Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6652
Short Communication(ISSN: 2637-6652) 
Foraminifera Distribution in Itapitangui River Mangrove, Cananeia (Sao Paulo, Brazil) and its Environmental Relations Volume 3 - Issue 1
Patricia Pinheiro Beck Eichler1*, Camilla Gomes1, Rogério Costa1, Audrey Amorim1, Moab Praxedes Gomes2 and Helenice Vital2
- 1Postgraduate Program in Environmental Sciences at the University of Southern Santa Catarina (UNISUL) University City, Brazil
- 2Graduate Program in Geodynamics and Geophysics (PPGG), Federal University of Rio Grande do Norte (UFRN). University Campus, Brazil
Received:September 27, 2019; Published:October 17, 2019
Corresponding author:Patricia Pinheiro Beck Eichler, Postgraduate Program in Environmental Sciences at the University of Southern Santa Catarina (UNISUL) University City, Brazil
DOI: 10.32474/MAOPS.2019.02.000151
Abstract
Investigation of foraminifera assemblage through thirtyt-wo surface samples along the transect in Itapitangui river mangrove was done in july / 89. Foraminiferal fauna composed mainly of Ammotium salsum, Arenoparrella Mexicana, Haplophragmoides wilberti, Miliammina fusca and Trochammina inflata occur on mangrove area of Cananeia-Iguape estuary-lagoon system at bottom salinity range from 6 to 30, bottom temperature range from18 -9°C and organic carbon percentage values range from 0.47 to 5.7. The low diversity pattern of foraminiferal population inhabiting the mangrove area of Itapitangui River and the lack of foraminiferal zonation is similar to that encountered in mangroves from all over the world.
In July 1989, a survey of foraminifera zoning was carried out by means of thirty-two surface samples along a transect in the Itapitangui river mangrove, Cananéia-Iguape estuarine-lagoon system. The foraminifera fauna found is mainly composed of Ammotium salsum, Arenoparrella mexicana, Haplophragmoides wilberti, Miliammina fusca and Trochammina inflata. This fauna has been found in muddy and sandy sediments where the bottom salinity ranges from 6 to 30, the bottom temperature ranges from 18oC to 19oC, and the organic carbon content ranges from 0.47% to 5.70%. The diversity pattern of the foraminifera population inhabiting the Itapitangui river mangrove area and the absence of zonation of foraminifera species are similar to those found in the mangroves worldwide.
Keywords: Association of foraminifera; Mangroves; Salinity; Foraminifera zonation
Introduction
Lagoons and estuaries situated between sea and continental domains are under the influence of hydrological and chemical fields’ variability. Those environments contain ecosystems that are very rich and diverse, where sea and freshwater species can reproduce and develop, maintaining the ecological balance of coastal regions. The Cananeia-Iguape estuary-lagoon system is one of the largest complex environments from south of Brazil. Benthic foraminifera fauna is used as biological indicators because these assemblies receive influences from several environmental factors such as salinity, temperature, substratum type, organic carbon, pH, Eh and ebb energy. Environmental variability control foraminifera assemblages delimiting particular microhabitats and establishing ecologically different environments. From these environments, the estuaries have characteristics of complex systems, where various environments parameters are responsible for the change of faunal composition in small geographic areas [1]. A remarkable characteristic resulting from the foraminifera assemblages is a transition towards the sea from agglutinated to a calcareous fauna. This faunal change could be gradual, but generally is a sudden fact. The characteristics of stressing conditions in estuaries environments, such as daily ebb oscillations that provokes abrupt changes in salinity allow only a few species, highly adapted, can tolerate these changes. Meanwhile, the species that are able to tolerate such changes and dominate the environment will get abundance food and low competition among species, dominating with high reproduction [2-4].
The foraminifera fauna distribution of estuaries has been widely studied and developed by the micropaleontology group from the Oceanographic Institute of Sao Paulo, through master’s degree dissertations, PhD thesis and papers resulting from international cooperation projects. Among these works, we can summarize: [5-10,11,12]. Our main objective is to add to the knowledge of the ecology of benthic foraminifera species from Itapitangui river mangrove in the Cananeia-Iguape estuary-lagoon system. This area remains well preserved from anthropic action, and it is believed that the respective fauna is subjected only to the natural stress typical from transitional environment. Resulting data from well preserved natural mangrove environments and its foraminifera fauna are valuable to compare with other mangroves from all over the world forming a basis to study the distribution of foraminifera fauna [13].
Study Area
The Cananeia-Iguape estuary-lagoon system is located south of Sao Paulo state and it is a low-energy environment formed by a complex group of islands. In the north part of it is delimited by the estuary of Ribeira de Iguape river, and in the south by Trapande Bay, where many rivers flow into the system the estuary-lagoon system is separated from adjacent ocean by 70km long barrier island (Island) Long). The Itapitangui river is located in the southeast part of Cananeia city (Figure 1). The Itapitangui river mangrove fringes are colonized by Laguncularia racemosa and Rhizophora Mangle. It can also be found isolated species of Avicennia shaueriana and fields of Spartina sp. The mangrove vegetation has an area of 2,000ha as interpreted in aerophotogrametrics by [14]. This area is microtidal with mean tidal range about 1.5m. The sampling area is well sheltered from severe wave activity and is subjected to periodic flooding as a result of fluctuations of the adjacent water body. Bottom sediments are mostly mud and sand. Rainfall, local tributaries rivers and tidal channels control area discharge.
Materials and Methods
Thirty-two surface samples (10cm3 area, 1 cm deep) were collected along a transect across the Itapitangui river mangrove in July / 89 (Figure 1). The first sample was taken on the channel, and the subsequent ones toward mangrove vegetation at every 50m. Samples were taken from sediment retained around supporting roots from Rizophora Mangle and Laguncularia Racemosa. To collect the foraminiferal samples, it was used a small, serrated corer, as described by [15]. This corer cuts the mangrove material, mainly roots, without compressing anything. Samples containing sediment were fixed with 5% buffered formalin, stained with rose Bengal, washed over at 0.063-micron sieve and dried. In order to separate foraminifera from sediment each sample were floated in carbon tetrachloride and examined for foraminiferal specimens. All specimens of foraminifera were identified and counted. Sediment samples for organic carbon analysis were also collected and performed the Gaudette [16-20] method. Salinity measurements were obtained from interstitial water in bottom sediment. These values were determined using Goldberg 10.433 portable refractometer. The temperature was obtained with manual thermometer. Species diversity index of Shannon-Wiener function H and Simpson Dominance were calculated for each station. The physical and chemical data (salinity and amount of organic carbon) in sedimentation were correlated with foraminifera data (diversity and dominance) through “nonparametric” correlation method of Spearman, accepting as the criterion for recognition, significant differences <0.05. The number of individuals was evaluated to group species and stations. The matrix was logarithmized (log x+1) and submitted to Bray Curtis index. A few samples and rare species were not taken into account for the matrix data. Samples and species that were removed from the calculation were samples 3, 18 and 31, and the foraminifera species Ammobaculites exigus, Ammodiscus sp., Glomospira compressa and Trochammina squamata. The grouping was obtained by minimum variation. The final calculation and dendogram were done by Fitopac program.
Results
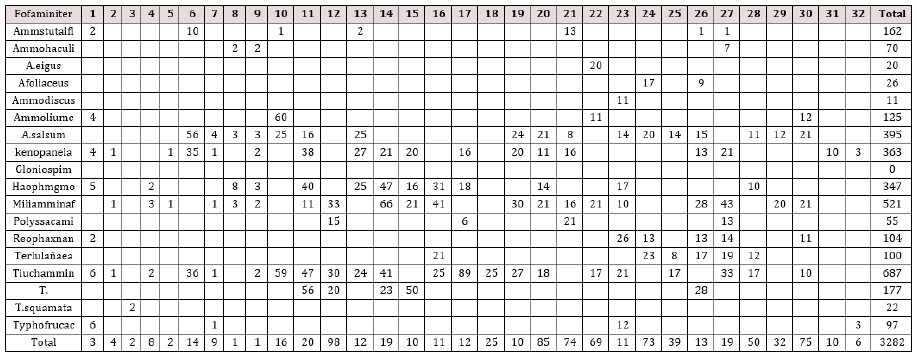
In the 32 samples collected along the Itapitangui river mangrove transect, was found a total abundance of 3282 individuals, belonging to 18 foraminifera species (Table 1). The foraminiferal fauna from this area is dominated by agglutinated species belonging to Textulariina sub order. Ammotium salsum, Arenoparrella mexicana, Haplophragmoides wilberti, Miliammina fusca and Trochammina inflata are the dominant foraminiferal assemblages found in the mangrove-studied area. The diversity and dominance species index, salinity, and organic carbon values found in Itapitangui mangrove are shown in (Table 2). Minimum diversity values were in sample 29 (0.662) and maximum diversity values were in sample 26 (2.011). The minimum dominance values were in sample 26 (0.137) and the maximum diversity values were in sample 29 (0.516). Bottom salinity values range from 6 to 30. The highest values occur near Itapitangui Sea at the tidal channel, and the lowest value in the upper mangrove region. Bottom water temperature values remaining between 18 and 19 °C. Organic carbon percentage values range from 0.47 and 5.7.
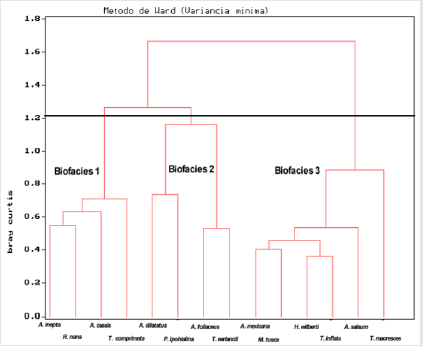
The cluster analysis, performed on the 32 samples containing 18 species, defined 3 biofacies (Figure 2). The found species of biofacies 1 are Ammoastuta inepta, Reophax nana, Ammotium cassis and Typhotrocha comprimata. Biofacies 2 presents Ammobaculites dilatatus, A. foliaceus, Polysaccamine hypohyaline and Textularia earlandi. Biofacies 3 consists of Arenoparrella mexicana, Milammina fusca, Haplophragmoides wilberti, Ammotium salsum, Trochamina inflata and T. macrescens. The biofacies 3 is characterized by the dominance of true mangrove foraminifera fauna. These findings suggest that foraminiferal zonation is unclear and change in foraminiferal assemblage may not be directly associated with mangroves. On the other hand, mangrove foraminiferal assemblage is rather controlled by others environmental factors such as salinity, temperature and substrate organic content [21]. The physical chemical data (bottom salinity and organic carbon percentage) were correlated with biological data (diversity and dominance). The results obtained in this study have not revealed any clear relations between the environmental parameters and foraminiferal fauna.
Discussion and Conclusion
The mangrove foraminiferal assemblage found in this study presents a low faunistic diversity, probably due to environmental changes such as daily salinity and temperature variations. The foraminifer’s patchy distribution contributes to recognizing several faunal provinces suggesting different microenvironments along the samples collected from the mangrove transect. The foraminiferal fauna identified in Itapitangui river mangrove consists of all agglutinated species, characteristics from mixohaline environments. It was not found any calcareous species from ocean adjacent areas. This result may suggest that there is a minimum ocean influence, through ebb flows, capable of transporting foraminifer’s tests. The high organic carbon percentage present in the transect mangrove, may be responsible for the development of true mangrove fauna entirely agglutinated. Besides that, bottom salinity seems to be another influential factor upon foraminiferal distribution in Itapitangui mangrove. Many authors are in agreement with that, and the importance of this attribute is well discussed [13,16]. The occurrence of agglutinated foraminifera is also probably due to freshwater discharge in the system. According to [17], the impact of fresh water, draining into the estuarine environments, promotes a reduction in calcareous species and increase in agglutinated individuals’ number and diversity. Diversity pattern expected for mangrove area is always lower than coastal regions, due to freshwater dilution from rivers. In hyposaline waters, the number of species varies about 10 species, mostly agglutinated species. In the other hand, in regions where discharge of fresh water is less significant, this number increases over 25, and calcareous shapes predominate. The low number of foraminifera species found in study area is a good confinement indicator of marine influence in mangrove area. Many authors have obtained reductions in the number of species when sampling stations are far from the adjacent ocean region [3,7]. Dominance and diversity results obtained from the foraminiferal fauna, seem to be at random, not showing any kind of distribution pattern. The salinity role upon foraminifera functioning system is getting started.
It is known, however, that effects from reduction of salt in waters may have a great influence on habitat restriction of mixohaline species. In the same way, the lack of inorganic salts availabilty in fresh water is an extreme limiting factor, mainly to the calcareous species, because calcareous species takes from the chemical elements necessary to construct its tests. The assemblage found in this study is considered a true mangrove fauna by the dominance of Mexican, Arenoparrella Milammina fusca, Haplophragmoides wilberti, Ammotium salsum and Trochamina inflata. These species dominate mangroves assemblages in Sumatra. The pattern found in the Itapitangui mangrove indicates that zonation does not seem to exist in this particular environment and foraminiferal distribution shows a uniform assemblage pattern along the study area. Usually, benthic foraminifera can be used as indicator of freshwater influence and gradient of marine influence in mangrove area, but in the present study salinity variation might have not been noted in the area and has not provided any control in foraminifera assemblage able to delimit ecologically different environments.
References
- Alve E (1995) Benthic Foraminiferal Responses to Estuarine Pollution: A Review. The Journal of Foraminiferal Research 25(3): 190-204.
- Biswas B (1976) Bathymetry of Holocene Foraminifera and Quaternary Sea-Level Changes on The Sunda Shelf. Journal of Foraminiferal Research 6(2): 107-133.
- Bonetti C, Eichler BB (1997) Benthic foraminifera and thecamoebians as indicators of river/sea gradients in estuarine zone of Itapitangui river-Cananeia/SP, Brazil. Anais da Academia Brasileira de Ciencias 69(4): 545-563.
- Boltovskoy E, Wright R (1976) Recent Foraminifera. Junk, The Hague, 515.
- Debenay JP (1991) Benthic Foraminifera Used as Indicators of a Gradient of Marine Influence in Paralic Environments of Western Africa. Journ of African Earth Sciences 12(1-2): 335-340.
- Debenay JP, Eichler BB, Sousa, ECPM Et Lesourd M (1995) How Rubratella Intermedia (Foraminifera) Can Resist High Energy in Ubatuba, Sp, (Brazil). Rèvue De Palèobiologie De Genève 14(2): 473-478.
- Debenay JP, Eichler BB, Guillou JJ, Eichler Coelho PB, Coelho C, et al. (1997) Behavior of Peuplements Of Foraminifères Et Comparison Avec L'Avifaune Dans a Lagune Fortement Stratifiée: La Lagoon of Conception (Sc, Bresil). Rèvue De Palèobiologie, Genève 16(1): 55-75.
- Debenay JP, Eichler BB, Duleba W, Bonetti C, Eichler PB (1998) Water Stratification in Coastal Lagoons. Its Influence on Foraminiferal Assemblages in Two Brazilian Lagoons. Marine Micropaleontology 35(1-2): 67-89.
- Duleba W (1997) Variations in Associations of Sub-Recent Tecamebas, Foraminifers and Ostracodes of the CananéiaIguape Lagoon Region 236.
- Duleba W, Debenay JP, Eichler BB, Mahiques MM (1999) Holocen Environmental and Water Circulation Changes; Foraminifera Morphogroups Evidence in Flamengo Bay (Sp, Brazil). Journal Coastal Research 15(2): 554-571.
- Eichler BB, Debenay JP, Bonetti C, Duleba W (1995) Répartition Des Foraminifères Benthiques Dans La Zone Sud-Ouest Du Système estuarien lagunaire d'iguape Cananéia (Brésil). Brazilian Journal of Oceanography 43(1): 1-17.
- Eichler, BB, Bonetti C (1993) Distribution of Foraminifers and Tecamebas Occurring in Baguaçu Mangrove and Their Relations with Some Environmental Parameters. Canaanite-Iguape Region, Sp, Brazil. In: Brazilian Congress of Paleontology Xiii. Summaries, São Leopoldo 225.
- Eichler Coelho PB (1996) Study of foraminifera and weaver associations in the estuarine lagoon region of Cananéia Iguape (SP) and their application in determining the ecological impact of Valo Grande. Oceanographic Institute of the University of São Paulo, Brazil.
- Gaudette HF, Flight WR, Toner L, Folger DW (1974) An Inexpensive Titration Method for The Determination of Organic Carbon in Recent Sediments. J Sedim Petrology 44(1): 248-253.
- Geraque, EA (1997) Ostracodes From the Estuarine Lagunar Region of Cananéia Iguape, Sao Paulo, Brazil, p. 118.
- Herz R (1991) Mangroves from Brazil. Sao Paulo. Special Publication Oceanographic Institute, University of Sao Paulo, Brazil, p. 227.
- Murray JW (1973) Distribution and Ecology of Living Benthic Foraminiferids. Crane Russak & Co., New York, USA, xiii: 274.
- Phleger FB (1970) Foraminiferal Populations and Marine Marsh Processes. Limnol Oceanogr 15(4): 522-534.
- Redois F, Debenay JP (1996) Influence Du Confinement Sur La Répartition des Foraminiféres Benthiques. Example of L'estran D'une Ria Mésotidale Of Bretagne Méridionale. Revue De Paléobiologie, Geneve 15(1): 243-260.
- Sen Gupta B (1999) Foraminifera in marginal marine environments- Modern foraminifera 141-159.
- Todd R, Brönnimann P (1957) Recent Foraminifera and Thecamoebina from the eastern Gulf of Paria. Cushman Foundation for Foraminiferal Research 3: 1-43.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...