Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6628
Mini Review(ISSN: 2637-6628) 
Systematic Review of a Series of Home-Grown Studies Concerning Thymoleptics Benefit Re Deficit Syndrome of Schizophrenia Volume 5 - Issue 2
Saeed Shoja Shafti*
- Full Professor of Psychiatry, University of Social Welfare and Rehabilitation Sciences (USWR), Razi Psychiatric Hospital, Iran
Received: February 08, 2021 Published:February 22, 2021
Corresponding author: Saeed Shoja Shafti, Full Professor of Psychiatry, University of Social Welfare and Rehabilitation Sciences (USWR), Razi Psychiatric Hospital, Iran
DOI: 10.32474/OJNBD.2021.05.000208
Abstract
Introduction
Though negative symptoms are a real barricade against effective recovery in schizophrenia, their management by add-on antidepressants has produced changeable consequences. In the current study, some of the local systematic published studies have been the subject of a systematic review, to assess the effectiveness of adjunctive antidepressants on negative symptoms of schizophrenia.
Methods
After probing in identified database, 8 home-based appropriate randomized clinical assessments, containing two hundreds and seventy-seven patients, was nominated. All chosen samples were among the long-lasting patients, with diagnosis of schizophrenia in line with “Diagnostic and Statistical Manual of Mental Disorders”, fourth edition, text revision. Following selection, they entered into a series of double-blind trials for random assignment to placebo or an antidepressant, along with their prescribed antipsychotic medicines. In the said trials, the “Scale for Assessment of Negative Symptoms” was the primary scale for evaluation of negative symptoms. Moreover, response was demarcated as a decrease of ⩾20percentage in the score of the said measure (as total item or subscales).
Results
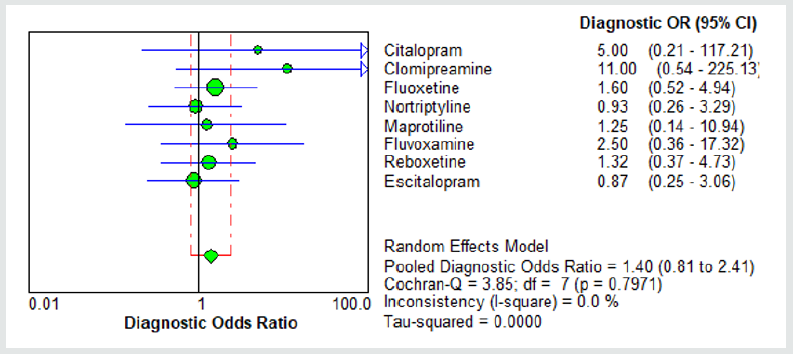
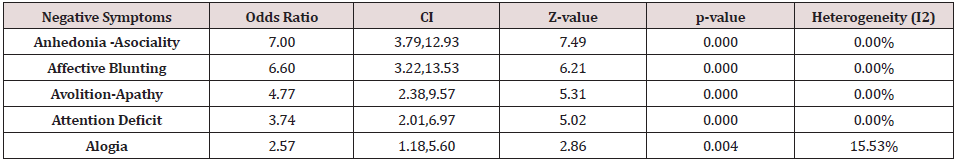
Analysis of “Combined Effect Size” showed a significant positive influence of antidepressant medications on negative symptoms. Similar results, too, were palpable separately with reference to different sub-scales of primary outcome measure. “Anhedonia– Asociality” displayed the best response, followed by “Affective Blunting”, “Avolition Apathy”, “Attention Deficit”, and finally “Alogia”. Heterogeneity of the said enquiry was insignificant and acceptable.
Conclusion
Antidepressant drugs have promising sound effects with regard to improvement of negative symptoms of schizophrenia.
Keywords: Deficit Syndrome of Schizophrenia; Negative Symptom of Schizophrenia; Adjuvant Antidepressant Medications; Pharmacotherapy; Serotonergic Antidepressant Drugs; Noradrenergic Antidepressant Drugs
Introduction
Schizophrenia is a heterogeneous syndrome characterized by cognitive, positive, and negative symptoms [1-2]. While positive symptoms talk about new psychological incidents outside of the normal experience (like, delusions and hallucinations,), negative symptoms imply impairment or loss of everyday functions. Negative symptoms include apathy, poverty of speech and thought, anhedonia, blunting of affect, loss of motivation, inattention to social or cognitive input, reduced social drive, and finally lack of social interest [3]. Attentiveness in negative symptoms has increased quickly over the last years, in parallel with a developing concern in practical and clinical retrieval [4]. Traditionally, negative symptoms are believed to be treatment-resistant and as the major backer to lower functional levels in most patients with schizophrenia, in comparison with their premorbid position [5]. Severe and persistent negative symptoms constitute the deficit syndrome, which is evident in around 25 percent of schizophrenic patients. While amelioration of positive symptoms does not automatically turn into efficient recovery, full functional/social retrieval happens in less than 15 percent of individuals with schizophrenia [6]. An associated impediment is secondary negative symptoms - loss of interest, social drive and expressiveness that results from depression, fear of social stigma, social anxiety, or the extra-pyramidal adverse effects of antipsychotic medications [1]. While negative symptoms are thoroughly connected to the thought deficits, the boundary between negative and cognitive symptoms, as well, is not sharp enough [7]. Schizophrenics do unwell on tests of mental confidence and elasticity, particularly word fluency and the capacity to sustain attention and shift its focus when needed [8]. While debate continues regarding the interconnection and influence of negative vs. cognitive symptoms to functional deficiency, it is recognizable that their impression is considerable [4]. Undoubtedly, the introduction of the antipsychotic medications in the 1950’s heralded a novel epoch in the management of schizophrenia. About 70 – 80 % of individuals with schizophrenia who take antipsychotic drug will find that their positive symptoms (delusions and hallucinations) reduce and sometimes may vanish totally [9]. Nevertheless, while antipsychotic drugs are very effective against the positive symptoms, such an operative treatment for the negative symptoms unhappily remains beyond our knowledge [2]. Maybe it is so because the roots of the negative symptoms are more complex than the positive ones and ingrained in psychological factors as in chemical alterations within the brain cells [10]. In the 1980s, the second-generation antipsychotics were introduced and are now the drugs of first choice for treating psychosis [11].
In the beginning, it gave the impression that these new drugs were not only as effective as the first-generation antipsychotic medications in treating the positive symptoms, but they were possibly effective against negative symptoms. But, in fact it was not so. Despite inconsistency in outcomes of published researches, certainly if there is any beneficial effect from the atypical antipsychotics on negative symptoms it is far less striking than their effect on positive symptoms [12]. Some practitioners have used antidepressants in the management of negative symptoms and have claimed that in addition to their effectiveness in combating the depression they may also increase the blood levels of the antipsychotic drugs and hence increase their effectiveness, and not only help with the depressive symptoms but also with the negative symptoms [4,9]. On the other hand, some scholars believe that no one of medications now accessible is an exact management for schizophrenia. All antipsychotic drugs are, approximately, similarly good at subduing psychotic symptoms, and in the same way similarly unsuccessful regarding the amelioration of negative symptoms [13]. Therefore, pharmacotherapy of negative symptoms by supplementary antidepressant medications is not without discrepancy. For example, while scholars like Möller ;[14-18] have faith in helpfulness of adjunctive antidepressants, other researchers like Kissling et al. [19-23] see that an unpredictable, ambiguous or useless approach. Therefore, in the current study and according to our evaluative query (are antidepressant drugs valuable in the management of negative symptoms?), some of the local systematic published studies have been the topic of a new inquiry, to assess over again the efficacy of auxiliary antidepressants on management of negative symptoms of schizophrenia.
DMethodse
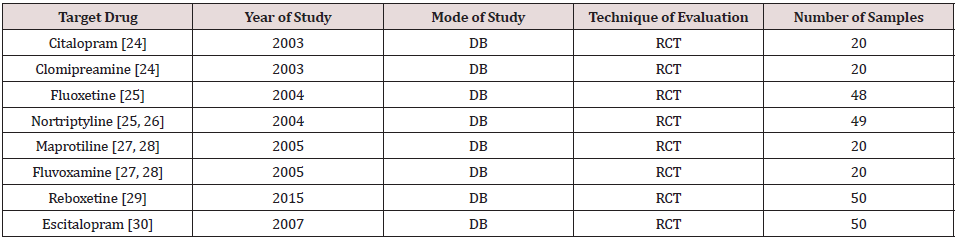
For evaluation, articles in all languages were searched through internet from these databanks: MEDLINE, EMBASE, PsycINFO, LILACS, ISI Web of Science, Cochrane Schizophrenia Group’s Specialized Register, The Cochrane Central Register of Controlled Trials, and PubMed. The selection criteria encompassed all homebased, pertinent randomized trials, comparing placebo with antidepressant medications, with reference to amelioration of negative symptoms. In conclusion, 8 studies were designated, which were done during the last 14 years in Razi psychiatric hospital, with a total of 277 male participants [24-30] (Table 1). Unpublished try-outs, non-methodical assessments, open trials, or disciplined estimations by medicines other than antidepressant drugs were excluded from the present study. In the said evaluations the primary analyses were performed in line with the “intentionto- treat”, “Last Observation Carried Forward (LOCF) approach”. Furthermore, all the cases were chronic schizophrenic inpatients, as accessible sample. Diagnosis of schizophrenia, as well, was in line with the criteria of “Diagnostic and Statistical Manual of Mental Disorders”, fourth edition, text revision [31]. In addition, while the studies were carried out consistent with the ‘Declaration of Helsinki and Ethical Principles for Medical Research Involving Human Subjects’ [32], the patients were educated regarding the procedure, and a signed informed consent was received from those who had in participated in the studies. Duration of at least 2 years of illness, too, was necessary for inclusion of samples. Moreover, cases with co-morbidities such as mental retardation, major depressive disorder, medical problems, and neurological ailments, and cases with diagnosis of schizoaffective disorder or patients, who had been prescribed long-acting depot antipsychotic drug (during the last 6 months) or atypical antipsychotic medications, antidepressant medicines or lithium, were excluded. The “Scale for Assessment of Negative Symptoms” (SANS) [33] was the primary assessment tool for estimation of negative symptoms. Besides, the “Scale for Assessment of Positive Symptoms” (SAPS) [34], “Simpson Angus Scale” (SAS) [35], “Hamilton Rating Scale for Depression” (HAM-D) [36], and “Mini-Mental Status Examination” (MMSE) [37], were utilized for assessment of confounding parameters. In this regard, SANS, ⩾66, SAPS, ⩾96, and SAS, ⩽10 were the basis of inclusion criteria. In addition, HAM-D and MMSE had been used, for exclusion of depression and cognitive disturbances, separately. So, HAM-D >10 and MMSE <25 were acknowledged as probable depression and cognitive trouble and could cause elimination. After a washout period of one week, patients were prescribed haloperidol (5-10 mg/ day), and after that had been randomized to receive either placebo or antidepressant drug, as add-on augmentative maneuvers. For prevention of escalation of psychosis, only the lower dosages of antidepressant preparations were permissible. For preserving blindness of the protocol, all add-on medications were inserted into empty and similar capsules. The surveyor, and personnel, too, was unmindful about the said panel and the nature of medicines set for each group. Patients had been evaluated at starting point and definite intervals by the main and ancillary scales. Also, appraisal of heterogeneity, publication bias analysis and, finally, grading quality of evidence (GRADE) [38], had been performed, on behalf of additional illumination of meta-analysis.
Statistical Analysis
In the present analysis, response was defined as a reduction of ⩾20 percent in the severity of SANS’ score (total and subscales). Confidence Interval (CI), Odds Ratio (OR), and combined effect size had been examined for each trial and symptom, independently. Meta-DiSc Version 1.4 [39] and Meta-Essentials Version 1.0 [40] were the statistical software tools for analysis.
Results
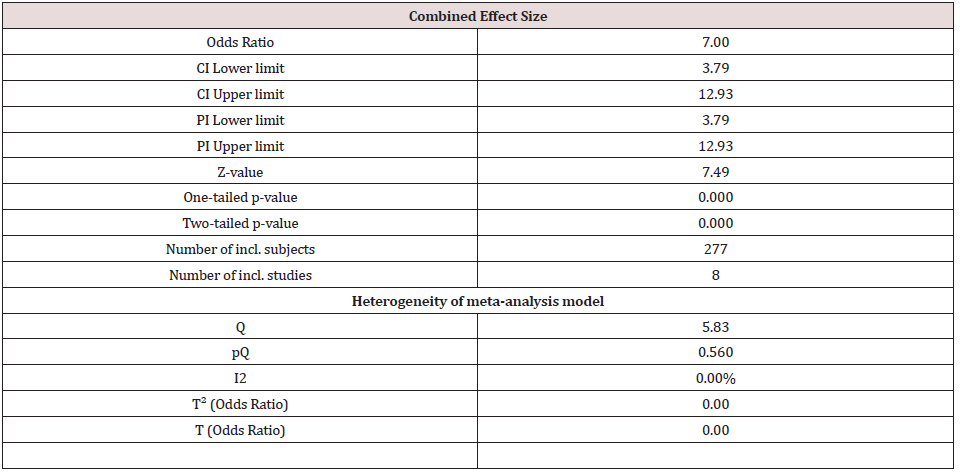
While the “Intention-to-treat” analysis was the primary protocol, there was not any significant missing data in the designated surveys. On the word of the outcomes, while the combined effect size of said trials had exposed a significant efficiency as regards the success of antidepressant medications on negative symptoms (OR = 7.00, CI= 3.79 - 12.93, z= 7.49, p<0.000), heterogeneity of the present metaanalysis was appropriate (<25%) (Table 2) (Figure 1). Similarly, analogous effects were discernible concerning different sub-scales of SANS, or symptoms of deficit syndrome of schizophrenia, like “Attention Deficit”, “Alogia”, “Avolition – Apathy”, “Anhedonia- Asociality“, and “Affective Blunting”. In this regard, “Anhedonia– Asociality” exhibited the best response, followed by “Affective Blunting”, “Avolition–Apathy”, “Attention Deficit”, and “Alogia” , in order (Table 3). Heterogeneity of all previously mentioned subscales, too, was minimal and thus proper. Based on a domain-based appraisal, because no apparent bias (pertaining to detection, choice, performance, reporting and attrition) was evident, the possibility of bias in this respect was in general small. In addition, the publication bias analysis revealed an adjusted combined effect size=1.95, in comparison with the detected combined effect size=1.76 (variance = 0.19), which, while minimal, does not disregard the requirement for further systematic investigations. Because of randomized trials, palpable directness, acceptable consistency, no serious limitation to study quality, low chance of reporting bias, and shortage of imprecise or sparse data ( except than 3 trials with wide confidence intervals), from one hand, and tough indication of association with noteworthy Odds Ratio ( >2 ), based on reliable proof from at least 2 assessments, with no reasonable confounders, Grading Quality of Evidence and Strength of Recommendations (GRADE) for this survey looks helpful and acceptable [38].
Discussion
Prevalent occurrence of negative symptoms and their resistance against available treatments, while they denote the most incapacitating and obstinate feature of schizophrenia, make them tough to overlook. Accordingly, interest in negative symptoms resuscitated in the 1980s-90s, with passionate struggles to better comprehend them and manage them more efficiently [41]. So, negative symptoms stay significant since they are the main barricade against a better life for schizophrenic patients [2]. To this last topic, work focusing on the prodromal phase of schizophrenia specifies that deficit symptoms and cognitive deficiency are evident during the first psychotic breakdown [42], while persistent negative symptoms are recognizable in around 25-30 percent of individuals with long-lasting schizophrenia [43]. Generally, the four major clinical subgroups of negative symptoms are classifiable as affective, communicative, conational, and relational [44]. On the other hand, though deficit syndrome has a main influence on quality of life of the patients [45], they are often linked with an inadequate response to drug treatments [46]. Thus far, studies specially probing negative symptoms in schizophrenia are inadequate [47]. Therefore, proper assortment of therapy of negative symptoms remains a major ignored medical requirement [48], and, according to the diversity of the clinical appearance of schizophrenia, management of negative symptoms, along with therapy for other related symptoms, should be mostly personalized along with a patient’s particular physiognomies [49]. Though from 1950s the introduction of operative pharmacological managements for positive symptoms has deliberately moved the diagnostic route to a positive symptombased model [50], through the 1980s, a quantity of categorizations of schizophrenia based on negative symptoms were presented [51], which exposed, nonetheless, several constraints, comprising the absence of diagnostic steadiness over time and the unfortunate prognostic importance [52]. Back to our discussion and as said by the results of the existent evaluation, antidepressant medications have substantial value for improvement of negative symptoms. Additionally, the highest effect was palpable in regard to “Anhedonia – Asociality”, and the least effect had been revealed with reference to “Alogia”.
Therefore, our deductions are compatible with Möller [14-16] found antidepressant drugs useful for amelioration of negative symptoms. In addition, it is somewhat compatible with the conclusions of Kissling et al. [19], who found mixture of antipsychotic drugs and antidepressant medications useful in handling negative symptoms, though as said by him the quantity of evidence was too inadequate to let any strong deduction. Incidentally, although they did not find any significant difference between ‘leaving the study early due to inefficacy’ and ‘leaving the study early due to adverse events’ between the two treatment groups, contributors treated with the antipsychotic medications in addition to antidepressant prescriptions exhibited, meaningfully, better improvement in negative symptoms than those treated with only antipsychotic drugs. Also, significant difference for the blend therapy was observable in different types of negative symptoms, like “Avolition”, “Alogia” and “Affective flattening”, which was again comparable to the outcomes of the current evaluation. Similarly, our conclusions were coherent with the outcomes of Rummel et al. [17-18], who found that antidepressants were effective in reducing negative symptoms, with a medium to high effect size, and such a reduction may be at least partially free from the antidepressant influence. In contrary, our deductions are not harmonious with the outcomes of Stahl et al. [20], who indicated that assessment and treatment trials’ method for the appraisal of negative symptoms demands more sophistication before therapeutic cheerfulness that superior managements for negative symptoms can be acknowledgeable. In addition, in contrary to our result, which comprised both Serotonin-Specific Reuptake Inhibitors (SSRIs) and tricyclic antidepressants, it is not consonant with Potvin et al. [21], who did not find any significant efficacy as regards the SSRIs. Nevertheless, when their studies were separated in line with the severity of disorder, a moderate and significant effect size appeared for the studies concerning the alleged ‘long-lasting patients’. Anyway, along with their conclusion, the accomplished review could not bring any comprehensive backing for the SSRIs. Maybe, as said by Czobor et al., while general effectiveness results appear to be optimistic, evidence for efficacy of present psychopharmacological medicines is hard to evaluate owing to technical difficulties and unstable results [53]. Also, the current evaluation is not in harmony with the outcomes of Hinkelmann et al. [54], who did not find any significant dissimilarity between serotonergic and noradrenergic antidepressants, as augmentative treatment for negative symptoms. Likewise, our results were not consistent with the conclusions of Singh et al. [24], and Fusar-Poli et al. [25], who believed that in spite of usual reportage of helpful responses, most trials often have not used a rigorous standard for description and control of negative symptoms. Thus, the issue of secondary negative symptoms could not be ruled out.
Therefore, efforts for better identification and measurement of negative symptoms should be continued [44]. For example, by studying endophenotypes, experts anticipate to learn how these processes work - not merely in people with schizophrenia, but in other persons who may or may not have a schizophrenia spectrum disorder. As investigation associates subtle signs and symptoms of illness to an individual’s basic genetic character, a better understanding of this range of ailments will be imaginable in near future [55]. However, the mechanism by which addition of antidepressant medications to antipsychotic drugs may cause lessening of negative symptoms is still vague [45]. More recently, the National Institute of Mental Health (NIMH) Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) twisted its care to negative symptoms. From a therapeutic standpoint, it is notable that the MATRICS consensus statement makes reference to stubborn negative symptoms and designates that the distinction between primary and secondary negative symptoms is not indispensable [7], which is many times unachievable [56-59]. Surely, restricted quantity of systematic native studies, short-term period of evaluations, gender-based sampling, and diverse sampling sizes were among the weaknesses of the current study, which together may limit the generalizability of the findings.
Conclusion
Antidepressant drugs have promising sound effects with regard to improvement of negative symptoms of schizophrenia.
References
- Carpenter WT, Heinrichs DW, Wagman AMI (1988) Deficit and nondeficit forms of schizophrenia: The concept. Am J Psychiatry 145(5): 578-583.
- Tandon R, Jibson M (2002) Negative symptoms of schizophrenia: How to treat them most effectively. Current Psychiatry 1(9): 36-42.
- Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT (1989) The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res 30(2): 119-124.
- Remington G, Foussias G, Fervaha G, Agid O, Takeuchi H, et al. (2016) Treating Negative Symptoms in Schizophrenia: An Update. Curr Treat Options Psych 3:133-150.
- Andreasen NC (1982) Negative symptoms in schizophrenia: Definition and reliability. Arch Gen Psychiatry 39(7): 784-788.
- Austin SF, Mors O, Secher RG, Hjorthoj CR, Albert N, et al. (2013) Predictors of recovery in first episode psychosis: the opus cohort at 10-year follow-up. Schizophr Res 150(1): 163-168.
- Kirkpatrick B, Fenton WS, Carpenter WT (2006) The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull 32(2): 214-219.
- Barch DM (2005) The Relationships among Cognition, Motivation, and Emotion in Schizophrenia: How Much and How Little We Know. Schizophrenia Bulletin 31(4): 875-881.
- Meltzer HY, Sommers AA, Luchins DJ (1986) The effect of neuroleptics and other psychotropic drugs on negative symptoms in schizophrenia. J Clin Psychopharmacol 6(6): 329-338.
- Tandon R, Greden JF (1989) Cholinergic hyperactivity and negative schizophrenic symptoms. Arch Gen Psychiatry 46(8): 745-753.
- Breier A, Buchanan RW, Kirkpatrick B (1994) Effect of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry 151(1): 20-26.
- DeQuardo JR, Tandon R (1998) Do atypical antipsychotic medications favorably alter the long-term course of schizophrenia? J Psychiatric Res 32(3-4): 229-242.
- Hunter R, Barry S (2012) Negative symptoms and psychosocial functioning in schizophrenia: neglected but important targets for treatment. Eur Psychiatry 27(6): 432-436.
- Möller HJ (2003) Management of the negative symptoms of schizophrenia: New treatment options. CNS Drugs 17(11): 793-823.
- Silver H (2003) Selective serotonin re-uptake inhibitor augmentation in the treatment of negative symptoms of schizophrenia. Int Clin Psychopharmacol 18(6): 305-313.
- Bauer A, Godemann F, Reischies FM, Selig F, Schlattmann P, et al. (2005) Negative symptoms of schizophrenia are improved by the addition of paroxetine to neuroleptics: A double-blind placebo-controlled study. Int Clin Psychopharmacol 20(1): 27-31.
- Rummel C, Kissling W, Leucht S (2005) Antidepressants as add-on treatment to antipsychotics for people with schizophrenia and pronounced negative symptoms: A systematic review of randomized trials. Schizophr Res 80 (1): 85-97.
- Helfer B, Samara MT, Huhn M (2016) Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: A systematic review and meta-analysis. Am J Psychiatry 173(9): 876-886.
- Kissling W, Leucht S Rummel C (2006) Antidepressants for the negative symptoms of schizophrenia. Cochrane Database Syst Rev 19(3): CD005581.
- Stahl SM, Buckley PF (2007) Pharmacological treatment of negative symptoms of schizophrenia: therapeutic opportunity or cul-de-sac? Acta Psychiatr Scand 115(2): 93-100.
- Potvin S, Elie R, Stip E, Sepehry AA (2007) Selective Serotonin Reuptake Inhibitor (SSRI) add-on therapy for the negative symptoms of schizophrenia: A meta-analysis. J Clin Psychiatry 68(4): 604-610.
- Singh SP, Singh V, Kar N, Chan K (2010) Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: Meta-analysis. Br J Psychiatry 197(3): 174-179.
- Fusar-Poli P, Papanastasiou E, Stahl D (2015) Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull 41(4): 892-899.
- Shoja Shafti S (2003) Effectiveness of Citalopram, Alprazolam and Clomipramine in amelioration of negative symptoms in Schizophrenia. Journal of Rehabilitation 3(12): 42-49.
- Shoja Shafti S (2004) Effectiveness of Bromocriptine, Flouxetine and Nortriptyline in ameliorating the negative symptoms of Schizophrenia. Journal of Rehabilitation 5(1-2): 58-63.
- Shoja Shafti S (2006) Treatment-responsiveness of negative symptoms in schizophrenia: a double-blind placebo-control clinical trial. Iranian J Med Sci 31(3): 135-138.
- Shoja Shafti S (2004) Rehabilitation of Schizophrenia: Adjunctive Therapy of Negative Symptoms. Iranian Rehabilitation Journal 1(2): 22-31.
- Shoja Shafti S (2005) Drug Specific Responsiveness of Negative Symptoms. International Journal of Psychosocial Rehabilitation 10 (1): 177-186.
- Shoja Shafti S, Jafarabad MS, Azizi R (2015) Amelioration of deficit syndrome of schizophrenia by norepinephrine reuptake inhibitor. Therapeutic Advances in Psychopharmacology 5(5): 263.
- Shoja Shafti S (2007) Amelioration of negative symptoms in schizophrenia by escitalopram: a double blind, placebo-controlled clinical trial. Clin Schizophrenia Related Psychosis 1: 255-258.
- American Psychiatric Association (APA) (2000) Diagnostic and Statistical Manual of Mental Disorders. (4th ed), Washington, DC: American Psychiatric Association.
- World Medical Association (2013) World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310(20): 2191-2194.
- Andreasen N (1981) The Scale for Assessment of Negative Symptoms (SANS), University of Iowa , Iowa.
- Andreasen N (1984) The Scale for Assessment of Positive Symptoms (SAPS), University of Iowa ,Iowa.
- Simpson GM and Angus JW (1970) A Rating Scale for Extrapyramidal Side Effects. Acta Psychiatr Scand 212 (suppl 44): 11-9.
- Hamilton M (1960) Rating scale for depression. J Neurol Neurosurg Psychiatry 23(1): 56-62.
- Folstein MF, Folstien SE, McHugh PR (1975) Mini- mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3): 189-198.
- GRADE Working Group (2004) Grading quality of evidence and strength of recommendations. BMJ 328(7454): 1490.
- Zamora J, Abraira V, Muriel A, Khan KS, Coomarasamy A (2006) Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Medical Research Methodology 6: 31.
- Van Rhee HJ, Suurmond R, Hak T (2015) User manual for Meta-Essentials: Workbooks for meta-analysis (Version 1.0) Rotterdam, The Netherlands: Erasmus Research Institute of Management 2015.
- Carpenter WT, Heinrichs DW, Alphs LD (1985) Treatment of negative symptoms Schizophrenia. Bull 11(3): 440-452.
- Bora E, Lin A, Wood SJ, Yung AR, Mc Gorry PD, et al. (2014) Cognitive deficits in youth with familial and clinical high risk to psychosis: a systematic review and meta-analysis. Acta Psychiatr Scand 130(1): 1-15.
- Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT (2001) A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry 58(2): 165-171.
- Cerveri G, Gesi C, Mencacci C (2019) Pharmacological treatment of negative symptoms in schizophrenia: Update and proposal of a clinical algorithm. Neuropsychiatric Disease and Treatment 15: 1525-1535.
- Blanchard JJ, Kring AM, Horan WP (2011) Toward the next generation of negative symptom assessments: The collaboration to advance negative symptom assessment in schizophrenia. Schizophr Bull 37 (2): 291-299.
- Leucht S, Davis JM (2017) Schizophrenia, primary negative symptoms, and soft outcomes in psychiatry. Lancet 389(10074): 1077-1078.
- Németh G, Laszlovszky I, Czobor P (2017) Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: A randomised, double-blind, controlled trial. Lancet 389(10074): 1103-1113.
- Corponi F, Serretti A, Montgomery S, Fabbri C (2017) Cariprazine specificity profile in the treatment of acute schizophrenia: A meta-analysis and meta-regression of randomized-controlled trials. Int Clin Psychopharmacol 32(6): 309-318.
- Malaspina D, Walsh-Messinger J, Gaebel W (2014) Negative symptoms, past and present: A historical perspective and moving to DSM-5. Eur Neuropsychopharmacol 24(5): 710-724.
- Crow TJ (1980) Molecular pathology of schizophrenia: More than one disease process? Bmj 280(7): 66-68.
- Andreasen NC, Olsen S (1982) Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry 39(7): 789-794.
- Peralta V, de Leon J, Cuesta MJ (1992) Are there more than two syndromes in schizophrenia? A critique of the positive-negative dichotomy. Br J Psychiatry 161: 335-343.
- Czobor P, Möller HJ (2015) Pharmacological treatment of negative symptoms in schizophrenia. Eur Arch Psychiatry Clin Neurosci 265(7): 567-578.
- Hinkelmann K, Yassouridis A, Kellner M, Jahn H, Wiedemann K (2013) No effects of antidepressants on negative symptoms in schizophrenia. J Clin Psychopharmacol 33(5): 686-690.
- Cannon TD (2006) Endophenotypes in the Genetic Analyses of Mental Disorders. Annual Review of Clinical Psychology 2: 267-290.
- Shoja Shafti S (2015) Odyssey of ‘Negative Symptoms’ of Schizophrenia: Rehabilitation vs Stigmatization. Current Psychopharmacology 4(1): 1-12.
- Shoja Shafti S (2019) Primary Negative Symptoms: Appraisal of a Misty Outlook. American Journal of Biomedical Science & Research 2(2): 317-319.
- Shoja Shafti S (2019) Deficit Syndrome of Schizophrenia: Specific Serotonin Reuptake Inhibitors Set Against Tricyclic Antidepressants. International Journal of Psychiatry Research 1(1): 12-14.
- Shoja Shafti S (2019) Evaluation of Assemblage and Manageability of Negative Symptoms of Schizophrenia: A New Stance. Journal of Psychiatry Studies 2(1): 1-6.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...