Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6652
Review Article(ISSN: 2637-6652) 
Applications of Minichannels in Gas Absorption: A Review Volume 2 - Issue 5
Mohammad Amin Makarem, Mohammad Farsi* and Mohammad Reza Rahimpour*
- Department of Chemical Engineering, Shiraz University, Iran
Received:August 13, 2019; Published:August 27, 2019
Corresponding author:Mohammad Farsi and Mohammad Reza Rahimpour, Department of Chemical Engineering, Shiraz University, Iran
DOI: 10.32474/MAOPS.2019.02.000149
Abstract
Channels with small hydraulic diameters are novel contactors that enhance mass transfer rate due to great interfacial area to volume ratio and flow hydrodynamics. One of the interesting applications of small channels is gas absorption processes including oxygen absorption, carbon dioxide capturing, methane and hydrogen sulfide absorption and volatile organic compounds (VOCs) sequestration. This research presents a brief review on the recent developments and advances in application of Minichannels in gas absorption process, flow hydrodynamics and mass transfer characteristics in Minichannels. The major studies have been conducted on the CO2 capture and separation by aqueous solution of amines due to effect of CO2 emission on the global warming and popularity of CO2 separation units. Although many researches have been conducted on gas absorption in mini contactors, there is a long way to industrialize this technology.
Keywords:Small scale channels; Mass transfer; Flow regime; Gas absorption
Introduction
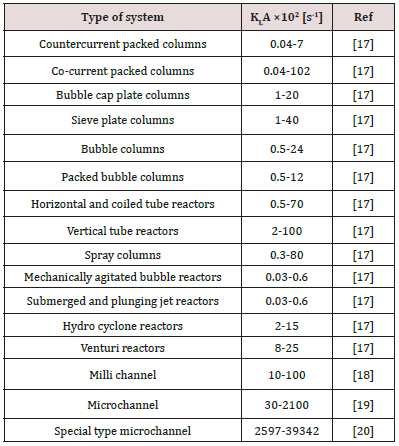
Absorption is one of the most important processes applied in the chemical, petrochemical and refinery units to separate gaseous mixtures and purifying streams. Through the absorption process, the desired components in a gaseous stream is dissolved in a liquid phase and captured. Industrially, the packed beds and tray columns [1,2], rotating packed beds [3], falling film columns [4], agitated vessels [5], bubble columns [6], spray towers [7] and membranes [8,9] are conventional devices used in the gas absorption processes. One of the main challenges in the gas absorption technology is the low rate of mass transfer in the contactors, which influence the number of stages, contact time and solvent circulating rate [10]. Increasing mass transfer coefficient in the gas-liquid contactors enhances the rate of mass transfer between phases and results in the lower contact time in the absorbers. Many researchers investigated the effects of fluid hydrodynamics, operating condition and tower specifications on the mass transfer coefficient in the absorbers and proposed novel structures to improve the rate of mass transfer. One of the novel structures introduced in the recent decade is mini-scale channels that present a large interfacial area caused by capillary force and surface tension [11]. The small-scale channels could yield both environmental and economic benefits compared to the conventional contactors by reducing the capital cost and equipment size [12,13] Figure 1 shows the schematic diagram of a small scale channel. Typically, the channels are categorized based on the hydraulic diameter and dimension. Based on the classification of Kandlikar and Grande, the hydraulic diameter of conventional channels is upper than 3mm, while the diameter of Minichannels and microchannels are in the ranges 3000-200μm and 200-10μm, respectively [14]. Mehendale et al. described the diameter range from 1 to 100μm as microchannels, 100μm to 1mm as mesochannels, 1 to 6mm as compact tube, and upper than 6 mm as conventional contactor [15]. The small scale channels present lower mass transfer resistance and higher pressure drop compared to the conventional contactors. Therefore, optimization of process conditions and channel dimensions is a practical solution to maintain pressure drop at moderate levels [16]. Table 1 compares the mass transfer coefficient in small scale channels with common equipment. It appears that channels can improve the mass transfer coefficient up to 1000 times more than other devices. This research presents a short review on the gas absorption topic including flow patterns and mass transfer rate in Minichannels from millimetric ranges to microscale ones, and their applications in gas separation and purification. In this regard, the absorption of gases including O2, CO2, CH4, H2S and VOCs by selective solvents is reviewed briefly.
Flow Hydrodynamics
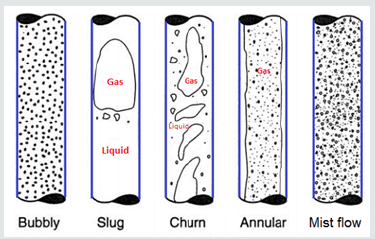
The flow hydrodynamics has a considerable effect on the mass transfer rate in two phase flow systems. Generally, four major flow regimes including bubbly flow, Taylor flow, churn flow, and annular flow are observed when the gas and liquid flow simultaneously in Minichannels [17]. The capillary and gravitational forces, gas to liquid ratio and channel dimensions determine the flow regime type in the channels. The effect of capillary and gravitational forces can be described by Bond number as the ratio of the channel diameter to capillary constant. Although reducing channel hydraulic diameter changes transition between flow patterns to higher superficial gas and liquid velocities, channel orientation does not has any effect on flow pattern and transition lines in mini-channels [18,19]. Figure 2 shows the flow pattern in a two phase flow system [20].
In the bubbly flow regime, the gas to liquid ratio is small, and small bubbles are randomly distributed in a continuous liquid phase. Tylor flow regime as the most common flow regime in small scale channels consists of frequent gas and liquid slugs. In the Taylor regime the small bubbles coalesce and form large slugs at the same size and shape. It provides a high mass transfer coefficient, large surface area, low axial dispersion, sharp residence time distribution and low pressure drop in the channel [21]. Currently, the Taylor flow regime has attracted more attention compared to other regimes, due to its potential to intensify fast reactions which are controlled by mass transfer such as CO2 absorption by chemical solvents. In the churn flow the bubbles form an unstable and nonuniform path in the center line of channel and pushes liquid. In the annular flow regime, the liquid develops a uniform wavy layer on the pipe walls and gas flows in the channel continuously. Chinnov et al. presented a good review on the hydrodynamics of two phase flow and flow patterns in mini and microchannels [22].
Mass Transfer Performance
The rate of heat and mass transfer in small scale channels depends on the fluid hydrodynamics and explained as volumetric mass transfer coefficient. Generally, there is two basic methods to predict flow regimes, mass transfer coefficient, gas and liquid slug lengths, gas and liquid holdups, and liquid film thickness in the small-scale channels, including experimental equations and theoretical approaches. In Taylor flow regime, which is dominant and desirable in minichannels, the overall mass transfer coefficient is usually expressed based on the operating parameters such as gas slug velocity, gas slug length, liquid slug length, dynamic gas holdup and liquid film thickness between the gas bubble and the channel wall. Besides, the mass transfer coefficient can be expressed as Sherwood, Reyonds and Schmit dimensionless numbers. Haase et al. presented a comprehensive overview on the hydrodynamics, flow regime and mass transfer in the minichannels and summarized the available correlations to predict Taylor flow characteristics, as well as mass transfer coefficients in gas-liquid systems [23].
Experimental Approach
The gas absorption from bubble phase toward the liquid results in a slug shrinking as it flows along the channel and the rate of reduction in the slug size and velocity are used to calculate the mass transfer coefficient [24]. Usually, the channels are made from famous polymeric materials such as poly–methyl-methacrylate and poly-dimethyl-siloxane through micro-mechanical cutting, wet and dry etching, lithography, embossing and imprinting, injection molding, and different laser methods [25]. Gas and liquid streams usually flows to the channels from different entrances and join together in a Y or T junctions. The rate of bubble shrinkage and bubble position are recorded by a high-speed camera, and finally, gas and liquids are separated in a micro separator. The rate of gas absorption is determined either by titrating the accumulated liquid or analyzing the changes of the bubble volume through the channel [26]. Typically, the experimental based correlations could be utilized with an acceptable precision when the process conditions are near to the base experiment and similarity condition is satisfied. Sattari et al. reviewed the experimental studies on mass transfer in gas –liquid and liquid–liquid small-scale channels and explained the effects of operating conditions and the channel geometry on the mass transfer [27]. Typically, the mass transfer coefficient is calculated based on the balance of mass transfer rate from the gas bubble into the interface with the mass transfer rate from interface to the liquid slug as:
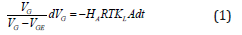
If the experimental measurements are based on the shrinkage of the gas bubbles, the molar rate and saturation concentration in above equation should be related to the gas slug volume with the aid of ideal gas assumption and Henry’s law, respectively. This lead to the following result as:
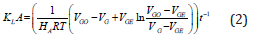
By knowing the initial and final volume of gas bubbles and applying the value of Henry’s constant at the experimental condition, KLA is calculated [28]. On the other hand, if the titration method is selected to determine the amount of absorption, formulations should be written based on the liquid concentration as below [29]:
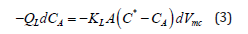
By a simple integration over the channel, the following equation is obtained.
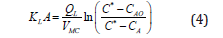
The initial concentration of gas in liquid is zero and the concentration of outlet liquid is found by titration. As aforementioned, after calculating the mass transfer coefficient, it can be re-correlated based on the either the effective parameters or dimensionless numbers. Table 2 summarize some correlations to calculate the gas-liquid mass transfer coefficient in minichannels. Alternatively, the gas holdup could be computed from the gas and liquid slug lengths, liquid film thickness and bubble curvature in axial direction. These equations are explained based on Bankoff coefficient and gas input volume fraction [30]. In addition, the slug length is explained based on hydraulic diameter of channel, superficial gas and liquid velocities, gas and liquid holdups and dimensionless numbers including Reynolds, Bond and Capillary. Table 3 shows developed experimental and semi-experimental equations to calculate the slug length in the Minichannels.
Theoretical Approach
In the second method, the hydrodynamics of a slug flow, slug length and rate of mass transfer in the gas–liquid systems are calculated based on the modeling approach using the continuity and the momentum conservation equations coupled with mass balance and the reactions expressions [31]. In this approach, the bubble length and shrinkage through are calculated by solving two phase flow equations for species concentrations [32]. In this regard, the Navier–Stokes and continuity equations are written as:
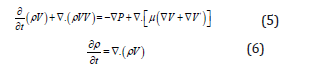
For the boundary conditions, the axial velocity (z direction) at the outer tube wall is set to the bubble velocity (Vz = VB) while it is assumed to be zero at radial (r direction) coordination (Vr = 0). Besides, free slip boundary condition is used normal to the bubble surface. By solving Eqs. 8 and 9, the velocity distribution along the channel will be found. The convection–diffusion equation is used to determine the rate of mass transfer from gas phase to the liquid phase, as shown below [33]. In this equation, the velocity component is found from the solution of Eqs. 8 and 9.
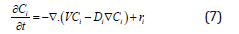
In the above equation, all terms with i index should be written for both species, i.e. the absorbing gas component and the liquid side reactant. Besides, the equation should be considered equal to zero for taking into account the physical absorption. A more detailed description on the continuity and momentum equations could be found in the literature [34].
Applications of Minichannels
In the field of chemical engineering, channels can be used in nanoparticles synthesis [35], producing organic products [36,37] polymers synthesis [38], biodiesel production [39], phase change including boiling and condensation [40-42], and separation processes including distillation, stripping and absorption [12]. In this section, the application of Minichannels in the gas absorption process is discussed.
Oxygen Absorption
Due to non-reactivity and physical absorption, oxygen absorption in water is a common system to evaluate flow hydrodynamic, pressure drop and heat and mass transfer rates in channels [43-45]. Based on the conducted experiments on the oxygen absorption in water, the dominant flow regime in Minichannels is Taylor flow and liquid film is completely saturated when the channel length is large. Besides, the liquid slug length can be estimated from pressure drop [46].
CO2 Capture
Carbon dioxide as the main greenhouse gas has a considerable effect on the global warming. Therefore, CO2 separation and conversion are important topics from academic and industrial viewpoints [47,48]. Due to years, different solvents were proposed to absorb CO2 from gas mixture in the conventional and intensified processes. Currently, carbon dioxide capture has been proposed as one of the most important application of Minichannels in the field of gas absorption due to high mass transfer rate, interfacial area to volume ratio and desirable flow hydrodynamics. Typically, the small-scale channels are economically competitive to conventional systems and result in the lower capital cost when the plant capacity is below than 50 MMSCFD [48]. However, at higher capacities the operating costs of Minichannels is considerable compared to conventional technologies and this technology is not efficient.
CO2 Capture by Sodium Hydroxide
Since sodium hydroxide is a strong base, the CO2 is chemically absorbed in the aqueous solution of NaOH and related salt is produced in the liquid phase. This reaction is very fast and mass transfer controls the rate of CO2 absorption. Thus, enhancing mass transfer coefficient in CO2 absorption process is a practical solution to decrease plant size and improving process performance. Generally, the channels with small hydraulic diameter yield a considerable reduction in the residence time, and reactor size [46]. This reduction is enhanced by applying higher solvent concentration and operating temperature. Yue et al. studied the absorption of CO2 in aqueous solution of NaOH in a minichannel with hydraulic diameter of 667 μm [19]. They measured pressure drop in the channel and reported that the liquid mass transfer coefficient in the volumetric basis is upper than 21s-1. Shao et al. simulated CO2 capture in NaOH solution by Computational Fluid Dynamics in channels with diameter less than 1mm and showed that physical absorption increases with the channel diameter, while the reverse is true in the case of chemisorption [47]. Aoki et al. investigated the effect of flow rate, channel dimension, and gas holdup on CO2 absorption by sodium hydroxide solution [49]. They concluded that the slug length depends on the channel diameter in the tee junction. Besides, the contacting angel less than 90˚c decreases the slug length at low flow rates. Tan et al. concluded that by curving the channel, the mass transfer rate will be enhanced in CO2 absorption by aqueous solution of sodium hydroxide [50].
CO2 Capture by Amines
Typically, the aqueous solution of amines such as monoethanolamine, diethanolamine, methyl diethanolamine, piperazine and their mixtures are primary absorbers for CO2 capture [51,52]. In industrial processes, carbon dioxide is absorbed by the aqueous solvent, and the CO2-rich amine stream feeds to the stripper. In that unit the amine mixture is regenerated with heat and steam, and lean amine is recycled to the main bed [53]. Spray towers, packed beds, tray columns mixers and falling film column are conventional apparatus used in gas sweetening and flue gas purification processes to separate CO2 from a gaseous mixture. Although the kinetics of CO2 absorption by primary and secondary amines is fast, desired purification level can only be achieved by very large columns due to the limited interfacial area between gas and liquid phases and mass transfer coefficient. Currently, small scale channels are proposed as the high-performance contactors to use in the CO2 capturing process due to high interfacial area and hence enhanced mass transfer characteristics [54-56]. Chunbo et al. investigated CO2 absorption into aqueous solution of monoethanolamine in a T-type minichannel [57]. They investigated the effect of mass transfer driving force, operating temperature, contact time, and enhancement factor on the absorption rate and capacity. It was concluded that the rate of chemical reaction plays an important role on the absorption rate. Since the monoethanolamine and CO2 react fast and absorption is controlled by diffusion, the Minichannels are good candidate for CO2 capturing. Kundu et al. studied CO2 chemisorption in aqueous solution of diethanolamine in circular millimetric channels [58]. They studied the effect of feed composition and channel diameter on the CO2 absorption rate. It was found that flow regime has a significant effect on the absorption performance, and the optimal feed rate and channel dimensions could be founded by formulation and optimization problem. Yang et al. showed that rate of CO2 absorption in diethanolamine enhances when the gas and liquid phase superficial velocities increase in Minichannels and the maximum mass transfer coefficient is achieved in the slug and churn flow regimes [59]. Since absorption rate of MDEA solution is low, it is used with an activator in CO2 capturing process. Pan et al. investigated the CO2 absorption in aqueous solution of MDEA and PZ in a microchannel [60]. They reported that the volume mass transfer coefficient reaches to 1.70 s-1 when Minichannels is used as gas-liquid contactor. Liu et al. investigated the desorption rate of CO2 from MDEA in a minichannel contactor [61]. The mass transfer coefficient of desorption was in the range of 0.36-2.68 s-1, which is in the same order with that of absorption and much greater than conventional equipment.
CO2 Absorption by Ammonia
Ammonia is an potential solvent for CO2 capture due to low energy consumption for solvent regeneration, low degradation rate, high CO2 removal efficiency and loading capacity [62]. Kittiampon et al. presented a comprehensive comparison between performance of microchannels, packed bed column, spray tower, bubble column and multistage spraying tower to absorb CO2 by aqueous solution of ammonia, in terms of operating conditions, absorption rate, and mass transfer coefficient [63]. The hourly volumetric flow rate of gas and liquid mass transfer coefficient in the small channel system were exceptionally high, indicating the potential of aqueous solutions of ammonia for CO2 absorption. Therefore, the absorber volume can be greatly reduced by changing the type of contacting device to Minichannels.
CO2 Absorption into Alcohols
Although alcohols are not primary absorbers for carbon dioxide, they can be used as working fluids to study CO2 absorption performance in Minichannels. Ji et al. presented a novel method to measure mass transfer coefficient in liquid phase through the Pressure-Volume-Temperature correlation of the gas phase [64]. They used CO2-H2O, CO2-ethanol and CO2-n-propanol systems to evaluate performance of proposed method in square channels with dimensions of 40×240μm, 100×800μm, and 100×2000μm. The results showed that the channel diameter and the capillary number are effective parameters on the mass transfer rate, and the maximum value of the mass transfer coefficient is achieved in annular flow regime. For short timescales, below 0.001s, gas bubble diffuses very fast and the initial dissolution rate is found proportional to the Henry’s constant and diffusion coefficient [65].
CO2 Absorption by Potassium Carbonate Solutions
Recently, carbonate solutions are the alternative of MEA solutions for carbon dioxide capture from power plants and natural gas due to the availability, low volatility, degradation resistance and environmental friendliness [65]. The main drawbacks are the slow CO2 absorption rate and difficulty to handle solid participation. The solubility and kinetics of carbonate solutions to absorb CO2 is the subjects of various studies [66,68]. In this family, aqueous solution of K2CO3 is one of the oldest solvents for CO2 capture from natural gas utilized in Benfield process [69]. The performance of carbonate solutions in small channels were rarely studied. Sobieszuk et al. investigated the mass transfer rate of CO2 from its mixture with N2 into aqueous mixture of K2CO3 and KHCO3 [70]. The volumetric mass transfer coefficient in the liquid phase was determined in terms of superficial velocities of gas and liquid. Besides, the rate of absorption was measured and the Dankwerts plot were generated. Finally, a correlation to predict mass transfer coefficient were presented [71].
CO2 Absorption by Novel and Green Solvents
Recently, finding green and efficient solvents for CO2 capturing is an attractive area from academic viewpoint. Between considered solvents, deep eutectics[72], ionic liquids [73-75] and amino acid ionic liquids [76] are the most important ones. Currently, the efficiency of mixture of MEA and [Bmim][BF4][77], aqueous solution of [Bmim] [BF4][75], and [Emim][BF4] [78] have been proved for physical absorption of carbon dioxide in small channels. The main benefits of such solvents are that the regeneration can be easily performed at moderate conditions.
CH4 Absorption
While the majority of the articles used CO2 as the absorbing gas, a few studies were reported to absorb methane in the Minichannels. Special type of ionic liquids can absorb high amount of methane and utilized in a multichannel system for separating it from a mixture with nitrogen [79]. As mentioned before, since the ionic liquid mixture can be regenerated only by heat, the suggested structure contains the regeneration unit as well.
H2S Absorption
Hydrogen sulfide removal is an important economic and environmental challenge faced by the oil and gas industries. Shah et al. reviewed H2S separation using reactive and non-reactive absorption and adsorption, membranes, and cryogenic distillation [80]. Generally, a few studies have been conducted H2S absorption in microchannels. Su et al. studied the mass transfer rate of H2S into MEA solution in a T-junction microchannel [81]. It is concluded that T-junctions lead to higher mass transfer rate in the gas side and H2S can be removed up to 99.5 % in such channels. Pan et al. focused on the absorption of H2S and CO2 mixture into MDEA in a microchannel reactor [82]. The results showed that the efficiency of the system could be up to 99.85% and the H2S removal efficiency increases with increasing the MDEA concentration, liquid flow rate and decreasing gas flow rate.
VOCs Sequestration
The emission of volatile organic compounds in atmosphere causes serious issues in the environment and diseases in humans such as cancer and various allergies [83]. Therefore, VOC capturing and conversion including adsorption on activated carbon, zeolite and MOF [84-86], thermal or catalytic incineration [87], membrane separation [88], biological conversion [89], and absorption by selective solvents [90] are the topic of many researches. One of the important categories of VOCs is chlorinated VOCs such as tetrachloroethylene (TCE), dichloromethane (DCM), and chlorobenzene (CB) emitted mainly from cleaning industries. The absorption by di-wasethylhexyl-adipate (DEHA), di-ethylhexylphthalate (DEHP), and Tetra-Ethylene-Glycol-Dimethyl-Ether (TEGDME) is common method to separate chlorinated VOCs from gas mixtures [91,92]. Monnier et al. studied the absorption of TCE by DEHA in plates containing microchannels and compared the results with unstructured plates [93]. The results showed a considerable increase in mass transfer rate by utilizing mini structure plates, especially when liquid flow rate is low. Mhiri studied TCE absorption from air by DEHA at atmospheric pressure in a micro-absorber [94]. The effects of gas flow rate and cavity thickness on absorption performance were investigated and it was found that reducing cavity thickness, especially at low gas velocity, improves the gas side mass transfer coefficient around 7 times.
Scale Up
Typically, small scale channels suffer from low capacity, and thousands to tens-of-thousands of small channels are necessary to achieve required resistance times and throughput for industries [95-99] Therefor their application in industrial scale is a serious challenge. Several structures, such as parallel and series channels and channels dimension enlargement have been suggested to overcome these issues [100]. Among them, parallel numberingup is the most common method for scaling up Minichannels. The performance of these structures are highly related to the phases distribution at the entrance of channel [101]. Usually internal and external methods are suggested for scaling-up small channels. In the first method, two phases are mixed together and distributed in all small channels, afterwards. The main challenge of this method is that the flow pattern and pressure drop will vary from one channel to another. Although, the bigger manifolds can be used to uniform the pressure across all channels, it results in deviation from Taylor flow pattern. The second solution is to enter both phases separately into each channel. Although the flow distribution will be more similar to a single channel, the equipment size and cost increased a lot. Therefore, an intermediate approach is proposed considering internal method for one phase and external method for two phase contacting. In other words, one phase is distributed in all channels while the other one will be injected to them separately [102]. This method will reduce both size and cost of the equipment and uniforms the flow pattern as high as possible [103-110].
Conclusion and Future Outlooks
In this work, a brief review on recent advances in application of Minichannels in the gas separation processes including O2, CO2, CH4, H2S and VOCS absorption were presented [111-114]. In all cases, a considerable enhancement in mass transfer rates were observed due to high surface area to volume ratio and desirable flow hydrodynamic regime. Between considered absorption processes, carbon dioxide absorption by conventional amines in Minichannels has attracted more attentions because of CO2 on global warming and environmental issues. Although CO2 absorption by green solvents such as deep eutectic solvents and ionic liquids is an active research area, a few researches have been conducted on the application of green solvents in Minichannels. Some structures suggested to scale up Minichannels, by putting many of them together and designing a parallel multichannel system. In spite of conducted efforts on industrialization of small-scale channels, more theoretical and experimental studies are still essential in the field.
References
- Baniadam M, Fathikalajahi J, Rahimpour M (2009) Comparison of Separation Performance of a Structured Packed Column with a Tray-Type Column for H2S and CO2. Oil & Gas Science and Technology 64(2): 179-190.
- Chilton TH, Colburn AP (1935) Distillation and absorption in packed columns A convenient design and correlation method. Industrial & Engineering Chemistry 27(3): 255-260.
- Lin CC, Kuo YW (2016) Mass transfer performance of rotating packed beds with blade packings in absorption of CO2 into MEA solution. International Journal of Heat and Mass Transfer 97: 712-718.
- Gheni SA, Abed MF, Halabia EK, Ahmed SR (2018) Investigation of carbon dioxide (CO2) capture in a falling film contactor by computer simulation. Oil & Gas Science and Technology-Revue d’IFP Energies nouvelles 73(43): 1-22.
- Yagi H, Yoshida F (1975) Gas absorption by Newtonian and non-Newtonian fluids in sparged agitated vessels. Industrial & Engineering Chemistry Process Design and Development 14(4): 488-493.
- Shulman HL, Molstad M (1950) Gas-bubble columns for gas-liquid contacting. Industrial & Engineering Chemistry 42(6): 1058-1070.
- Kuntz J, Aroonwilas A (2008) Performance of spray column for CO2 capture application. Industrial & Engineering Chemistry Research 47(1): 145-153.
- Masoumi S, Rahimpour MR, Mehdipour M (2016) Removal of carbon dioxide by aqueous amino acid salts using hollow fiber membrane contactors. Journal of CO2 Utilization 16: 42-49.
- Bernardo P, Drioli E, Golemme G (2009) Membrane gas separation: A review/state of the art. Industrial & Engineering Chemistry Research 48(10): 4638-4663.
- Gaddis E (1999) Mass transfer in gas-liquid contactors. Chemical Engineering and Processing: Process Intensification 38(4-6): 503-510.
- Guangwen C, Jun Y, Quan Y (2008) Gas-liquid micro reaction technology: Recent developments and future challenges, Chinese Journal of Chemical Engineering 16(5): 663-669.
- Lam KF, Sorensen E, Gavriilidis A (2013) Review on gas-liquid separations in microchannel devices. Chemical Engineering Research and Design 91(10): 1941-1953.
- Ganapathy H, Shooshtari A, Dessiatoun S, Alshehhi M, Ohadi M (2014) Fluid flow and mass transfer characteristics of enhanced CO2 capture in a Minichannels reactor. Applied Energy 119(15): 43-56.
- Kandlikar SG, Grande WJ (2003) Evolution of microchannel flow passages--thermohydraulic performance and fabrication technology. Heat transfer engineering 24(1): 3-17.
- Mehendale S, Jacobi A, Shah R (2000) Fluid flow and heat transfer at micro-and meso-scales with application to heat exchanger design. Applied Mechanics Reviews 53(7): 175-193.
- Ohadi M, Choo K, Dessiatoun S, Cetegen E (2013) Next generation microchannel heat exchangers.
- Shao N, Gavriilidis A, Angeli P (2009) Flow regimes for adiabatic gas-liquid flow in microchannels. Chemical Engineering Science, 64(11): 2749-2761.
- Coleman JW, Garimella S (1999) Characterization of two-phase flow patterns in small diameter round and rectangular tubes. International Journal of Heat and Mass Transfer 42(15): 2869-2881.
- Ide H, Kariyasaki A, FukanoT (2007) Fundamental data on the gas-liquid two-phase flow in Minichannels. International Journal of Thermal Sciences 46(6): 519-530.
- McQuillan K, Whalley P (1985) Flow patterns in vertical two-phase flow. International Journal of Multiphase Flow 11(2): 161-175.
- Angeli P, Gavriilidis A (2008) Hydrodynamics of Taylor flow in small channels: A Review. Proceedings of the Institution of Mechanical Engineers Part C: Journal of Mechanical Engineering Science 222(5): 737-751.
- Chinnov E, Ron’shin F, Kabov OA (2015) Regimes of two-phase flow in micro-and Minichannels. Thermophysics and aeromechanics 22(3): 265-284.
- Haase S, Murzin DY, Salmi T (2016) Review on hydrodynamics and mass transfer in minichannel wall reactors with gas–liquid Taylor flow. Chemical Engineering Research and Design 113: 304-329.
- Durgadevi A, Pushpavanam S (2018) An experimental and theoretical investigation of pure carbon dioxide absorption in aqueous sodium hydroxide in glass millichannels. Journal of CO2 Utilization 26: 133-142.
- Prakash S, otIo ME Kumar SJP (2015) Fabrication of microchannels: A Review. Part B: Journal of Engineering Manufacture 229(8): 1273-1288.
- Zhu C, Li C, Gao X, Ma Y, Liu D (2014) Taylor flow and mass transfer of CO2 chemical absorption into MEA aqueous solutions in a T-junction microchannel. International Journal of Heat and Mass Transfer 73: 492-499.
- Sattari Najafabadi M, Esfahany MN, Wu Z, Sunden B (2018) Mass transfer between phases in microchannels: A Review. Chemical Engineering and Processing-Process Intensification 127: 213-237.
- Tan J, Lu Y, Xu J, Luo G (2012) Mass transfer performance of gas-liquid segmented flow in microchannels. Chemical Engineering Journal 181(1): 229-235.
- Yue J, Chen G, Yuan Q, Luo L, Gonthier Y (2007) Hydrodynamics and mass transfer characteristics in gas–liquid flow through a rectangular microchannel. Chemical Engineering Science 62(7): 2096-2108.
- Sowiński J, Dziubiński M, Fidos H (2009) Velocity and gas-void fraction in two-phase liquid-gas flow in narrow mini-channels. Archives of Mechanics 61(1): 29-40.
- Abiev RSh, Dymov AV (2013) Modeling the hydrodynamics of slug flow in a Minichannels for liquid-liquid two-phase system. Theoretical Foundations of Chemical Engineering 47(4): 299-305.
- Ganapathy H, Al Hajri E, Ohadi M (2013) Mass transfer characteristics of gas-liquid absorption during Taylor flow in mini/microchannel reactors. Chemical Engineering Science 101: 69-80.
- Shao N, Gavriilidis A, Angeli P (2010) Mass transfer during Taylor flow in microchannels with and without chemical reaction, Chemical Engineering Journal 160(3): 873-881.
- Abiev RS (2008) Simulation of the slug flow of a gas-liquid system in capillaries. Theoretical Foundations of Chemical Engineering 42(2): 105-117.
- Edel JB, Fortt R (2002) Microfluidic routes to the controlled production of nanoparticles. Chemical Communications 21(10): 1136-1137.
- Inoue T, Kikutani Y, Hamakawa S, Mawatari K, Mizukami F, et al. (2010) Reactor design optimization for direct synthesis of hydrogen peroxide. Chemical Engineering Journal 160(3): 909-914.
- Takizawa E, Nagaki A, Yoshida Ji (2012) Flow microreactor synthesis of tricyclic sulfonamides via N-tosylaziridinyllithiums. Tetrahedron Letters 53(11): 1397-1400.
- Iwasaki T, Yoshida Ji (2005) Free radical polymerization in microreactors. Significant improvement in molecular weight distribution control. Macromolecules 38(4): 1159-1163.
- Madhawan A, Arora A, Das J, Kuila A, Sharma V, et al. (2017) Microreactor technology for biodiesel production: A review. Biomass Conversion and Biorefinery.
- Li W, Wu Z (2010) A general criterion for evaporative heat transfer in micro/mini-channels, International Journal of Heat and Mass Transfer 53(9-10): 1967-1976.
- Hapke I, Boye H, Schmidt J (2000) Onset of nucleate boiling in Minichannels. International Journal of Thermal Sciences 39(4): 505-513.
- Garimella S (2006) Condensation in Minichannels and microchannels. Heat Transfer and Fluid Flow in Minichannels and Microchannels, pp. 227-408.
- Vandu CO, Liu H, Krishna R (2005) Mass transfer from Taylor bubbles rising in single capillaries, 60(22): 6430-6437.
- Yue J, Luo L, Gonthier Y, Chen G, Yuan Q (2009) An experimental study of air-water Taylor flow and mass transfer inside square microchannels. Chemical Engineering Science 64(16): 3697-3708.
- Heiszwolf JJ, Kreutzer MT, Van Den Eijnden MG, Kapteijn F, Moulijn JAJCT (2001) Gas-liquid mass transfer of aqueous Taylor flow in monoliths. Catalysis Today 69(1-4): 51-55.
- Koytsoumpa EI, Bergins C, Kakaras EJTJoSF (2018) The CO2 economy: Review of CO2 capture and reuse technologies. The journal of Supercritical Fluids 132: 3-16.
- Olajire AA (2010) CO2 capture and separation technologies for end-of-pipe applications-a review. Energy 35(6): 2610-2628.
- Yang Z, Khan TS, Alshehhi M, AlWahedi YF (2018) Economic assessment of carbon capture by minichannel absorbers. AIChE Journal 64(2): 620-631.
- Aoki N, Tanigawa S, Mae K (2011) Design and operation of gas-liquid slug flow in miniaturized channels for rapid mass transfer. Chemical engineering science 66(24): 6536-6543.
- Tan J, Lu Y, Xu J, Luo G (2012) Mass transfer characteristic in the formation stage of gas-liquid segmented flow in microchannel. Chemical Engineering Journal 185-186: 314-320.
- Rao AB, Rubin ES (2002) A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environmental science & technology 36(20): 4467-4475.
- Yu CH, Huang CH, Tan CS (2012) A review of CO2 capture by absorption and adsorption. Aerosol Air Quality Research 12: 745-769.
- Dutcher B, Fan M, Russell AGJAam (2015) Interfaces, Amine-based CO2 capture technology development from the beginning of 2013 A Review. ACS Publications 7(4): 2137-2148.
- Yao C, Zhu K, Liu Y, Liu H, Jiao F, et al. (2017) Intensified CO2 absorption in a microchannel reactor under elevated pressures. Chemical Engineering Journal 319: 179-190.
- Bangerth S, Ganapathy H, Ohadi M, Khan TS, Alshehhi M (2014) Study of CO2 Absorption Into Aqueous Diethanolamine (DEA) Using Microchannel Reactors. in: ASME 2014 International Mechanical Engineering Congress and Exposition American Society of Mechanical Engineers pp V06AT07A010-V006AT007A010.
- Lin G, Jiang S, Zhu C, Fu T, Ma Y (2019) Mass transfer characteristics of CO2 absorption into aqueous solutions of N-methyldiethanolamine+ diethanolamine in a T-junction microchannel. ACS Sustainable Chemistry & Engineering.
- Chunbo Y, Guangwen C, Quan Y (2012) Process characteristics of CO2 absorption by aqueous monoethanolamine in a microchannel reactor. Chinese Journal of Chemical Engineering 20(1): 111-119.
- Kundu A, Basu J, Das G (2012) A novel gas-liquid contactor for chemisorption of CO2. Separation and purification technology 94(9): 115-123.
- Yang Z, Khan TS, Alshehhi MAlWahedi YF (2016) Mass Transfer Characterization of Chemical Absorption Of CO2 In Microchannel Absorbers.
- Pan MY, Qian Z, Shao L, Arowo M, Chen JF, et al. (2014) Absorption of carbon dioxide into N-methyldiethanolamine in a high-throughput microchannel reactor. Separation and Purification Technology 125: 52-58.
- Liu H, Yao C, Zhao Y, Chen G (2017) Desorption of carbon dioxide from aqueous MDEA solution in a microchannel reactor. Chemical Engineering Journal 307: 776-784.
- Rhee CH, Kim JY, Han K, Ahn CK, Chun HDJEP (2011) Process analysis for ammonia-based CO2 capture in ironmaking industry. Energy Procedia 4: 1486-1493.
- Kittiampon N, Kaewchada A, Jaree A (2017) Carbon dioxide absorption using ammonia solution in a microchannel. International Journal of Greenhouse Gas Control 63: 431-441.
- Ji X, Ma Y, Fu T, Zhu C, Wang D (2010) Experimental investigation of the liquid volumetric mass transfer coefficient for upward gas-liquid two-phase flow in rectangular microchannels. Brazilian Journal of Chemical Engineering 27: 573-582.
- Sun R, Cubaud T (2011) Dissolution of carbon dioxide bubbles and microfluidic multiphase flows, Lab on a Chip 11(17): 2924-2928.
- Knuutila H, Svendsen HFAnttila (2009) CO2 capture from coal-fired power plants based on sodium carbonate slurry; a systems feasibility and sensitivity study. International Journal of Greenhouse Gas Control 3(2): 143-151.
- Astarita G, Savage DW, Longo JMJCES (1981) Promotion of CO2 mass transfer in carbonate solutions. Chemical Engineering Secience 36(3): 581-588.
- Cents A, Brilman DWF, Versteeg GJCES (2005) CO2 absorption in carbonate/bicarbonate solutions: The Danckwerts-criterion revisited. Chemical Engineering Secience 60(21): 5830-5835.
- Vericella JJ, Baker SE, Stolaroff JK, Duoss EB, Hardin IV JO, et al. (2015) Encapsulated liquid sorbents for carbon dioxide capture. Nature Communications 6: 6124.
- Sobieszuk P, Cygański P, Pohorecki Rte. (2008) Volumetric liquid side mass transfer coefficient in a gas-liquid microreactor. Chemical and Process Engineering 29: 651-661.
- Sobieszuk P, Pohorecki R, Cygański P, Grzelka J (2011) Determination of the interfacial area and mass transfer coefficients in the Taylor gas-liquid flow in a microchannel. Chemical engineering science 66(23): 6048-6056.
- Sarmad S, Xie Y, Mikkola JP, Ji X (2017) Screening of Deep Eutectic Solvents (DESs) as green CO2 sorbents: from solubility to viscosity. New Journal of Chemistry 41(1): 290-301.
- Kamgar A, Rahimpour MR (2016) Prediction of CO2 solubility in ionic liquids with QM and UNIQUAC models. Journal of Molecular Liquids 222: 195-200.
- Baghban A, Mohammadi AH, Taleghani MS (2017) Rigorous modeling of CO2 equilibrium absorption in ionic liquids. International Journal of Greenhouse Gas Control 58: 19-41.
- Aghaie M, Rezaei N, Zendehboudi S (2018) A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renewable and Sustainable Energy Reviews 96: 502-525.
- Yang Q, Wang Z, Bao Z, Zhang Z, Yangn Y et al. (2016) New Insights into CO2 Absorption Mechanisms with Amino‐Acid Ionic Liquids. ChemSusChem 9(8): 806-812.
- Yin Y, Fu T, Zhu C, Guo R, Ma Y et al. (2019) Dynamics and mass transfer characteristics of CO2 absorption into MEA/[Bmim][BF4] aqueous solutions in a microchannel. Separation and Purification Technology 210(8): 541-552.
- Qin K, Wang K, Luo R, Li Y, Wang T (2018) Dispersion of supercritical carbon dioxide to [Emim][BF4] with a T-junction tubing connector. Chemical Engineering and Processing-Process Intensification 127: 58-64.
- Tonkovich ALY, Litt RD, Dongming Q, Silva LJ, Lamont MJ et al. (2011) Methods for applying microchannels to separate methane using liquid absorbents, especially ionic liquid absorbents from a mixture comprising methane and nitrogen.
- Shah MS, Tsapatsis M, Siepmann JI (2017) Hydrogen sulfide capture: From absorption in polar liquids to oxide, zeolite, and metal-organic framework adsorbents and membranes. Chem Rev 117(14): 9755-9803.
- Su H, Wang S, Niu H, Pan L, Wang A et al. (2010) Mass transfer characteristics of H2S absorption from gaseous mixture into methyldiethanolamine solution in a T-junction microchannel. Separation and Purification Technology 72(3): 326-334.
- Pan MY, Li T, Zhou Y, Qian Z, Shao L et al. (2015) Selective absorption of H2S from a gas mixture with CO2 in a microporous tube-in-tube microchannel reactor. Chemical Engineering and Processing: Process Intensification 95:135-142.
- Molhave LJIA (1991) Volatile organic compounds, indoor air quality and health. International Journal of Indoor Environment and Health 1(4): 357-376.
- Das D, Gaur V, Verma N (2004) Removal of volatile organic compound by activated carbon fiber. Carbon 42(14): 2949-2962.
- Brosillon S, Manero MH, Foussard JN (2001) Mass transfer in VOC adsorption on zeolite: experimental and theoretical breakthrough curves. Environ Sci Technol 35(17): 3571-3575.
- Yang K, Xue F, Sun Q, Yue R, Lin D (2013) Adsorption of volatile organic compounds by metal-organic frameworks MOF-177. Journal of Environmental Chemical Engineering 1(4): 713-718.
- Van der Vaart D, Vatvuk W, Wehe A (1991) Thermal and catalytic incinerators for the control of VOCs. Journal of the Air & Waste Management Association 41(1): 92-98.
- Zhiliang LRXJX, Xiuyun WLLJS (2008) Membrane-based gas absorption for separation of VOCs/N. Chinese Journal of Environmental Engineering.
- Edwards FG, Nirmalakhandan N (1996) Biological treatment of airstreams contaminated with VOCs: An overview. Water Science and technology 34(3-4): 565-571.
- Hadjoudj R, Monnier H, Roizard C, Lapicque F (2004) Absorption of chlorinated VOCs in high-boiling solvents: determination of Henry's law constants and infinite dilution activity coefficients. Ind Eng Chem Res 43(9): 2238-2246.
- Hadjoudj R, Monnier H, Roizard C, Lapicque FJI (2004) Absorption of chlorinated VOCs in high-boiling solvents: Determination of Henry's law constants and infinite dilution activity coefficients. Ind Eng Chem Res 43(9): 2238-2246.
- Gossett JMJES (1987) Technology, Measurement of Henry's law constants for C1 and C2 chlorinated hydrocarbons. Environ Sci Technol 21(2): 202-208.
- Monnier H, Mhiri N, Falk L (2010) Falling liquid film stability in microgas/liquid absorption. Chemical Engineering and Processing: Process Intensification 49(9): 953-957.
- Mhiri N, Monnier H, Ces Falk LJ (2011) Intensification of the G/L absorption in microstructured falling film application to the treatment of chlorinated VOC's. Part III: Influence of gas thickness channel on mass transfer. Chemical Engineering Science 66(23): 5989-6001.
- Kockmann N, Gottsponer, Cej Roberge DMJ (2011) Scale-up concept of single-channel microreactors from process development to industrial production. The Chemical Engineering Journal 167(2): 718-726.
- Tonkovich A, Kuhlmann D, Rogers A, McDaniel J, Fitzgerald S (2005) Microchannel technology scale-up to commercial capacity. Chemical Engineering Research and Design 83(6): 634-639.
- Yue J, Boichot R, Luo L, Gonthier Y, Chen G, (2010) Flow distribution and mass transfer in a parallel microchannel contactor integrated with constructal distributors. AIChE journal 56(2): 298-317.
- Constantinou A, Gavriilidis A (2009) CO2 absorption in a microstructured mesh reactor. Ind Eng Chem Res 49(3): 1041-1049.
- Ganapathy H, Steinmayer S, Shooshtari A, Dessiatoun S, Ohadi MM (2016) Process intensification characteristics of a microreactor absorber for enhanced CO2 Applied energy 162(C): 416-427.
- Zhang J, Wang K, Teixeira AR, Jensen KF, Luo Gs (2017) Design and scaling up of microchemical systems: a review. Annual review of chemical and biomolecular engineering 8: 285-305.
- Haase S, Bauer T, Lange RJCOCT (2015) Numbering-up of mini-and microchannel contactors and reactors 33: 2.
- Kashid MN, Gupta A, Renken A, Kiwi Minsker LJCEJ (2010) Numbering-up and mass transfer studies of liquid–liquid two-phase microstructured reactors, Chemical Engineering Journal 158(2010): 233-240.
- Charpentier JC (1981) Mass-transfer rates in gas-liquid absorbers and reactors. Advances in chemical engineering 11: 1-133.
- Liu D, Wang S (2010) Gas-Liquid Mass Transfer in Taylor Flow through Circular Capillaries. Industrial & Engineering Chemistry Research 50(4): 2323-2330.
- Ganapathy H, Shooshtari A, Dessiatoun S, Ohadi M, Alshehhi M (2015) Hydrodynamics and mass transfer performance of a microreactor for enhanced gas separation processes. Chemical Engineering Journal 266(15): 258-270.
- Bercˇicˇ G, Pintar A (1997) The role of gas bubbles and liquid slug lengths on mass transport in the Taylor flow through capillaries. Chemical Engineering Science 52(21-22): 3709-3719.
- Irandoust S, Ertlé S, Andersson B (1992) Gas‐liquid mass transfer in Taylor flow through a capillary. The Canadian Journal of Chemical Engineering 70(1): 115-119.
- Abolhasani M, Kumacheva E, Günther A (2015) Peclet number dependence of mass transfer in microscale segmented gas-liquid flow. Industrial & Engineering Chemistry Research 54(36): 9046-9051.
- Yao C, Dong Z, Zhao Y, Chen G (2014) An online method to measure mass transfer of slug flow in a microchannel. Chemical Engineering Science 112: 15-24.
- Laborie S, Cabassud C, Durand Bourlier L, Laine JJCESS (1999) Characterisation of gas-liquid two-phase flow inside capillaries. Chemical Engineering Science 54(23): 5723-5735.
- Kreutzer MT (2003) Hydrodynamics of Taylor flow in capillaries and monolith reactors.
- Liu H, Vandu CO, Krishna R (2005) Hydrodynamics of Taylor flow in vertical capillaries: Flow regimes, bubble rise velocity, liquid slug length, and pressure drop. Industrial & engineering chemistry research 44(14): 4884-4897.
- Qian D, Lawal A (2006) Numerical study on gas and liquid slugs for Taylor flow in a T-junction microchannel. Chemical Engineering Science 61(23): 7609-7625.
- Shao N, Salman W, Gavriilidis A, Angeli P (2008) CFD simulations of the effect of inlet conditions on Taylor flow formation. International journal of heat and fluid flow 29(6): 1603-1611.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...