Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6628
Opinion(ISSN: 2637-6628) 
Rhinal Cortices Connecting the Papez and Yakovlev Circuits: An Integrated Viewpoint Through MRI Epidemiology to Animal Experiment for Better Understanding Clinical Features of Alzheimer Disease and Vascular Dementia Volume 4 - Issue 4
Kenichi Meguro*
- Geriatric Behavioral Neurology, Tohoku University New Industry Creation Hatchery Center, Tohoku University, Tohoku University Graduate School of Medicine, Japan
Received: September 28, 2020; Published: October 07, 2020
Corresponding author: Kenichi Meguro, Project Leader and Professor, Geriatric Behavioral Neurology Project, Tohoku University New Industry Creation Hatchery Center, Cyclotron Radioisotope Center, Tohoku University, Tohoku University Graduate School of Medicine, Seiyo-machi, IDAC, Aoba-ku, 980-8575 Sendai, Japan
DOI: 10.32474/OJNBD.2020.04.000193
Introduction
I herein present a unique statement, not focusing on the specific area but present an integrated viewpoint through MRI epidemiology to animal experiment. The aim is to provide a perspective for better understanding clinical features of Alzheimer disease (AD) and vascular dementia (VaD). It is well known that AD and VaD are two major dementing diseases [1,2], and the clinical features are different; grossly, AD shows disintegrated emotion and cognitive function, and VaD shows apathy. First, I will present two typical VaD cases.
Case Reports of VaD with Thalamic Infarctions
Case-1: A 71-year-old right-handed woman [3]
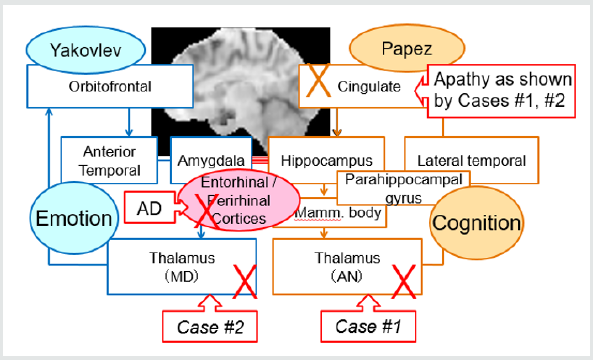
She was originally scrupulous in her performance of domestic chores. She suddenly developed difficulty finding words and used many pronouns in her communication. She exhibited apathy and did her housework in a careless manner. Although her MMSE score was 23, she could not recall episodic memory of her daily life. Neurologically, she exhibited very slight right hemiparesis. MRI showed infarction in the left thalamic anteroventral nucleus (AN), and SPECT revealed decreased bilateral frontal and anterior cingulate blood flow. The precuneus and parietotemporal areas, characteristic of AD, were intact. Thus, the diagnosis is VaD, and her amnesia was explained by the disturbed Papez circuit involving AN as illustrated in the right part of (Figure 1).
Case-2: An 82-Year-Old Right-Handed Man [3]
He had been active and enjoyed his hobbies every day. He suddenly developed difficulty finding words, gradually exhibited amnesia and apathy. His MMSE score was 22. He could poorly remember episodic memory of his daily life. Neurologically, he exhibited slight right hemiparesis. MRI showed infarction in the left thalamic dorsomedial nucleus (MD), and SPECT revealed decreased bilateral frontal and anterior cingulate blood flow. The precuneus and parietotemporal areas were intact. Thus the diagnosis is VaD; however, one of the fronto-subcortical circuits involving MD cannot explain amnesia, since clinically we know that the limited frontal lesions cannot manifest amnesia. The MD is involved in the Yakovlev circuit responsible for emotion. Then how we can consider his amnesia? To get straight to the point, preserved rhinal cortices can connect the Papez and Yakovlev circuits, and explain amnesia as a remote effect as illustrated in (Figure 1). Then I will present MRI epidemiology on the entorhinal cortex.
MRI Epidemiology Disclosed an Importance of Entorhinal Cortex
To investigate MRI findings of questionable dementia, 485 participants were selected in a community including 113 Clinical Dementia Rating (CDR) 0.5 people in my early study [4]. We found that each part of the brain showed atrophy compared with healthy adults. An amygdala atrophy was the only finding indicating CDR effect but no age effect. The amygdala or anterior entorhinal atrophy was found to be important for discriminating questionable dementia from healthy elderly. Using more sophisticated methodology [5], we computed cerebral cortical thickness, correlating with memory performance in questionable dementia people. We found that poor episodic memory was associated with thinner cortices in the left entorhinal region. Then the entorhinal cortex can affect remote cortical areas through neuronal network?
Functional Neuroimaging and an Animal Experiment
Hippocampal area (including entorhinal cortex) atrophy and
hypometabolism in the posterior association neocortex are two
well-known neuroimaging features of AD. The latter was already
revealed in the CDR 0.5 stage further decline to AD [6]. Analyzing
the data of MRI based atrophy and PET based glucose metabolism,
we found a positive neurobiologically expected correlation
between hippocampal width and angular gyrus metabolism.
Hippocampal-neocortical disconnection due to medial temporal
pathology may at least partly explain the posterior association
cortex hypometabolism found in AD [7,8].
To exclude an indirect dementia severity effect and directly
prove the effect of neuronal network, we performed an animal
experiment [9,10]. Namely, the remote metabolic effects of bilateral
neurotoxic lesions of rhinal cortices was assessed. Using PET,
glucose metabolism was measured before surgery and a couple
months afterward. Compared with sham-operated baboons, the
lesioned animals showed a long-lasting metabolic decline in
the parietal and temporal cortices, and posterior hippocampal
region, all specific to AD. This shows the temporoparietal and
hippocampal hypometabolism found in AD may partly result from
neuroanatomical disconnection with the rhinal cortex. Neuronal
damage and dysfunction in the rhinal cortices is suggested to play
a major role in the expression of AD. Then finally, the concept for
better understanding clinical features of AD and vascular VaD is
summarized in (Figure 1).
Functional Neuronal Networks for Better Understanding of AD and VaD
Clinically AD patients frequently shows behavioral and psychological symptoms of dementia (BPSD), despite relatively preserved cognitive function in the mild stage. This imbalance between emotion and cognition may be based on the disconnection between the Yakovlev and Papez circuits, which are responsible for emotional and cognitive functions, respectively.
On the contrary, VaD patients show mainly apathy and less frequently such unbalanced, disintegrated emotional changes disproportionally to cognitive level unlikely to AD patients. This is probably due to spared rhinal cortices.
References
- Meguro K, Ishii H, Yamaguchi S, Ishizaki J, Shimada M, et al. (2002) Prevalence of dementia and dementing diseases in Japan: The Tajiri Project. Archives of Neurology 59(7): 1109-1114.
- Meguro K, Tanaka N, Kasai M, Nakamura K, Ishikawa H, et al. (2012) Prevalence of dementia and dementing diseases in the old-old population in Japan: The Kurihara Project. Implications for Long-Term Care Insurance Data. Psychogeriatrics 12(4): 226-234.
- Meguro K, Akanuma K, Ouchi Y, Meguro M, Nakamura K, et al. (2013) Vascular dementia with left thalamic infarction: Neuropsychological and behavioral implications suggested by involvement of the thalamic nucleus and the remote effect on cerebral cortex. The Osaki-Tajiri Project. Psychiatry Research: Neuroimaging 213(1): 56-62.
- Ishii H, Meguro K, Yamaguchi S, Hirayama K, Tabuchi M, et al. (2006) Different MRI findings for normal elderly and very mild Alzheimer's disease in a community: Implications for clinical practice. The Tajiri Project. Archives of Gerontology and Geriatrics 42(1): 59-71.
- Fujishima M, Maikusa N, Nakamura K, Nakatsuka M, Matsuda H, et al. (2014) Mild cognitive impairment, poor episodic memory, and late-life depression are associated with cerebral cortical thinning and increased white matter hyperintensities. Frontiers in Aging Neuroscience 6: 306.
- Ishii H, Ishikawa H, Tashiro M, Yamaguchi S, Meguro K (2009) Decreased cortical glucose metabolism in converters from CDR 0.5 to Alzheimer’s disease in a community: The Osaki-Tajiri Project. International Psychogeriatrics 21(1): 148-156.
- Yamaguchi S, Meguro K, Itoh M, Hayasaka C, Shimada M, et al. (1997) Decreased cortical glucose metabolism correlated with hippocampal atrophy in Alzheimer’s disease as shown by MRI and PET. Journal of Neurology Neurosurgery and Psychiatry 62(6): 596-600.
- Meguro K, LeMestric C, Landeau B, Desgranges B, Eustache F, et al. (2001) Relations between hypometabolism in the posterior association neocortex and hippocampal atrophy in Alzheimer’s disease: A PET/MRI correlative study. Journal of Neurology Neurosurgery and Psychiatry 71(3): 315-321.
- Meguro K, Blaizot X, Kondoh Y, LeMestric C, Baron JC, et al. (1999) Neocortical and hippocampal glucose hypometabolism following neurotoxic lesions of the entorhinal and perirhinal cortices in the non-human primate as shown by PET: Implications for Alzheimer’s disease. Brain 122(Pt 8): 1519-1531.
- Blaizot X, Meguro K, Millien I, Baron JC, Chavoix C (2002) Correlations between visual recognition memory and neocortical and hippocampal glucose metabolism after bilateral rhinal cortex lesions in the baboon: Implications for Alzheimer’s disease. J Neurosci 22(21): 9166-9170.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...