains
The isolates obtained were then screened for bioremediation of aromatic hydrocarbons (xylene and toluene) using mineral salt medium (MSM) (1.8g K2HPO4, 4.0 g NH4CI, 0.2g MgSO4.7H2O, 0.1g NaCl, 0.01g FeSO4.7H2O in 1 L distilled water) in the presence and absence of surfactant (Tween 40). About 10 ml of the inoculum was added to 250 ml conical flask containing 100 ml of mineral salt liquid medium.
Flask 1 : MSM + Inoculum (control)
Flask 2 : MSM + Inoculum + AH
Flask 3 : MSM + Inoculum + AH + Tween
Flask 4 : MSM + Inoculum + Tween
Flask 5 : MSM + AH (negative control)
The experiments were done in triplicates and the flasks were incubated at 37°C for 21 days. The samples were analyzed at regular intervals (7th day, 14th day and 21st day) for pH of the medium, growth curve, total proteins and rhamnolipid production.
Total Protein Estimation
Total protein of the cultures was estimated by following Lowry’s method. Briefly, 1ml of the culture was taken from each sample and centrifuged at 10,000rpm for 20 min. The pellet obtained was washed with Ringer’s solution and the pellets were resuspended in 4.6 M NaOH to boiling temperature for 10 mins. Total protein estimation was estimated by Lowry’s method using BSA as standard.
Rhamnolipid Extraction and Quantification
Rhamnolipid from the bacterial culture was extracted by centrifuging the culture (10,000 g) and extracting the supernatant with 1 ml of chloroform and ethanol (2:1 v/v). The organic phase was evaporated to dryness and the precipitated was dissolved in 0.5 ml of water. The quantification of rhamnolipids was done by adding 0.9 mL of 0.19% orcinol (in 53% H2SO4) to 0.1 ml of sample. The solution mixture was heated for 30 min at 80°C and the samples were cooled at room temperature. The absorbance was measured at 421 nm in a UV-Vis spectrophotometer and the rhamnolipid concentration was calculated from standard curves prepared with L-rhamnose (0-50 mg/L) and expressed as rhamnose equivalent [14].
16s rRNA Sequencing
The colonies isolated and tested positive for the hydrocarbon degrading assay were purified for identification. DNA from the isolates was extracted from the colonies and 16s rRNA sequencing was carried out using universal primers 27F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1392R (5’-GGTTACCTTGTTACGACTT-3’) (Eurofins, Bangalore).
Results
The predominant colonies appeared during initial screening was labeled as X2 (xylene degrader) and T1 (toluene degrader) and subjected to hydrocarbon degradation assay. The assay was carried out in four different flasks having various combinations as mentioned earlier. Both the medium and inoculum were withdrawn at 7th, 14th and 21st day of incubation and analyzed for degradation assays.
pH of the Medium
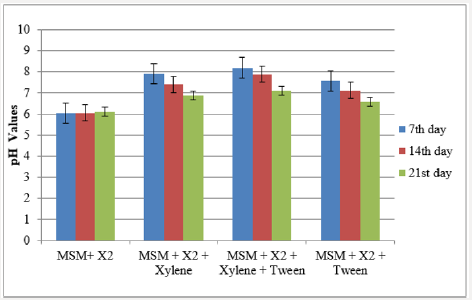
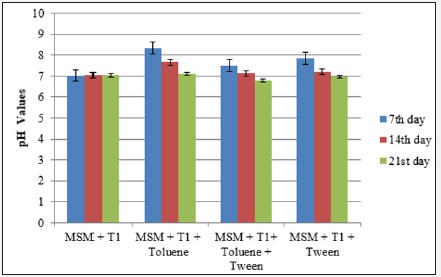
Medium from the inoculated flasks was collected at regular intervals to determine the pH as a function of bioremediation process. In all the flasks, pH was reduced with increased incubation period and the pH of the flask that has mineral salt medium (MSM)+inoculum+xylene+tween had a pH of 8.2, 7.89 and 7.12 at 7th, 14th and 21st days of incubation respectively. In the case of toluene degradation, flask with MSM+inoculum+toluene had a pH of 8.35 at 7th day and was reduced to a pH of 7.11 at the end of incubation period. pH of the other flasks during the cultivation period is represented in Figure 1a & 1b
Bacterial Growth Curve
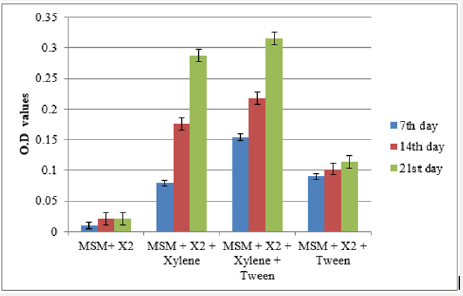
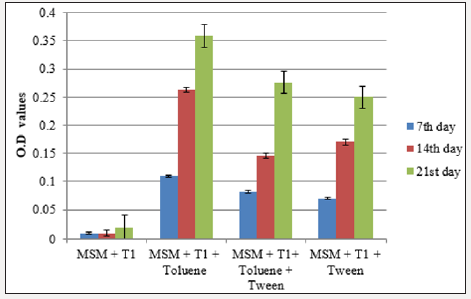
Bacterial growth in the inoculated flasks was determined at regular intervals through colorimetric estimation using the flask containing mineral salt medium and inoculum as blank at 620 nm. The optical density values were increased with incubation period indicating the growth of bacteria in the medium. Highest OD value was observed in flask containing MSM+inoculum+xylene+tween which was followed by MSM+inoculum+xylene. For toluene degradation, flask containing MSM+inoculum+toluene recorded the highest OD value (Figure 2a & 2b).
Total Protein Estimation
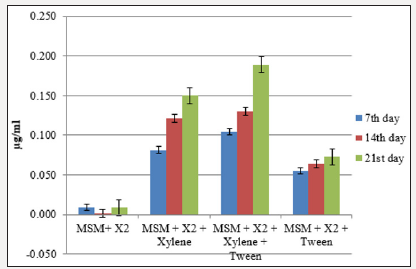
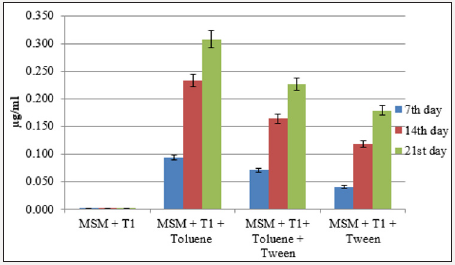
The total protein content of aromatic hydrocarbon degrading bacteria grown under various combinations was calculated using BSA as standard (R2=0.993). Total protein concentration of strain X2 from the flask containing MSM+X2+xylene+tween was in the range of 0.105-0.189 μg ml-1. In the flask containing MSM+T1+toluene, highest protein content was recorded (0.307 μg ml-1) at the end of cultvation period (Figure 3a & 3b).
Rhamnolipids Synthesis
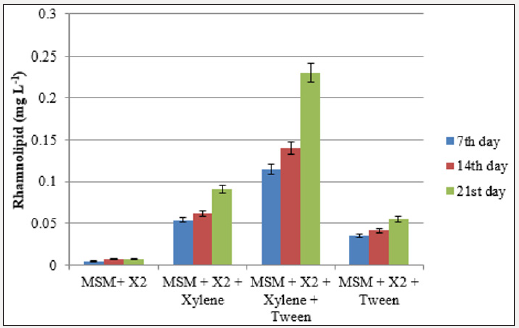
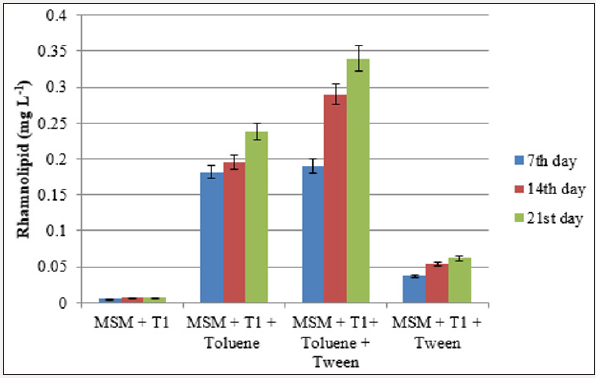
Synthesis of rhamnolipids by the bacterial isolate grown in the presence of aromatic hydrocarbon was quantified by colorimetric method. The presence of synthetic surfactant (tween 40) in the growth medium had increased the production of rhamnolipids in both xylene and toluene containing media. The concentration of rhamnolipid in xylene degrading bacteria was in the range of 0.14- 0.23mg l-1 and the production was three-fold increased with the addition of tween (Figure 4a & 4b). However, there was a 42.8% increase in the rhamnolipid synthesis by the toluene degrading bacteria at the end of 21 days incubation period in the flask containing MSM+inoculum+toluene+tween (0.34mg l-1) than flask without tween (0.238mg l-1).
16s rRNA Sequencing
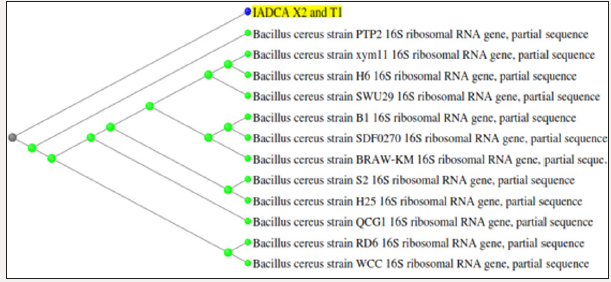
The colonies that were tested positive for xylene and toluene degradation were identified by 16s rRNA sequencing. The sequence obtained was then checked for sequence similarity among the organisms for identity. The sequence was then compared using Blastn and the query was found to have 99% identity with Bacillus cereus (Figure 5a & 5b).
Figure 5b: Neighbor-joining method cladogram showing phylogenetic relationship between strains (strains X1 and T2) and other related reference microorganisms based on the 16S rRNA gene sequence analysis.
Discussion
Soils contaminated with aromatic hydrocarbons are a major threat to the soil ecosphere. The low bioavailability of polycyclic hydrocarbons to microorganisms makes them difficult to undergo bioremediation [15]. There are several microbes which are found useful in biodegrading such contaminants. Bacterial consortium involving Bacillus subtilis and Pseudomonas aeruginosa was reported to degrade aromatic hydrocarbons effectively [16]. In this study, bacterial isolates from the soil samples were screened for the bioremediation activity, and only two isolates were found to degrade the tested aromatic hydrocarbons. On the basis of morphological features and 16S rRNA analysis both strains were assigned to Bacillus Cereus. In order to find the efficacy of degradation, flasks with different combinations of hydrocarbons were used. Tween 40 was used as surfactant to solubilize the hydrocarbons and four different flasks were used separately for both xylene and toluene degradation assay. The presence of chemical surfactant enhanced the growth of bacteria and its bioremediation activity indicating the possible role of the tween in bioremediation process. For bioremediation, the optimal pH is between 6.5 and 8.5. Most of the microbial processes are associated with the addition of electron donors and as such tends to decrease the pH. The pH of all the experimental samples were maintained at about pH 6.04- pH 8.2, which was the optimum for bioremediation. The pH of all samples was found to be gradually decreased from 7th day to 21st day. The reduction in pH levels in the experiment in agreement with [17] who indicated the degradation of hydrocarbons is greater in neutral medium. On the contrary, [18,19] found that basic pH is suitable for bacterial degradation of hydrocarbons. The effect of aromatic hydrocarbons on the growth of bacterial isolate was studied in MSM containing toluene and xylene with or without surfactant. The results showed that addition of hydrocarbons could enhance the bacterial growth as confirmed by increasing optical density values as presented in Figure 2a and 2b. The bacterial growth curve was increased in the medium supplemented with both xylene and toluene which proved bioremediation of the added organic hydrocarbons. It also showed an enhanced activity in the presence of tween, which might say of the possible role of the tween as an enhancer of the bioremediation.
Increase in protein content of the bacterial isolate in this study during the cultivation period indicated its metabolic activity in the presence of hydrocarbons. This is in agreement with the findings of Das and Mukherjee [20] and Madri and Lin [21] who had reported that protein content was increased with incubation time. Similar result was also reported by Sepahi [22] where the total protein estimation showed maximal increase during bioremediation. Surfactants increase the solubility and bioavailability of hydrocarbons thereby increasing their biodegradation [23,24]. Biosurfactants are produced by a variety of microorganisms and rhamnolipids are the most studied for bioremediation of organic pollutants [25- 30], reported the rhamnolipids enhanced biodegradation of oil by bacterial consortium. In this study, increase in the rhamnolipid concentration of B. cereus is indicating the role of rhamnolipids in the biodegradation of xylene and toluene. Bacillus species are more tolerant to high levels of hydrocarbons and hence could be effective in bioremediation of oil spills [31]. The identification of soil isolate as Bacillus Cereus in this study is evident that the organism could be effective in clearing hydrocarbon contamination. However, no tests were performed during this study to identify if the bacterial isolate was a true hydrocarbon degrader by determining the aromatic hydrocarbon levels and their metabolic products at the end of cultivation period [32]. The addition of aromatic hydrocarbons into the mineral salt medium induced the bacterial growth and rhamnolipid synthesis in Bacillus Cereus. The inclusion of chemical surfactant has increased bioavailability of xylene and toluene to the bacterial isolate which was evidenced by the higher levels of growth, protein content and rhamnolipid synthesis in B. cereus.
References
- Martin Alexander (1999) Biodegradation and Bioremediation (2nd edn). Academic Press, San Diego, USA, pp. 325-327.
- Evans WC (1963) The microbiological degradation of aromatic compounds. J Gen Microbiol 32: 177-184.
- Jindrova E, M Chocova, K Demnerova, V Brenner (2002) Bacterial aerobic degradation of benzene, toluene, ethylbenzene and xylene. Folia Microbiol (Praha) 47(2): 83-93.
- Foght J (2008) Anaerobic biodegradation of aromatic hydrocarbons: Pathways and Prospects. J Mol Microbiol Biotechnol 15(2): 93-120.
- Allen J, M Coombs (1980) Covalent binding of polycyclic aromatic compounds to mitochondrial and nuclear DNA. Nature 287(5776): 244- 245.
- Sikkema J, J de Bont, B Poolman (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59(2): 201-222.
- Brigmon RL, D Camper, F Stutzenberger (2002) Bioremediation of compounds hazardous to health and the environment-An Overview. In: Biotransformation: Bioremediation Technology for Health and Environment Protection, Singh VP, RD Stapleton (Eds.). Elsevier Science Publishers, Netherlands, 36: 1-28.
- Agency for Toxic Substance and Disease Registry (ATSDR) (1993) Toxicological profile for xylene. USA.
- Muller MM, JH Kugler, M Henkel, M Gerlitzki, B Hormann, et al. (2012) Rhamnolipids-next generation surfactants? J Biotechnol 162(4): 366- 380.
- Lawniczak L, R Marecik, L Chrzanowski (2013) Contributions of biosurfactants to natural or induced bioremediation. Appl Microbiol Biotechnol 97(6): 2327-2339.
- Chakrabarty AM (1985) Genetically-manipulated microorganisms and their products in the oil service industries. Trends Biotechnol 3(2): 32- 38.
- Toribio J, AE Escalante, G Soberon Chavez (2010) Rhamnolipids: Production in bacteria other than Pseudomonas aeruginosa. Eur J Lipid Sci Technol 112(10): 1082-1087.
- Bogardt AH, BB Hemmingson (1992) Enumeration of phenanthrene degrading bacteria by an overlay technique and its use in evaluation of petroleum contaminated sites. Appl Environ Microbiol 58(8): 2575- 2582.
- Chandrasekaran EV, JN Bemiller (1980) Constituent analyses of glycosaminoglycans. In: Methods in carbohydrate chemistry. Whistler Roy L, Wolfrom ML (Eds.) pp. 89-96.
- Zhong H, Z Wang, Z Liu, Y Liu, M Yu, et al. (2016) Degradation of hexadecane by Pseudomonas aeruginosa with the mediation of surfactants: Elation between hexadecane solubilization and bioavailability. Int Biodeterior Biodegrad 115: 141-145.
- Mukherjee AK, NK Bordoloi (2012) Biodegradation of benzene, toluene, and xylene (BTX) in liquid culture and in soil by Bacillus subtilis and Pseudomonas aeruginosa strains and a formulated bacterial consortium. Environ Sci Pollut Res 19(8): 3380-3388.
- Leahy JG, RR Colwell (1990) Microbial degradation of hydrocarbons in the environment. Microbiol Rev 54(3): 305-315.
- Dibble JT, R Bartha (1979) Effect of environmental parameters on the biodegradation of oil sludge. Appl Environ Microbiol 37(4): 729-739.
- Hambrick GA, RD Delaune, WH Patrick Jr (1980). Effect of estuarine sediment pH and oxidation-reduction potential on microbial hydrocarbon degradation. Appl Environ Microbiol 40(2): 365-369.
- Das K, AK Mukherjee (2007) Crude petroleum-oil biodegradation efficiency of Bacillus subtilis and Pseudomonas aeruginosa strains isolated from a petroleum-oil contaminated soil from North-East India. Bioresour Technol 98(7): 1339-1345.
- Mandri T, J Lin (2007) Isolation and characterization of engine oil degrading indigenous microrganisms in Kwazulu-Natal, South Africa. Afr J Biotechnol 6(1): 23-27.
- Sepahi AA, ID Golpasha, M Emami, AM Nakhoda (2008) Isolation and characterization of crude oil degrading Bacillus spp. Journal of Environmental Health Science and Engineering 5(3): 149-154
- Tang S, J Bai, H Yin, J Ye, H Peng, et al. (2014) Tea saponin enhanced biodegradation of decabromodiphenyl ether by Brevibacillus brevis. Chemosphere 114: 255-261.
- Liu Y, G Zen, H Zhong, Z Wang, Z Liu, et al. (2017). Effect of rhamnolipid solubilization of hexadecane bioavailability: enhancement and reduction. J Hazard Mater 322: 394-401.
- An CJ, GH Huang, J Wei, H Yu (2011) Effect of short chain organic acids on the enhanced desorption of phenanthrene by rhamnolipid biosurfactant in soil-water environment. Water Res 45(17): 5501-5510.
- Hoskova M, O Schreiberova, R Jezdik, J Chudoba, J Masak, et al. (2013) Characterization of rhamnolipids produced by non-pathogenic Acinetobacter and Enterobacter bacteria. Bioresour Technol 130: 510- 516.
- Soares Dos Santos A, N Pereira, DM Freire (2016) Strategies for improved rhamnolipid production by Pseudomonas aeruginosa pA1. Peer J 24: 2078.
- Roy S, S Chandni, I Das, L Karthik, G Kumar, et al. (2014) Aquatic model for engine oil degradation by rhamnolipid producing Nocardiopsis VITSIB. 3 Biotech 5(2): 153-164.
- Rooney AP, NP Price, KJ Ray, TM Kuo (2009) Isolation and characterization of rhamnolipid producing bacterial strains from a biodiesel facility. FEMS Microbiol Lett 295(1): 82-87.
- Chen Q, M Bao, X Fan, S Liang, P Sun (2013) Rhamnolipids enhance marine oil spill bioremediation in laboratory system. Mar Pollut Bull 71(1-2): 269-275.
- Ghazali FM, RNZA Rahman, AB Salleh, M Basri (2004) Biodegradation of hydrocarbons in soil by microbial consortium. Int Biodeterior Biodegradation 54(1): 61-67.
- Lowry OH, NJ Rosebrough, AL Farr, RJ Randall (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1): 265- 275.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...