
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6628
Research Article(ISSN: 2637-6628) 
Distribution of Multilayered Fiber Terminals in the Human Cerebellar Cortex. Visualization by Immunohistochemistry for Histamine Volume 2 - Issue 3
Anna Rizzi, Matteo Saccia and Vincenzo Benagiano*
- Department of Basic Medical Science Neuroscience and Sensory Organs, University of Bari, Italy
Received: February 12, 2019 ; Published: February 21, 2019
Corresponding author: Vincenzo Benagiano, Department of Basic Medical Science Neuroscience and Sensory Organs, University of Bari, Italy
DOI: 10.32474/OJNBD.2019.02.000139
Abstract
The distribution of histamine in the human cerebellar cortex was studied by light microscope immunohistochemistry using a rabbit polyclonal antibody anti-histamine. In all layers of the cerebellar cortex, were revealed punctate immunoreactive elements related to putative histaminergic nerve terminals. These findings provide insights into the existence of a histaminergic system in the human cerebellar cortex, presumably involved in the cerebrocerebellar circuit, the feedback circuit through which the cerebellum is widely connected with the neocortex and the hypothalamus and intervene in the regulation of motor and non-motor functions.
Keywords: Cerebrocerebellar Circuit; Hypothalamus; Cerebellum; Multilayered Fibers; Histamine
Introduction
Since Schmahmann and Sherman described a clinical syndrome, the cerebellar cognitive-affective syndrome, characterized by mental disorders and determined by cerebellar dysfunction [1], the cerebellum is considered to be involved also in the regulation of non-motor functions, including psychic functions, instinctive and emotional behaviors, and visceral activities [2]. The anatomical basis of these regulatory roles of the cerebellum is represented by the cerebrocerebellar circuit, a feedback circuit that bidirectionally connects the main superior cerebral centers and the cerebellum. Two main cerebrocerebellar circuit have been described, one established between neocortex and cerebellum, neocorticocerebellar circuit (NCC), one, between hypothalamus and cerebellum, hypothalamocerebellar circuit (HCC) [2].
The HCC consists of a descending limb, formed by the hypothalamocerebellar fibers, and an ascending limb, formed by cerebellohypothalamic fibers [3]. The hypothalamocerebellar fibers arise from widespread nuclei/regions of the hypothalamus including the paraventricular nucleus [4,5], the ventromedial nucleus [4,6], the tuberomammillary nucleus and the lateral region of the posterior zone [4,7]. The hypothalamocerebellar fibers run along the midbrain tegmentum, and reach the cerebellum, mainly through the ipsilateral superior cerebellar peduncle; in the cerebellum, they send collaterals to all the cerebellar nuclei, and terminate in the cortex of all the cerebellar lobes [8,9]. On the other hand, the cerebellohypothalamic fibers originate from all the cerebellar nuclei, particularly from the fastigium nucleus, enter the superior cerebellar peduncle, and ascend in the midbrain tegmentum up to the hypothalamus, where they terminate on the same nuclei and regions at the origin of the descending limb [10].
Studies have indicated that the hypothalamocerebellar fibers end in the cerebellar cortex with different ways from the other fibers afferent to it: precisely, they end on all the cortical layers, multilayered fibers, and synapse on granule neurons, Purkinje neurons and interneurons of the molecular layer [9,10]. The behavior of these fibers is clearly different from that of the mossy or climbing fibers, which, instead, selectively end in the granular layer or in the molecular layer [11]. Another difference between the multilayered fibers and the mossy and climbing fibers is that some multilayered fibers use histamine as a chemical neurotransmitter, while the mossy and climbing fibers predominantly use glutamate[12]. Accordingly, physiological studies have reported that histamine exerts postsynaptic excitation on granule and Purkinje neurons of the rat cerebellar cortex [13,14], and a study conducted by PET techniques has revealed histamine receptors in the human cerebellar cortex [15].
It is noteworthy that the tuberomammillary nucleus, the only proven source of histaminergic fibers in the central nervous system, is also one of the hypothalamic nuclei at the origin of hypothalamocerebellar fibers [16]. Aim of the present study was to analyze the fine distribution of histamine-containing axon terminals in the human cerebellar cortex with the goal of defining the morphological organization of its histaminergic nervous circuits and evaluating the interactions between the histaminergic terminals and the wide distributed GABAergic and glutamatergic terminals. The study was carried out by light microscopy immunohistochemical techniques based on a highly specific polyclonal antibody against histamine.
Material and Methods
The cerebellum of 12 adult patients aged between 23 and 61 years (mean age: 41±7) with no clinical history of neurological disease were removed at autopsy within 24 to 36 h after death. Fragments of 40 to 80 mm3 were taken from lobules of both vermis and hemisphere: central lobule (II-III), anterior quadrangulate lobule (HIV), simplex lobule (HVI), crus I and crus II (HVII), pyramid (VIII), tonsilla (HIX), flocculus (HX) (the lobules were designed according to nomenclature reported by Benagiano et al. [2]).
The fragments were fixed by immersion for 2-4 h depending on their size in saturated solution of picric acid containing 4% paraformaldehyde [17] and embedded in a semi-synthetic paraffin wax. Paraffin blocks were serially cut into 5-mm sections orthogonally oriented towards the cerebellar surface. The sections were incubated with polyclonal antibodies, developed in rabbit using histamine conjugated to succinylated KLH as immunogen (Sigma, MO, USA), diluted 1:50 in PBS. The immunoreactions were revealed by the streptavidinperoxidase complex (Dako LSAB kit, Dako, CA, USA), using 3-amino-9-ethyl-carbazole (Vector Laboratories, CA, USA) as a chromogen.
Negative controls: total abolition of the immunolabeling was obtained by treating sections with non-immune serum or omitting the primary antibodies.
Results
The histamine immunoreactivity was observed in all the layers of the human cerebellar cortex exclusively in punctate elements. The qualitative pattern of distribution of the immunoreactivity did not show significant difference in the different lobes/lobules examined. The immunoreactive puncta were interpreted as terminals of histamine-containing fibers, selectively revealed by the immunoreactions probably due to their greater amount of histamine, compared to that of the fibers to which they belong. No immunoreactivity was detected in neuronal bodies and processes of the cortex, suggesting that all the immunoreactive axon terminals observed here were of extrinsic origin.
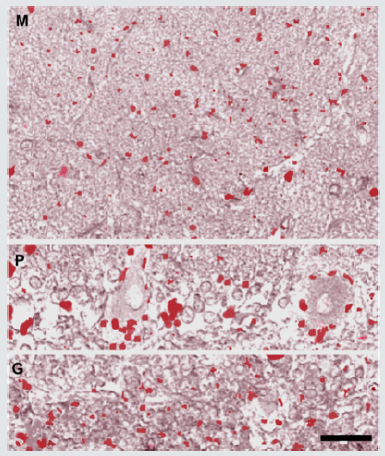
In the molecular layer, only a small number of isolated immunoreactive puncta was observed in the neuropil or on the surface of neuronal bodies and processes of the layer; they appeared uniformly concentrated throughout the layer. In the Purkinje neuron layer, some puncta were observed on the body surface of a few of Purkinje neurons, concentrating on their deep pole (preaxon region). In the granular layer, a relatively larger number of immunoreactive puncta was observed, isolated or gathered in small groups, in the islet of neuropil comprised among the granules (Figure 1).
Figure 1: Histamine immunoreactivity was detected in puncta distributed in all the layers of the human cerebellar cortex (tonsilla). In the molecular layer (M), a small number of isolated immunoreactive puncta was observed mainly in the neuropil. In the Purkinje neuron layer (P), some puncta were observed on the body surface of a number of Purkinje neurons, concentrating on their deep pole. In the granular layer (G), the puncta were observed isolated or gathered in small groups in the islet of neuropil comprised among the granules. Scale bar: 40x.
Discussion
The results of the present study demonstrated the existence of terminals immunoreactive for histamine in the human postmortem cerebellar cortex, presumably belonging to a contingent of hypothalamocerebellar fibers, the descending limb of the HCC. This is a feedback circuit that bidirectionally connects hypothalamus and cerebellum and is believed to be involved in regulation of affective psychic functions, instinctive and emotional behaviors, and visceral activities [2]. The histamine-containing terminals were detected in all cerebellar lobes/lobules and distributed in all layer of the cerebellar cortex, multilayered fibers. This indicated that histamine action is exerted ubiquitously in the cerebellar cortex. However, the detection of a relatively small number of terminals, indicated that this action, although ubiquitous, is rather selective, unlike that of the glutamate, which is widely exerted through mossy and climbing fibers [12].
In other terms, the histamine-immunoreactive terminals act on selected neuronal groups intervening in the processing of afferents from hypothalamus and originating efferents constituting the ascending limb of the HCC. The latter is firstly represented by corticonuclear fibers projecting on cerebellar nuclei. Since they originate from all cerebellar lobes, these corticonuclear fibers project on all the cerebellar nuclei, namely the fastigium, interpositum and dentate nucleus. These data agree with those indicating an involvement of all the cerebellar nuclei in the HCC [8]. The selected nuclear neurons give rise to the cerebellohypothalamic fibers, the closing section of the ascending limb. It is interesting that neurons in all the cerebellar nuclei received histaminergic terminals from collaterals of the hypothalamocerebellar fibers [18-20]. In practice, histamine would be important both in regulating some intrinsic circuits of the cortex, from which activity the efferents destined to the nuclei arise, and in regulating the activity of some nuclear neurons, at the origin of efferents to the hypothalamus.
The descending limb of HCC and NCC, the two components of the cerebrocerebellar circuit, terminates with different distributional patterns in the cerebellar cortex: HCC terminates ubiquitously, with few histaminergic terminals belonging to multilayered fibers, NCC terminates in the cortex of the cerebrocerebellum, with a greater number of glutamatergic terminals belonging to mossy fibers. It is likely that interactions between histaminergic terminals (i.e., HCC) and glutamatergic terminals (i.e., NCC) occur in the regions of cerebellar cortex where they converge. Such interactions would underlie the combined role of the cerebellum in the regulation of somatic and non-somatic functions. These results open new perspectives in the interpretation of the physiopathology of neurological and psychiatric disorders depending on dysfunction of the cerebellum and offer new therapeutic possibilities. In this view, the use of histamine has already been proposed for the treatment of cerebellar ataxia [21,22]. Further applications are needed in light of the involvement of the cerebellum in an increasing number of pathologies, including disorders of motor, psychic and visceral functions.
References
- Schmahmann J D, Sherman J C (1997) Cerebellar cognitive affective syndrome. Int Rev Neurobiol 41: 433-440.
- Benagiano V, Rizzi A, Lorusso L, Flace P, Saccia M, et al. (2018) The functional anatomy of the cerebrocerebellar circuit: A review and new concepts. J Comp Neurol 526(5): 769-789.
- Haines DE, Dietrichs E, Mihailoff GA, McDonald EF (1997) The cerebellar-hypothalamic axis: basic circuits and clinical observations. Int Rev Neurobiol 41: 83-107.
- Haines DE, Dietrichs E (1984) An HRP study of hypothalamocerebellar and cerebello-hypothalamic connections in squirrel monkey (Saimiri sciureus). J Comp Neurol 229(4): 559-575.
- Hefco V, Olariu A, Hefco A, Nabeshima T (2004) The modulator role of the hypothalamic paraventricular nucleus on immune response. Brain Behav Immun 18(2): 158-165.
- Okamoto S, Ibaraki K, Hayashi S, Saito M (1996) Ventromedial hypothalamus suppresses splenic lymphocyte activity through sympathetic innervation. Brain Res 739(1-2): 308-313.
- Baciu I, Hriscu M, Saulea G (2003) Hypothalamic mechanisms of immunity. Int J Neurosci 113(2): 259-277.
- Dietrichs E, Haines DE, Roste GK, Roste LS (1994) Hypothalamocerebellar and cerebellohypothalamic projections: circuits for regulating nonsomatic cerebellar activity? Histol Histopathol 9(3): 603-614.
- Panula P, Takagi H, Inagaki N, Yamatodani A, Tohyama M, et al. (1993) Histamine-containing nerve fibers innervate human cerebellum. Neurosci Lett 160(1): 53-56.
- Li B, Zhu JN, Wang JJ (2014) Histaminergic afferent system in the cerebellum: structure and function. Cerebellum Ataxias 1: 5.
- Cavdar S, Onat F, Aker R, Sehirli U, San T, et al. (2001) The afferent connections of the posterior hypothalamic nucleus in the rat using horseradish peroxidase. J Anat 198(Pt 4): 463-472.
- Benagiano V, Lorusso L, Flace P, Girolamo F, Rizzi A, et al. (2011) VAMP-2, SNAP-25A/B and syntaxin-1 in glutamatergic and GABAergic synapses of the rat cerebellar cortex. BMC Neurosci 12: 118.
- Li WC, Tang XH, Li HZ, Wang JJ (1999) Histamine excites rat cerebellar granule cells in vitro through H-1 and H-2 receptors. J Physiology Paris 93(3): 239-244.
- Tian L, Wen YQ, Li HZ, Zuo CC, Wang JJ (2000) Histamine excites rat cerebellar Purkinje cells via H2 receptors in vitro. Neurosci Res 36(1): 61-66.
- Ashworth S, Rabiner EA, Gunn RN, Plisson C, Wilson AA, et al. (2010) Evaluation of C-11-GSK189254 as a novel radioligand for the H-3 receptor in humans using PET. J Nucl Med 51(7): 1021-1029.
- Haas HL, Sergeeva OA, Selbach O (2008) Histamine in the nervous system. Physiol Rev 88(3): 1183-1241.
- Benagiano V, Flace P, Virgintino D, Rizzi A, Roncali L, et al. (2000) Immunolocalization of glutamic acid decarboxylase in postmortem human cerebellar cortex. A light microscopy study. Histochem Cell Biol 114(3): 191-195.
- Tang B, Zhang J, Yu L, Li HZ, Zhu JN, et al. (2008) Excitation of histamine on neuronal activity of cerebellar fastigial nucleus in rat. Inflamm Res 57(Suppl 1): S41-S42.
- Song YN, Li HZ, Zhu JN, Guo CL, Wang JJ (2006) Histamine improves rat rota-rod and balance beam performances through H(2) receptors in the cerebellar interpositus nucleus. Neuroscience 140(1): 33-43.
- Qin YT, Ma SH, Zhuang QX, Qiu YH, Li B, et al. (2011) Histamine evokes excitatory response of neurons in the cerebellar dentate nucleus via H2 receptors. Neurosci Lett 502(3): 133-137.
- Prokopenko SV, Rudnev VA, Afanasieva EV, Abramov VG (2004) A use of betaserc in ataxic syndromes. Zh Nevropatol Psikh 104(8): 41-45.
- Bardgett ME, Points M, Kleier J, Blankenship M, Griffith MS (2010) The H3 antagonist, ciproxifan, alleviates the memory impairment but enhances the motor effects of MK-801 (dizocilpine) in rats. Neuropharmacology 59(6): 492-502.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




