
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6628
Mini Review(ISSN: 2637-6628) 
Prosaposin and Its Receptors, GRP37 and GPR37L1, Protects Neurons Against In Vivo Neuropathological Disorders Volume 5 - Issue 1
Hiroaki Nabeka1*, Gao Hui-ling2, Xuan Li3, Chen Li3, Hiroyuki Wakisaka4, Joji Kunihiro1, Kana Unuma5, Miho Taniguchi1, Yuki Nakabayashi6, Md. Sakirul Islam Khan1, Tetsuya Shimokawa1, Farzana Islam1, Shouichiro Saito7, Fumihiko Hamada8, Naoto Kobayashi9 and Seiji Matsuda1
- 1Department of Anatomy and Embryology, Ehime University Graduate School of Medicine, Japan
- 2College of Life and Health Sciences, Northeastern University, Shenyang 110819, China
- 3Department of Immunology, China Medical University, Shenyang, China
- 4Department of Otorhinolaryngology, Ehime University Graduate School of Medicine Toon, Ehime, Japan
- 5Section of Forensic Medicine, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Bunkyo, Tokyo, Japan
- 6Department of Forensic Medicine, Ehime University Graduate School of Medicine, 454 Shitsukawa, Toon, Ehime 791-0295, Japan
- 7Laboratory of Veterinary Anatomy, Faculty of Applied Biological Sciences, Gifu University, Yanagido, Gifu, Japan
- 8Department of Human Anatomy, Oita University Faculty of Medicine, Yufu, Oita, Japan
- 9Department of Medical Education Center, Ehime University Graduate School of Medicine, Toon, Ehime, Japan
Received: December 08, 2020; Published:December 22, 2020
Corresponding author: Hiroaki Nabeka, Department of Anatomy and Embryology, Ehime Graduate School of Medicine, 454 Shitsukawa, Toon, Ehime 791-0295, Japan
DOI: 10.32474/OJNBD.2020.05.000202
Abstract
- Abstract
- PSAP Protects Against Ischemic Damage
- PSAP Rescues Neurons from Damage Induced by Neurotoxic Agents
- PSAP Shows Neuro- and Glio-Protection After Nerve Transection
- PSAP and Its Receptors in the Dorsal Root Ganglion (DRG)
- Decreased PSAP Expression in A Duchene Muscular Dystrophy Model
- Decreased PSAP Expression in the Lacrimal Glands of Adult Female Mice
- References
Prosaposin (PSAP) is both a precursor protein of saposin A–D and a neuroprotective factor. In response to neuropathological disorders such as ischemia, neurotoxins, and nerve transection, PSAP is up-regulated such that both PSAP immunoreactivity and PSAP mRNA levels in neurons increase significantly. PSAP and an 18-mer peptide (PS18) derived from its neurotrophic region were shown to significantly protect damaged neurons. Meyer et al. [1] characterized the PSAP receptors GPR37 and GPR37L1, both of which are involved in neuronal protection, although their expression and their interactions with PSAP have not been fully elucidated (see the review of Smith [2]). However, the increased expression of PSAP and its receptors in damaged neurons and the surrounding glia points to a role for these proteins in protecting damaged cells in the nervous system. This mini-review examines the neuro- and glio-protective functions of PSAP and its receptors, based mainly on data from neuropathological in vivo models. Additional information can be found in the review by Meyer et al. [3].
Keywords: Prosaposin; Neuroprotection; Kainic Acid; Parkinsonism; Nerve Transection; Dystrophin-Deficient Mdx
PSAP Protects Against Ischemic Damage
- Abstract
- PSAP Protects Against Ischemic Damage
- PSAP Rescues Neurons from Damage Induced by Neurotoxic Agents
- PSAP Shows Neuro- and Glio-Protection After Nerve Transection
- PSAP and Its Receptors in the Dorsal Root Ganglion (DRG)
- Decreased PSAP Expression in A Duchene Muscular Dystrophy Model
- Decreased PSAP Expression in the Lacrimal Glands of Adult Female Mice
- References
Prosaposin (PSAP) is a precursor protein of saposin A–D [4,5]. Unprocessed PSAP is found in cerebrospinal fluid [6,7], and PSAP mRNA is strongly expressed in the choroid plexus [8], which together suggest that PSAP is a secretory neurotrophic factor. O’Brien et al. [9] were the first to identify PSAP as a potent neurotrophic factor. Both PSAP and peptides containing the PSAP neurotrophic activity domain exhibit neuro- and glio-protective functions in vitro [9-15]. The enhanced expression and release of PSAP following ischemia suggest that it protects cells from ischemic damage [16-18]. In a study by our group, PSAP infusion into the lateral ventricles of gerbils prevented learning disabilities and ischemic neuronal death [19]. Similar results were obtained with PS18 [20,21]. These effects were accompanied by the protection of cells from apoptotic death [19,21]. Similar protective effects were observed in the thalamus following occlusion of the middle cerebral artery [22,23].
PSAP Rescues Neurons from Damage Induced by Neurotoxic Agents
- Abstract
- PSAP Protects Against Ischemic Damage
- PSAP Rescues Neurons from Damage Induced by Neurotoxic Agents
- PSAP Shows Neuro- and Glio-Protection After Nerve Transection
- PSAP and Its Receptors in the Dorsal Root Ganglion (DRG)
- Decreased PSAP Expression in A Duchene Muscular Dystrophy Model
- Decreased PSAP Expression in the Lacrimal Glands of Adult Female Mice
- References
Kainic acid (KA) is a glutamate analogue whose injection causes neurotoxicity in animals. At a dose of 5 mg/kg, a subcutaneous injection of KA stimulates neurons without causing cell death [24]. Following KA injection, PSAP expression in hippocampal pyramidal neurons and interneurons increased (Figure 1a-d). After KA injection (12 mg/kg), injured and healthy pyramidal neurons in the hippocampal CA1 region in rats with/without PS18 injection were counted (Figure 1e-g). The presence of fewer injured neurons and more healthy neurons in the PS18-injected rats indicated that PS18 rescues CA1 neurons from KA-induced degeneration. The detection of higher levels of PSAP in PV-positive GABAergic inhibitory interneurons and their axons around pyramidal neurons suggested the axonal transport of PSAP [24,25] (Figure 1h-i). Similarly, PSAP was strongly expressed in the Purkinje cells and interneurons of the cerebellum of KA-injected rats [26]. These findings provide insights into the role of PSAP in alleviating the neural damage caused by KA. PSAP is up-regulated in the substantia nigra of patients with Parkinson’s disease [27]. The ability of a PSAP-derived peptide to protect dopaminergic neurons from the neurotoxins MPP+ and MPTP in in vitro and in vivo models of Parkinson’s disease, respectively, was reported [28,29]. In the latter study, PSAP was shown to inhibit the MPTP-induced cleavage of caspase-3, downregulate the pro-apoptotic factor BAX, and up-regulate the antiapoptotic factor Bcl-2, consistent with an inhibition of apoptosis by PSAP [29].
Figure 1a-d: In situ hybridization of PSAP mRNA expression. Compared with normal control (a, b), the signals of the antisense probe in the hippocampus (a, c) and the choroid plexus (b, d) were significantly more intense after KA injection (c, d). The numbers on the bar indicate μm. Figures reproduced with permission from PLoS One [24].
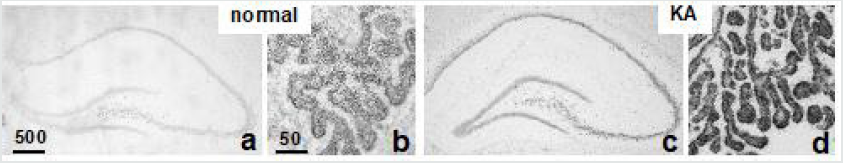
Figure 1e-g:Light microscopic analysis of hippocampal CA1. Photomicrographs of toluidine blue-stained hippocampal CA1 neurons in a normal control rat (e) and rats that received an injection of PBS (f), or 2.0 mg/kg PS18 (g) after a 12 mg/kg KA injection. Injured CA1 neurons were rescued by a PS18 treatment (g). Figures reproduced with permission from PLoS One [39].
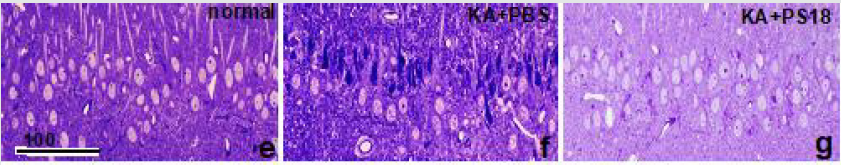
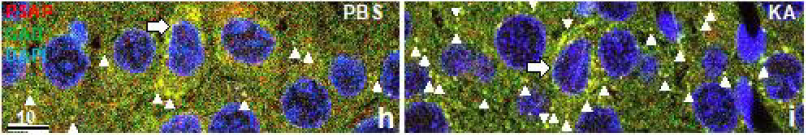
Figure 1h-i: Immunofluorescence light micrographs of the hippocampal CA1 neurons stained with anti-PSAP IgG, anti-GAD, and DAPI showing the PSAP-IR 3 days after PBS (h) or 5 mg/kg KA (i) injection. The arrows indicate interneurons with slender nuclei and very intense immunoreactivity both of PSAP and GAD. Note that the GAD-PSAP double positive axon terminals (arrowheads) are observed around almost all CA1 neurons after KA injection (i), but only some after PBS injection (h). Nuclei are stained with DAPI (blue), PSAP is shown red, and GAD is shown green. Figures reproduced with permission from IBRO Reports [24].
PSAP Shows Neuro- and Glio-Protection After Nerve Transection
- Abstract
- PSAP Protects Against Ischemic Damage
- PSAP Rescues Neurons from Damage Induced by Neurotoxic Agents
- PSAP Shows Neuro- and Glio-Protection After Nerve Transection
- PSAP and Its Receptors in the Dorsal Root Ganglion (DRG)
- Decreased PSAP Expression in A Duchene Muscular Dystrophy Model
- Decreased PSAP Expression in the Lacrimal Glands of Adult Female Mice
- References
PSAP and an PS18 facilitate regeneration following sciatic
nerve transection [20]. A PSAP-derived peptide was shown to
increase both the expression and enzymatic activity of GALT, an
enzyme predominantly found in myelinating Schwann cells [15],
and sulfatide concentrations in the brain and sciatic nerve of
developing rats [30]. Our group similarly found an increase in the
levels of intrinsic PSAP and its mRNA in the facial nerve nucleus
after nerve transection [31,32].
In rats, PSAP gene transcription generates two alternative
splicing forms of PSAP mRNA: Pro +9, containing a 9-base insertion,
and Pro +0 without the insertion. In the rat facial nucleus after
facial nerve transection, a peak in Pro +0 mRNA levels was detected
after 5–10 days whereas Pro +9 mRNA levels remained unchanged.
Moreover, during facial nerve regeneration PSAP mRNA expression
increased not only in facial motoneurons but also in microglia [32].
Meyer et al. [1] characterized the PSAP receptors GPR37
and GPR37L1, both of which are involved in neuronal protection
[33,34], although their expression and their interactions with PSAP
have not been fully elucidated (see the review of Smith [2]). We also
examined changes in the immunoreactivity of the PSAP receptors
GPR37 and GPR37L1 in the rat facial nucleus after facial nerve
transection. Strong GPR37L1 immunoreactivity was detected in
many of the microglia (Figure 1j-k) and astrocytes (Figure 1l-m) on
the operated side whereas GPR37 expression was mainly observed
in neurons [35].
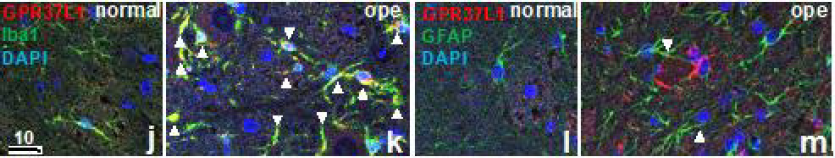
Figure 1j-m: Double-immunofluorescence staining in the facial nucleus 7 days after facial nerve transection. Double-staining
images for GPR37L1 (red) with glial markers (Iba1, GFAP, green) and DAPI (blue) are shown.
These images show that cells with a high GPR37L1-IR signal were located predominately on the operated (ope) side (k, m).
Double-staining with GPR37L1 and Iba1 (j, k) showed that GPR37L1-IR and Iba1-IR also increased on the operated side, and
high co-localization of GPR37L1 with Iba1 (arrowheads) was observed in many cells (k). Double-staining with GPR37L1 and
GFAP (l, m) showed that GPR37L1-IR and GFAP-IR increased intensely on the operated side, but co-localization of GPR37L1
with GFAP (arrowheads) was not frequent (m).
PSAP and Its Receptors in the Dorsal Root Ganglion (DRG)
- Abstract
- PSAP Protects Against Ischemic Damage
- PSAP Rescues Neurons from Damage Induced by Neurotoxic Agents
- PSAP Shows Neuro- and Glio-Protection After Nerve Transection
- PSAP and Its Receptors in the Dorsal Root Ganglion (DRG)
- Decreased PSAP Expression in A Duchene Muscular Dystrophy Model
- Decreased PSAP Expression in the Lacrimal Glands of Adult Female Mice
- References
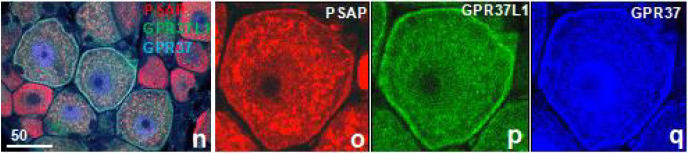
We examined the distribution of PSAP and its receptors in the developing DRG. PSAP colocalized with two receptors in the satellite cells and made very characteristic ring-shaped image around 8 weeks after birth, a period of intense growth of DRG (Figure 1nq). This ring-shaped image suggests that several satellite cells are synchronously activated.
Figure 1n-q: The immunofluorescence light micrographs of the rat DRG at 8 weeks clearly show the colocalization of PSAP and its receptors.
Decreased PSAP Expression in A Duchene Muscular Dystrophy Model
- Abstract
- PSAP Protects Against Ischemic Damage
- PSAP Rescues Neurons from Damage Induced by Neurotoxic Agents
- PSAP Shows Neuro- and Glio-Protection After Nerve Transection
- PSAP and Its Receptors in the Dorsal Root Ganglion (DRG)
- Decreased PSAP Expression in A Duchene Muscular Dystrophy Model
- Decreased PSAP Expression in the Lacrimal Glands of Adult Female Mice
- References
The dystrophin-deficient mdx mouse is a model of human Duchene muscular dystrophy, a disease characterized by disorders of the central nervous system, including mental retardation and metabolic damage, and damage to the neuromuscular system. However, whether PSAP is related to the loss of dystrophin and/ or to the brain abnormalities of the disease remains unclear. In a study of mdx mice, we found decreased levels of intrinsic PSAP and its mRNA in the brain [36], consistent with a report of low levels of PSAP in the muscles of these animals [37].
Decreased PSAP Expression in the Lacrimal Glands of Adult Female Mice
- Abstract
- PSAP Protects Against Ischemic Damage
- PSAP Rescues Neurons from Damage Induced by Neurotoxic Agents
- PSAP Shows Neuro- and Glio-Protection After Nerve Transection
- PSAP and Its Receptors in the Dorsal Root Ganglion (DRG)
- Decreased PSAP Expression in A Duchene Muscular Dystrophy Model
- Decreased PSAP Expression in the Lacrimal Glands of Adult Female Mice
- References
Lacrimal glands produce growth factors whose expression increases during ocular injuries, suggesting that they respond to pathological conditions. We showed that in young male and female mice the expression of colocalized PSAP and its receptors did not differ whereas in older females a decrease was determined. Whether this result reflects cross-talk between lacrimal PSAP and gonadal secretion, particularly in response to aging, when the levels of sex hormone decline, remains to be explored in further studies [38].
References
- Abstract
- PSAP Protects Against Ischemic Damage
- PSAP Rescues Neurons from Damage Induced by Neurotoxic Agents
- PSAP Shows Neuro- and Glio-Protection After Nerve Transection
- PSAP and Its Receptors in the Dorsal Root Ganglion (DRG)
- Decreased PSAP Expression in A Duchene Muscular Dystrophy Model
- Decreased PSAP Expression in the Lacrimal Glands of Adult Female Mice
- References
- Meyer RC, Giddens MM, Schaefer SA, Hall RA (2013) GPR37 and GPR37L1 are receptors for the neuroprotective and glioprotective factors prosaptide and prosaposin. Proc Natl Acad Sci USA 110(23): 9529-9534.
- Smith NJ (2015) Drug Discovery Opportunities at the Endothelin B Receptor-Related Orphan G Protein-Coupled Receptors, GPR37 and GPR37L1. Front Pharmacol 6: 275.
- Meyer RC, Giddens MM, Coleman BM, Hall RA (2014) The protective role of prosaposin and its receptors in the nervous system. Brain Res 1585: 1-12.
- O'Brien JS, Kretz KA, Dewji N, Wenger DA, Esch F, et al. (1988) Coding of two sphingolipid activator proteins (SAP-1 and SAP-2) by same genetic locus. Science 241(4869): 1098-1101.
- Sano A, Radin NS, Johnson LL, Tarr GE (1988) The activator protein for glucosylceramide beta-glucosidase from guinea pig liver. Improved isolation method and complete amino acid sequence. J Biol Chem 263(36): 19597-19601.
- Hineno T, Sano A, Kondoh K, Ueno S, Kakimoto Y, et al. (1991) Secretion of sphingolipid hydrolase activator precursor, prosaposin. Biochem Biophys Res Commun 176(2): 668-674.
- Hiraiwa M, Soeda S, Kishimoto Y, O'Brien JS (1992) Binding and transport of gangliosides by prosaposin. Proc Natl Acad Sci USA 89(23): 11254-11258.
- Saito S, Saito K, Nabeka H, Shimokawa T, Kobayashi N, Matsuda S (2014) Differential expression of the alternatively spliced forms of prosaposin mRNAs in rat choroid plexus. Cell and tissue research 356(1): 231-242.
- O'Brien JS, Carson GS, Seo HC, Hiraiwa M, Kishimoto Y (1994) Identification of prosaposin as a neurotrophic factor. Proc Natl Acad Sci USA 91(20): 9593-9596.
- Kondoh K, Sano A, Kakimoto Y, Matsuda S, Sakanaka M (1993) Distribution of prosaposin-like immunoreactivity in rat brain. J Comp Neurol 334(4): 590-602.
- Morales CR, Hay N, El-Alfy M, Zhao Q (1998) Distribution of mouse sulfated glycoprotein-1 (prosaposin) in the testis and other tissues. J Androl 19(2): 156-164.
- Campana WM, Eskeland N, Calcutt NA, Misasi R, Myers RR, O'Brien JS (1998) Prosaptide prevents paclitaxel neurotoxicity. Neurotoxicology 19(2): 237-244.
- Tsuboi K, Hiraiwa M, O'Brien JS (1998) Prosaposin prevents programmed cell death of rat cerebellar granule neurons in culture. Brain Res Dev Brain Res 110(2): 249-255.
- Hiraiwa M, Martin BM, Kishimoto Y, Conner GE, Tsuji S, et al. (1997) Lysosomal proteolysis of prosaposin, the precursor of saposins (sphingolipid activator proteins): its mechanism and inhibition by ganglioside. Arch Biochem Biophys 341(1): 17-24.
- Hiraiwa M, Campana WM, Mizisin AP, Mohiuddin L, O'Brien JS (1999) Prosaposin: a myelinotrophic protein that promotes expression of myelin constituents and is secreted after nerve injury. Glia 26(4): 353-360.
- Yokota N, Uchijima M, Nishizawa S, Namba H, Koide Y (2001) Identification of differentially expressed genes in rat hippocampus after transient global cerebral ischemia using subtractive cDNA cloning based on polymerase chain reaction. Stroke 32(1): 168-174.
- Hiraiwa M, Liu J, Lu AG, Wang CY, Misasi R, et al. (2003) Regulation of gene expression in response to brain injury: enhanced expression and alternative splicing of rat prosaposin (SGP-1) mRNA in injured brain. J Neurotrauma 20(8): 755-765.
- Costain WJ, Haqqani AS, Rasquinha I, Giguere MS, Slinn J, et al. (2010) Proteomic analysis of synaptosomal protein expression reveals that cerebral ischemia alters lysosomal Psap processing. Proteomics 10(18): 3272-3291.
- Sano A, Matsuda S, Wen TC, Kotani Y, Kondoh K, et al. (1994) Protection by prosaposin against ischemia-induced learning disability and neuronal loss. Biochem Biophys Res Commun 204(2): 994-1000.
- Kotani Y, Matsuda S, Wen TC, Sakanaka M, Tanaka J, et al. (1996) A hydrophilic peptide comprising 18 amino acid residues of the prosaposin sequence has neurotrophic activity in vitro and in vivo. J Neurochem 66(5): 2197-2200.
- Morita F, Wen TC, Tanaka J, Hata R, Desaki J, et al. (2001) Protective effect of a prosaposin-derived, 18-mer peptide on slowly progressive neuronal degeneration after brief ischemia. J Cereb Blood Flow Metab 21(11): 1295-1302.
- Igase K, Tanaka J, Kumon Y, Zhang B, Sadamoto Y, et al. (1999) An 18-mer peptide fragment of prosaposin ameliorates place navigation disability, cortical infarction, and retrograde thalamic degeneration in rats with focal cerebral ischemia. J Cereb Blood Flow Metab 19(3): 298-306.
- Lu AG, Otero DA, Hiraiwa M, O'Brien JS (2000) Neuroprotective effect of retro-inverso Prosaptide D5 on focal cerebral ischemia in rat. Neuroreport 11(8): 1791-1794.
- Nabeka H, Uematsu K, Takechi H, Shimokawa T, Yamamiya K, et al. (2014) Prosaposin Overexpression following Kainic Acid-Induced Neurotoxicity. PLoS One 9(12): e110534.
- Nabeka H, Saito S, Li X, Shimokawa T, Khan MSI, et al. (2017) Interneurons secrete prosaposin, a neurotrophic factor, to attenuate kainic acid-induced neurotoxicity. IBRO Reports 3: 17-32.
- Li X, Nabeka H, Saito S, Shimokawa T, Khan MSI, et al. (2017)Expression of prosaposin and its receptors in the rat cerebellum after kainic acid injection. IBRO Rep 2: 31-40.
- Miller RM, Kiser GL, Kaysser-Kranich TM, Lockner RJ, Palaniappan C, et al. (2006) Robust dysregulation of gene expression in substantia nigra and striatum in Parkinson's disease. Neurobiol Dis 21(2): 305-313.
- Liu J, Wang CY, O'Brien JS (2001) Prosaptide D5, a retro-inverso 11-mer peptidomimetic, rescued dopaminergic neurons in a model of Parkinson's disease. FASEB J 15(6): 1080-1082.
- Gao HL, Li C, Nabeka H, Shimokawa T, Saito S, et al. (2013) Attenuation of MPTP/MPP(+) toxicity in vivo and in vitro by an 18-mer peptide derived from prosaposin. Neuroscience 236: 373-393.
- Hiraiwa M, Campana WM, Wang CY, Otero DA, O'Brien JS (2001) A retro-inverso Prosaptide D5 promotes a myelination process in developing rats. Brain Res Dev Brain Res 128(1): 73-76.
- Unuma K, Chen J, Saito S, Kobayashi N, Sato K, et al. (2005) Changes in expression of prosaposin in the rat facial nerve nucleus after facial nerve transection. Neuroscience research 52(3): 220-227.
- Chen J, Saito S, Kobayashi N, Sato K, Terashita T, et al. (2008) Expression patterns in alternative splicing forms of prosaposin mRNA in the rat facial nerve nucleus after facial nerve transection. Neuroscience research 60(1): 82-94.
- Jolly S, Bazargani N, Quiroga AC, Pringle NP, Attwell D, et al. (2018) G protein-coupled receptor 37-like 1 modulates astrocyte glutamate transporters and neuronal NMDA receptors and is neuroprotective in ischemia. Glia 66(1): 47-61.
- Liu B, Mosienko V, Vaccari Cardoso B, Prokudina D, Huentelman M, et al. (2018) Glio- and neuro-protection by prosaposin is mediated by orphan G-protein coupled receptors GPR37L1 and GPR37. Glia 66(11): 2414-2426.
- Kunihiro J, Nabeka H, Wakisaka H, Unuma K, Khan MSI, et al. (2020) Prosaposin and its receptors GRP37 and GPR37L1 show increased immunoreactivity in the facial nucleus following facial nerve transection. PLoS One 15(12): e0241315.
- Gao HL, Li C, Nabeka H, Shimokawa T, Kobayashi N, et al. (2013) Decrease in prosaposin in the Dystrophic mdx mouse brain. PLoS One 8(11): e80032.
- Li C, Gao HL, Shimokawa T, Nabeka H, Hamada F, et al. (2013) Prosaposin expression in the regenerated muscles of mdx and cardiotoxin-treated mice. Histology and histopathology 28(7): 875-892.
- Islam F, Khan MSI, Nabeka H, Shimokawa T, Yamamiya K, et al. (2020) Age- and sex-associated changes in prosaposin and its receptors in the lacrimal glands of rats. Histology and histopathology 35(1): 69-81.
- Nabeka H, Shimokawa T, Doihara T, Saito S, Wakisaka H, et al. (2015) A prosaposin-derived Peptide alleviates kainic Acid-induced brain injury. PLoS One 10(5): e0126856.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




