Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6628
Research Article(ISSN: 2637-6628) 
The Relationship of the Trigemino-Cardiac Reflex to Sleep Bruxism Volume 1 - Issue 2
Ken Luco*
- Department of Dental Surgery, University of Alberta, Canada
Received: March 23, 2018; Published: April 06, 2018
Corresponding author: Ken Luco, Department of dental surgery, University of Alberta, Canada
DOI: 10.32474/OJNBD.2018.01.000106
Abstract
The trigemino-cardiac reflex is well researched in medicine. A newly proposed classification based upon the region stimulated elucidates sleep bruxism’s paradoxical effect on the trigemino-cardiac reflex, characterized by tachycardia, hyperpnea and hypertension. This article discusses this complex relationship and the resulting signs and symptoms reported in sleep bruxism, and how it meets these new criterion.
Keywords: Trigemino-cardiac reflex; Trigeminal cardiac reflex; Masseter inhibitory reflex; Sleep bruxism
Abbreviations: TCR: Trigemino Cardiac Reflex; MIR: Masseter Inhibitory Reflex; SB: Sleep Bruxism; GG: Gasserion (trigeminal) Ganglion; GERD: Gastro-Esophageal Reflux Disorder; OSA: Obstructive Sleep Apnea; RF: Reticular Formation; TMD: Temporomandibular Dysfunction; HR: Heart Rate (pulse); MABP Maximum Arterial Blood Pressure; 5-HT: Serotonin
Introduction
The trigemino-cardiac reflex (TCR) is a unique and powerful brainstem reflex that has received a great deal of research interest. Sleep bruxism (SB) is sleep disorder that affects the TCR as well as other brainstem reflexes via stimulation of the brainstem; at the level of the gasserion ganglion (GG). This paper will discuss the unusual relationship of the TCR and SB in addition to how well sleep bruxism meets a new proposed classification system for TCR activation.
Literary Research
Relevant literature was identified through searching PubMed; Research Gate; Google scholar database and Mendeley web library using the search terms “trigemino-cardiac reflex”; “sleep bruxism”; “GERD”; and “masseter inhibitory reflex”.
Discussion
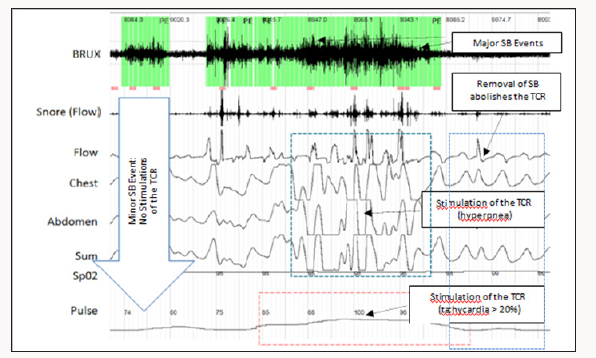
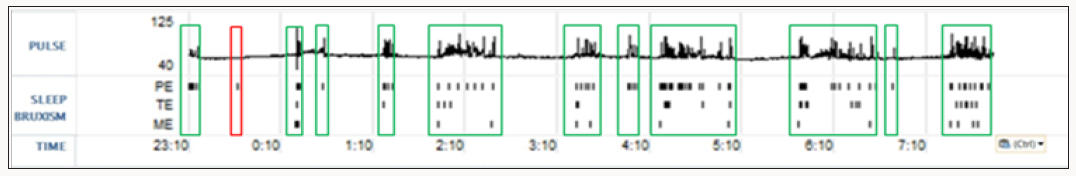
Sleep disorders are an increasing health problem in all countries that have a direct effect on quality of life and productivity/safety of workers [1-3]. Sleep bruxism (SB) is a movement type sleep disorder characterized by transient tachycardia; tachypnea and hypertension occurring slightly before; during or slightly after the bruxism event; resolving immediately after the event ceases [4].
This increase in sympathetic activity has been shown to result from stimulation of the TCR at the level of the gasserion ganglion (GGa) [5-7]. SB is seen to occur concurrently with sleep apnea; occurring immediately before or after apnea events; but may also be seen on EMG studies independently of OSA [8]. SB also results in micro-arousals from sleep; classifying it as a true sleep disorder. Epworth Sleepiness Scale scores of 4 to 9 are characteristic of SB whereas scores of 10 and higher are suggestive of OSA4. The daytime sleepiness (males) and tiredness (females) seen in SB have similar deleterious effects on alertness; productivity and quality of life to OSA. In addition; SB is associated with the inception of chronic myofascial pain [9] affecting the orofacial region; tension and migraine type headaches; and temporomandibular dysfunction syndrome (TMD) affecting the temporomandibular joints [9-10].
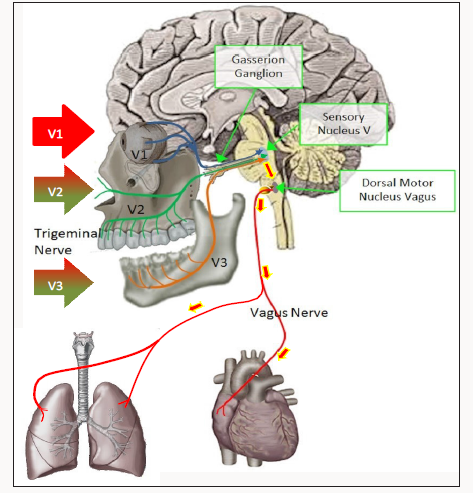
The TCR is a powerful brainstem reflex that manifests as a sudden onset of hemodynamic influences on heart rate (HR); blood pressure (MABP) and has been associated with cardiac arrhythmias; asystole; apnea and gastric mobility [11]. It is an oxygen-conserving reflex that was first discovered in 1999 [12]; with considerable research ensuing [13-17]. The reflex may be activated by mechanical or chemical stimulation of the trigeminal nerve at any course along its distribution. Stimulation of the TCR results in neuronal signals being transmitted via the trigeminal nerve to the GGa; continuing to the sensory nucleus of the trigeminal nerve (V5) in the brain stem (mesencephalic nucleus). Signals are then transmitted polysynaptically through the reticular formation (RF); via short internucial fibers; to the dorsal motor nucleus of the vagus nerve (X). This pathway is considered as an afferent to the TCR (Figures 1-3). Parasympathetic neurons comprise much of reflex; arising in the motor nucleus of V5. Stimulation of V5 results in bradycardia; hypotension; as well as apnea and gastric hypermobility [17]. The reflex is of utmost importance during surgical procedures adjacent to the branches of the trigeminal nerve as the TCR can inadvertently be stimulated compromising the surgical procedure [18-21]. In procedures near or in the GGa; the opposite effect may be encountered: tachycardia; tachypnea and hypertension and gastric hypomobility [22,23].
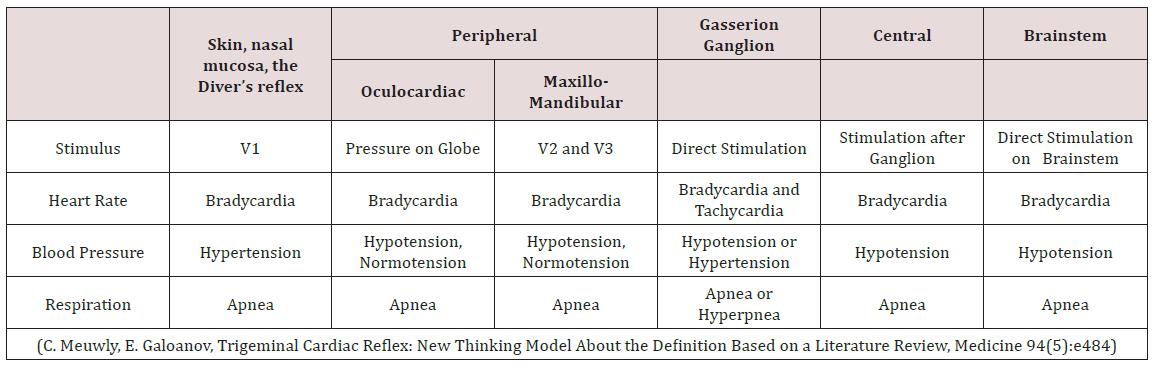
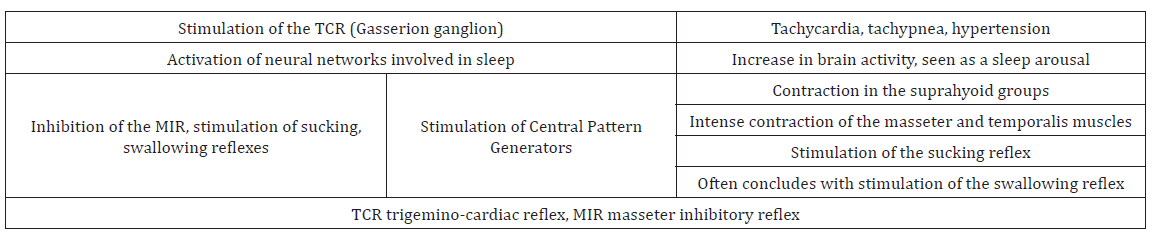
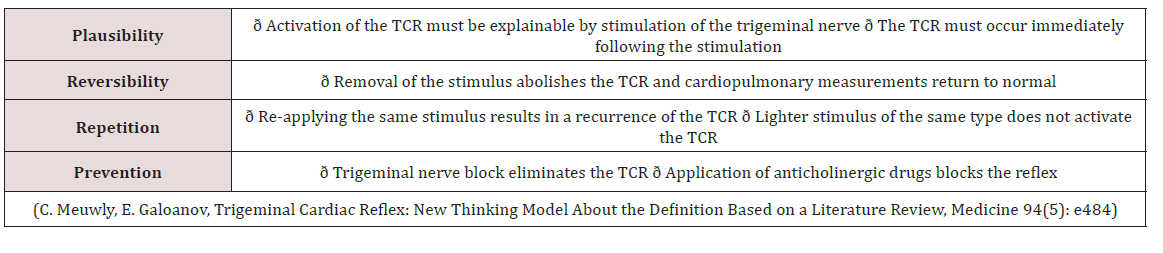
Meuwly; Galoanov et al have proposed a new classification for the TCR based upon where the trigeminal nerve is stimulated (Table 1) [17]. The classic “diver’s reflex” results from stimulation at the level of the first branch of the trigeminal nerve and the typical TCR response of bradycardia; hypotension and hypopnea. It is a cutaneous stimulation of the trigeminal nerve; on the ocular and nasal skin regions. The peripheral classification includes the actual orbit of the eye (by compressing the orbit); the maxillary branch (V2) and the mandibular branch (V3). Only the maxillary and mandibular stimulation regions result in the paradoxical sympathetic response of tachycardia; tachypnea and hypertension (Figures 1-3). In this classification; the only region of stimulation of the TCR occurs at the level of the GGa; all other regions react as expected. This stimulation at the level of the Gasserion ganglion can result in paroxysmal brady or tachycardia; hyper or hypotension and apnea or hyperpnea; depending upon the stimulus. A recent study described this phenomenon and concluded from the research that HR was the most significantly affected and the most useful measurement in the determination of TCR [24]. A diagnostic criterion that is generally accepted is of a heart rate decrease (or increase) of 20% or more from baseline; for a positive TCR diagnosis [17]. Table 2 lists other cause-effect relationship criterion proposed to diagnose and differentiate TCR proposed by Meuwly et al [17]. Sleep bruxism meets the first five of seven criteria of Table 2. GGa nerve blocks have an elevated risk profile and do not meet ethical standards for trials; and anticholinergic drugs fail to suppress the TGR effects of SB. By utilizing this additional criterion as well as the decrease (or increase in the case of SB) of HR by greater than 20% ; discrimination of other sympathetic and parasympathetic disorders that could affect HR and mimic the TCR is possible; rendering a more accurate diagnosis of this condition [24].
Table 2: Additional Criterion based upon Cause and Effect (2 Positive Criterion = Positive TCR Stimulation).
Research has also found a genetic commonalty in SB sufferers: a polymorphism of the HTR2A gene on chromosome [13-25,26]. This gene codes for 5-HT (serotonin) receptors in the brain and gut. The masseter inhibitory reflex (MIR); another brainstem reflex; is located in the mesencephalic nucleus and has the MIR reflex nucleus affected by this polymorphism [27,28]. The resulting hypersensitivity to serotonin results in loss of inhibition by the MIR as well as activation of central pattern generators involved with chewing. In SB; extreme muscle contractions of the masseter; temporalis and suprahyoid muscle groups result [29]. The HTR2A polymorphism; seen in SB; was found to occur frequently in other conditions including obstructive sleep apnea; tension headaches; migraine headaches (without auras) and a myriad of psychiatric disorders [30-33]. Interestingly; OSA has headaches as a commonly reported symptom; often upon waking (assumed to be due to hypercapnia). In recent studies of the TCR it was demonstrated that; at the connection at the level of the reticular formation and nucleus ambiguus; there appeared to be an endogenous modulation. It was also found that the 5-HTR1A and 5-HTR2A serotonin reception genes were involved in mediation (stimulation; depression) of the connections (antagonists altered the TCR) [17]. With the 5-HTR2A polymorphism known to exist in SB; it is reasonable to assume that this and other regions could also be similarly affected (potentially resulting in hypersensitivity of the TCR).
During SB bursts; there is an ensuing cascade of events that occurs (Table 3). There are a number of cranial reflexes involved including the TCR; the MIR; the sucking reflex 4 (which can result in trauma of the buccal mucosa and development of linea alba lesions); and the swallowing reflex [34-36]. Scalloped borders of the tongue are also a common finding in SB; resulting from activation of the genioglossus muscle pressing the tongue forcibly against the teeth [37,38]. In other research it has been shown that there is a commonalty between SB and gastro-esophageal reflux disorder (GERD); supporting SB’s influence on the vagus nerve [39,40]. This can manifest as tooth erosions on the inner or lingual surfaces of the teeth and increase susceptibility to dental caries [41].
With the suppression of the MIR; the forces generated during SB events far exceed those of normal chewing [29]. Damage to the teeth is common including:
a) Sensitive; loose or broken teeth [44]
b) Abfraction lesions [44]
c) Accelerated periodontal disease [45]
d) Formation of mandibular tori [46]
e) Eagle’s syndrome (calcification of the stylohyoid ligaments) [47]
f) Elongation of the coronoid processes [48]
g) Excessive compression of the TMJ; often initiating or accelerating degenerative changes [49-51].
As expected; these forces can be a contraindication to some dental procedures including porcelain crowns and inlays [52,53] and dental implants [54,55].
Conclusion
When considering how SB affects the TCR and MIR; the range of signs and symptoms observed during SB events can be readily interpreted. Restless leg syndrome (RLS) is another movement type sleep disorder that results in activation of the TCR similar to SB. Tachycardia; hyperpnea and hypertension (TCR stimulation); shown to also occur in RLS; has been shown to be a risk factor for heart disease in a number of studies [56,57]. To date SB has not been studied to the extent of RLS and it is not known at this time if SB is also a risk factor for heart disease. With the significant effect of SB on the TCR; Further research is certainly warranted.
References
- Carol M Baldwin, Kent A Griffith, Javier Nieto, O Connor GT, Walsleben JA, et al. (2001) The Association of Sleep-Disordered Breathing and Sleep Symptoms with Quality of Life in the Sleep Heart Health Study. Sleep 24(1): 96-105.
- Engleman H Douglas N, Thorax (2004) Sleep. 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnea syndrome 59 (7): 618-622.
- Sassani A, Findley L, Kryger M, Goldlust E, George C, et al. (2004) Reducing Motor-Vehicle Collisions, Costs, and Fatalities by Treating Obstructive Sleep Apnea Syndrome. Sleep 27(3): 453-458.
- Sateia MJ (2014) International classification of sleep disorders-third edition: highlights amd modifications. American Academy of Sleep Medicine 146(5): 1387-1394.
- Chowdhury T, Mendelowith D, Golanov E, Spiriev T, Arasho B, et al. (2015) Trigeminocardiac reflex: The current clinical and physiological knowledge. Journal of Neurosurgical Anesthesiology 27(2): 136-147.
- Chowdhury T, Bindu B, Singh G, Schaller B (2017) Sleep Disorders: Is the Trigemino-Cardiac Reflex a Missing Link? Front Neurolo 8: 63.
- Huynh N, Kato T, Rompré PH, Okura K, Saber M, et al. (2006) Sleep bruxism is associated to micro-arousals and an increase in cardiac sympathetic activity. J Sleep Res 15(3): 339-346.
- Oksenberg A, Arons E (2002) Sleep bruxism related to obstructive sleep apnea: the effect of continuous positive airway pressure, Sleep Medicine 3(6): 513-515.
- Schmitter M, Kares-Vrincianu A, Kares H, Bermejo JL, Schindler HJ (2015) Sleep-associated aspects of myofascial pain among Temporomandibular Disorder patients and controls. Sleep Medicine 16(9): 1056-1061.
- Fernandes G, Lucia Franco A, Gonçalves DA, Speciali JG, Bigal ME, et al. (2013) Temporomandibular disorders, sleep bruxism, and primary headaches are mutually associated. J Orofac Pain 27(1): 14-20.
- Schaller B, Cornelieus J, Prabhakar H, Koerbel A, Gnanalingham K, et al. (2009) The Trigemino-cardiac Reflex: An Update of the Current Knowledge. Journal of Neurosurgical Anesthesiology 21(3): 187-195.
- Sandu N Schaller B (2010) The trigemino-cardiac reflex: a view to the future. Arch Med Sci 16(1): 138-139.
- Schaller B, Chowdhury T, Rosemann T (2017) Editorial: The Trigeminocardiac Reflex: Beyond the Diving Reflex. Front Neurosci 1(11): 673.
- Meuwly C, Chowdhury T, Gelpi R, Erne P, Rosemann T, et al. (2017) The clinical surrogate definition of the trigeminocardiac reflex: Development of an optimized model according to a PRISMA-compliant systematic review. Medicine (Baltimore) 96(49).https://www.researchgate.net/publication/321653736_The_clinical_surrogate_definition_of_the_trigeminocardiac_reflex_Development_of_an_optimized_model_according_to_a_PRISMA-compliant_systematic_review
- Barloese M, Petersen AS, Guo S, Ashina M, Mehlsen J, et al. (2017) Sphenopalatine ganglion stimulation induces changes in cardiac autonomic regulation in cluster headache. Clin Physiol Funct Imaging.
- Lunt JM, Al Taee A, Rawal R, Buckhold FR (2017) Trigeminocardiac Reflex as the Presentation of Maxillary Sinus Adenocarcinoma. Am J Med 130(11): 505-506.
- Meuwly C, Chowdhury T, Sandu N, Golanov E, Erne P, et al. (2017) Definition and Diagnosis of the Trigeminocardiac Reflex: A Grounded Theory Approach for an Update. Front Neurol 9(8): 533.
- Shibao S, Kenawy K, Borghei Razavi H, Yoshida K (2017) The Trigeminocardiac Reflex During the Anterior Transpetrosal Approach. World Neurosurg 106: 939-944.
- Wang CM, Guan ZY, Zhang J, Cai CH, Pang QG, et al. (2015) Comparative study of trigeminocardiac reflex after trigeminal ganglion compression during total intravenous anesthesia. J Neurosurg Anesthesiol 27(1): 16- 20.
- Bhargava D, Thomas S, Chakravorty N, Dutt A (2014) Trigeminocardiac Reflex: A Reappraisal with Relevance to Maxillofacial Surgery. J Maxillofac Oral Surg 13(4): 373-377.
- Krishnan B (2011) Re: classification of potential risk factors for trigeminocardiac reflex in craniomaxillofacial surgery. J Oral Maxillofac Surg 69(4): 960.
- Arakeri G, Brennan PA (2010) Trigeminocardiac reflex: potential risk factor for syncope in exodontia? J Oral Maxillofac Surg 68(11): 2921- 2922.
- Seker A, Toktas ZO, Peker S, Batirel HA, Necmettin Pamir M (2009) Asystole due to trigemino-cardiac reflex: a rare complication of transsphenoidal surgery for pituitary adenoma. J Clin Neurosci 16(2): 338- 340.
- Schaller B (2005) Trigemino-cardiac reflex during microvascular trigeminal decompression in cases of trigeminal neuralgia. J Neurosurg Anesthesiol 17(1): 45-48.
- Meuwly C, Chowdhury T, Gelpi R, Erne P, Rosemann T, et al. (2017) The clinical surrogate definition of the trigeminocardiac reflex: Development of an optimized model according to a PRISMA-compliant systematic review. Medicine (Baltimore) 96(49).
- Bornhardt T, Iturriaga V, Salazar LA (2016) Genetic polymorphisms in the serotonergic system are associated with circadian manifestations of bruxism. Oporto GH 5th J Oral Rehabil 43(11): 805- 812.
- Abe Y, Suganuma T, Ishii M, Yamamoto G, Gunji T, et al. (2012) Association of genetic, psychological and behavioral factors with sleep bruxism in a Japanese population. J Sleep Res 21(3): 289-296.
- Hoashi Y, Okamoto S, Abe Y, Matsumoto T, Tanaka J, et al. (2017) Generation of neural cells using iPSCs from sleep bruxism patients with 5-HT2A polymorphism. J Prosthodont Res 61(3): 242-250.
- Minakuchi H, Sogawa C, Miki H, Hara ES, Maekawa K, et al. (2016) Sleep bruxism frequency and platelet serotonin transporter activities in young adult subjects. Sleep Breath 20(1): 271-276.
- Goiato MC, Zuim PRJ, Moreno A, Dos Santos DM, da Silva EVF, et al. (2017) Does pain in the masseter and anterior temporal muscles influence maximal bite force? Arch Oral Biol 83: 1-6.
- (2016) American Academy of Neurology. “Migraine, tension headaches and irritable bowel syndrome linked?.” ScienceDaily.
- Sundararajan T, Manzardo AM, Butler MG (2018) Functional analysis of schizophrenia genes using Gene Analytics program and integrated databases. Gene 641: 25-34.
- Gray JC, MacKillop J, Weafer J, Hernandez KM, Gao J, et al. (2018) Genetic analysis of impulsive personality traits: Examination of a priori candidates and genome-wide variation. Psychiatry Res 259: 398-404.
- Shi Y, Li M, Song C, Xu Q, Huo R, et al. (2017) Combined study of genetic and epigenetic biomarker risperidone treatment efficacy in Chinese Han schizophrenia patients. Transl Psychiatry 11(7): 7.
- İnan R, Şenel GB, Yavlal F, Karadeniz D, Gündüz A, et al. (2017) Sleep bruxism is related to decreased inhibitory control of trigeminal motoneurons, but not with reticulobulbar system. Neurol Sci 38(1): 75- 81.
- Huang H, Song YH, Wang JJ, Guo Q, Liu WC (2014) Excitability of the central masticatory pathways in patients with sleep bruxism. Neurosci Lett 558: 82-86.
- Gastaldo E, Quatrale R, Graziani A, Eleopra R, Tugnoli V, et al. (2006) The excitability of the trigeminal motor system in sleep bruxism: a transcranial magnetic stimulation and brainstem reflex study. J Orofac Pain 2006 Spring 20(2): 145-155.
- Pittman LJ, Bailey EF (2009) Genioglossus and intrinsic electromyographic activities in impeded and unimpeded protrusion tasks. J Neurophysiol 101(1): 276-82.
- Blumen MB, de La Sota AP, Quera Salva MA, Frachet B, Chabolle F, et al. (2004) Respir Physiol Neurobiol Tongue mechanical characteristics and genioglossus muscle EMG in obstructive sleep apnoea patients 140(2): 155-164.
- Castroflorio T, Bargellini A, Rossini G, Cugliari G, Deregibus A (2017) Sleep bruxism and related risk factors in adults: A systematic literature review. Arch Oral Biol 83: 25-32.
- Ohmure H, Kanematsu Hashimoto K, Nagayama K, Taguchi H, Ido A, et al. (2016) Evaluation of a Proton Pump Inhibitor for Sleep Bruxism: A Randomized Clinical Trial. J Dent Res 95(13): 1479-1486.
- Moshaverinia A, Kar K, Aalam AA, Takanashi K, Kim JW, et al. (2014) A multidisciplinary approach for the rehabilitation of a patient with an excessively worn dentition: a clinical report. J Prosthet Dent 111(4): 259-263.
- Palinkas M, De Luca Canto G, Rodrigues LA, Bataglion C, Siéssere S, et al. (2015) Comparative Capabilities of Clinical Assessment, Diagnostic Criteria, and Polysomnography in Detecting Sleep Bruxism. J Clin Sleep Med 11(11): 1319-1325.
- Nikolaos Tsiggos N, Tortopidis D, Hatzikyriakos A, Menexes G (2008) Association between self-reported bruxism activity and occurrence of dental attrition, abfraction, and occlusal pits on natural teeth. J Pros Dent 100(1): 41-46.
- Restrepo CC, Tirado M, Jimenez KJ (2016) Association of sleep bruxism and dental plaque factors on signs of periodontal disease in children in the mixed dentition. Int J Paediatr Dent 26(6): 477-485.
- M Bansal, (2012) “Diseases of the Ear, Nose and Throat.” JP Medical Ltd.
- Singh GD, Cranio (2010) On the etiology and significance of palatal and mandibular tori. 28(4): 213-215.
- Deepika Raina, Rajesh Gothi, and Sriram Rajan (2009) Eagle syndrome, , Indian J Radiol Imaging. 19(2): 107-108.
- Karen G. Raphael, Malvin N. Janal, David A, et al. (2013) Masticatory Muscle Sleep Background EMG Activity is Elevated in Myofascial TMD Patients. J Oral Rehabil 40(12): 883-891.
- Karen G Raphael, David A Sirois, Janal MN, Wigren PE, Dubrovsky B, et al. (2012) Sleep bruxism and myofascial temporomandibular disorders: A laboratory-based polysomnographic investigation. J Am Dent Assoc 143(11): 1223-1231.
- Karen G Raphael, Malvin N Janal, David A Sirois, Boris Dubrovsky, Jack Klausner J, et al. (2015) Validity of Self-reported Sleep Bruxism among Myofascial Temporomandibular Disorder Patients and Controls. J Oral Rehabil 42(10): 751-758.
- de Souza Melo G, Batistella EÂ, Bertazzo-Silveira E, Simek Vega Gonçalves TM, Mendes de Souza BD, et al. (2017) Association of sleep bruxism with ceramic restoration failure: A systematic review and meta- analysis. J Pros Dent 119(3): 354-362.
- Mengatto CM, Coelho de Souza FH, de Souza Junior OB (2016) Sleep bruxism: Challenges and restorative solutions. Clinical, Cosmetic and Investigational Dentistry 8: 71-77.
- Chrcanovic B, Kisch J (2017) Impact of Different Surgeons on Dental Implant Failure, Inter J Pros 30(5).
- Levin L, Refuat Hapeh Vehashinayim (2010) Dealing with Dental Implant Failures, (1993). 27(1): 6-12.
- Anke C Winter, Markus Schürks, Robert J Glynn, Julie E, Tobias Kurth, et al. (2013) Vascular risk factors, cardiovascular disease and restless legs syndrome in women. Am J Med 126(3): 220-227

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...