Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6628
Research Article(ISSN: 2637-6628) 
Association of IL-6 & C-Reactive Protein with Cognitive Decline in Dementia Volume 6 - Issue 1
Abhishek Khatri1, Om Prakash1, Suman Kushwaha2, Chandra Bhushan Tripathi3, Rachna Agarwal4*
- 1Department of Psychiatry, Institute of Human Behaviour & Allied Sciences, India
- 2Department of Neurology, Institute of Human Behaviour & Allied Sciences, India
- 3Department of Biostatistics, Institute of Human Behaviour & Allied Sciences, India
- 4Department of Neurochemistry, Institute of Human Behaviour & Allied Sciences, India
Received:August 12, 2021 Published:September 3, 2021
Corresponding author:Rachna Agarwal, Associate Professor, Department of Neurochemistry, Institute of Human Behaviour & Allied Sciences, India
DOI: 10.32474/OJNBD.2021.06.000226
Abstract
Inflammatory markers Interleukin-6 (IL-6) and C-Reactive Protein (CRP) associated are with high rates of cognitive decline. Inverse relation between IL-6 levels and Hindi Mental Status Examination (HMSE) scores has been studied. IL-6 is a multifunctional cytokine is a key regulator of CRP. A cross sectional study was performed to examine the association between inflammatory markers (including IL-6, CRP and albumin) and cognition in subjects attending Neurobehavior clinic. 66 Dementia patients with average age of 69 ± 0.06 years were included in the study (44 Alzheimer’s Disease & 22 Vascular Dementia). AD cases were diagnosed according to ICD-10 and National Institute of Neurological Disorders and Stroke-Alzheimer Disease and Related Disorders Criteria (NINCDS-ADRDS) for probable AD and Vascular dementia cases were diagnosed with NINDS-AIREN criteria. All dementia cases were assessed using Hindi Mental Status Examination (HMSE) for cognitive function and Clinical Dementia Rating Scale (CDR) was applied for staging severity of dementia. Out of three inflammatory biomarkers (IL-6, CRP and albumin), IL-6 showed strong positive correlation with duration of illness in dementia cases, whereas CRP had weak positive correlation with duration of illness. Albumin showed no such relationship in dementia cases. On elimination of confounding variables, age and duration of illness, strength of relationship between HMSE and IL-6 was moderately negative but significant. Such relationship was nonexistent between HMSE and CRP as well as albumin.
Introduction
Cognitive decline in dementia is associated with cytokine dysregulation. Neuroinflammation acts as independent pathological factor in early preclinical stages of Alzheimer’s disease, along with other risk factors like systematic infections [1,2], decreased physical activity [3,4] which also have an inflammatory component. Furthermore, some epidemiological studies demonstrate that non-steroidal anti-inflammatory drugs (NSAIDs) can prevent or retard AD cognitive decline [5], although other studies have found little improvement [6]. This slowing of cognitive decline may be attributed to decreased inflammation since NSAID therapy substantially reduces the number of activated microglia associated with plaques [7].
Interleukin-6 (IL-6) and C-reactive protein (CRP) have been most widely studied in population, showing IL-6 and CRP associated with high rates of cognitive decline. The health ABC study reported subjects with CRP and/or IL-6 levels in the upper tertile were at higher risk of cognitive decline compared to those in the lowest [8]. Rotterdam study also established associated risk of developing dementia including Alzheimer’s disease with raised IL-6 and CRP levels. However, elevated CRP levels had stronger association with vascular dementia [9]. Another longitudinal follow up study performed on population based samples of non disabled elderly people reported that subjects with higher IL-6 level were at a higher risk of developing future cognitive decline [10]. Another multi ethnic cohort study observed inverse relation between IL-6 levels and MMSE scores [11].
IL-6 is a multifunctional cytokine with dual role, predominantly pro-inflammatory [12] with some anti-inflammatory property.
It is also a key regulator of CRP. Microglia and astrocytes are the two main cell components of the CNS that play a pivotal role in the neuroinflammatory process responsible for neuro-biological homeostasis. During aging, there is pro-inflammatory shift in CNS leading to proliferation of activated microglia and astrocytes resulting in continuous secretion of IL-6 [13].The production of cytokines like IL-6 in brain of patients with AD not only increases the amyloid deposition in cortical areas, but also leads to blood brain barrier dysfunction. This increased permeability of blood brain barrier may cause peripheral spill over of these cytokines produced in the brain. As they enter the plasma, they stimulate the production of peripheral inflammatory mediators like IL-6 and CRP. This activation of peripheral inflammation leads to further amyloid deposition which further stimulates production of IL-6 [13,14]. These findings suggest that peripheral inflammation is not only the result also a causative agent in pathogenesis of AD. Bettcher et al, [15] has also reported modest associations between plasma and CSF levels for IL-6 (r=0.16, p=0.05). Similarly CRP, a marker of inflammation, lead to low grade chronic inflammation which may lead to cognitive impairment and predispose elderly for the development of dementia and act as risk factor for the development of AD and Vascular dementia [16].
Our study group earlier evaluated IL-6, CRP and albumin levels from the patients with AD and vascular dementia as compared with age and sex matched nondemented subjects to elucidate their role in the pathogenesis of dementia and found significantly high levels of IL-6 in AD as compared to controls (Paper under publication). In the current study, we performed a cross sectional study to examine the association between inflammatory markers (including IL-6, CRP and albumin) and cognition in subjects attending outpatient services after adjusting for age and duration of illness.
Material and Methods
Subjects
In the present cross sectional study, subjects were enrolled from outpatient services of Psychiatry and Neurology departments, Institute of Human Behavior & Allied Sciences (IHBAS), New Delhi, India. Sixty six Dementia patients (42 males and 24 females) were included in the study after applying inclusion and exclusion criteria. The study was approved by the ethical committee of IHBAS. Written consent was obtained from all the patients and controls.
Socio-Demographic Profile
A designed to collect personal information like religion, marital status, education level, occupation, smoking habit, alcohol use, family history of dementia along with clinical history including chronic illness (like dyslipidemia, DM etc), treatment history, history of substance use.
Clinical Assessment
AD cases were diagnosed according to ICD-10 and National Institute of Neurological Disorders and Stroke-Alzheimer Disease and Related Disorders Criteria (NINCDS-ADRDS) [17] for probable AD. Vascular dementia was diagnosed with NINDS-AIREN criteria [18]. All dementia cases were assessed using Hindi Mental Status Examination (HMSE) for cognitive function [19] and Everyday Abilities Scale for India (EASI) for activities of daily living [20]. Clinical Dementia Rating Scale (CDR) was applied for staging severity of dementia [21].
Laboratory Analysis
All subjects underwent estimation of Interleukin-6 (IL-6), C-Reactive Protein (CRP) and albumin along with routine laboratory tests in serum. At baseline non fasting serum samples were obtained from patients taking all the standard precautions. The specimen was centrifuged within 30 min of sample collection to separate the serum and examined for routine biochemistry. Serum samples for evaluation of IL-6 and CRP were stored at −20°C until analysis. Serum IL-6 was measured on by Electrochemiluminescence immunoassay Analyzer, Cobas e 611 (M/s Roche Diagnostics Asia Pacific Pvt. Ltd, Singapore) using sandwich immunoassay technique. CRP was measured using Random Access Discrete Auto Analyser, Pictus 700 from Spectrum Pvt. Ltd. using commercially available kits. The Coefficient of Variation (CV%) for IL-6 and CRP was less than 10%.
Statistical Analysis
Frequency, percentage and descriptive statistics (Mean, Standard deviation, Median and Inter quartile range) were calculated. Pearson’s correlation coefficients and their 95% confidence intervals were calculated to explore the linear relationship between two continuous variables. As Pearson’s correlation coefficient cannot eliminate the effect of confounding variables, so the partial correlation coefficients were calculated to find out absolute strength of relationship after adjusting the effect of confounding variables. The 95% confidence intervals for partial correlation coefficients were also calculated by bootstrapping method. All the analysis was carried out by SPSS 25.0 software (IBM SPSS Statistics for Windows, 2018).
Results
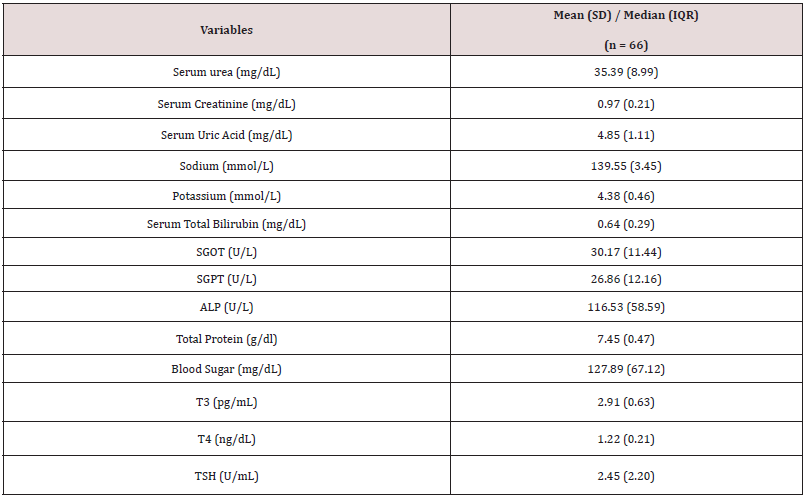
Table 1 shows the details of socio demographic profile of the study subjects. The average age (SD) of the study subjects was 69.03 (7.63) years and majority of subjects were males and were residing in rural area (74.20%). 80% subjects were married, whereas 45% were educated upto matric level. 74.20% subjects were nonsmokers and 86.40% were non alcoholic. The mean (SD) of duration of illness (years), body mass index, HMSE score, and CDR score were 3.80(2.19), 23.05(3.09), 13.76(3.95) and 5.69(3.74) respectively. 90.90% subjects had family history of dementia. Only 24.20% subjects had dyslipidemia and DM. Table 2 shows the result of biochemical variables measured in all study subjects.
As it was anticipated that age of subjects and duration of illness may have the relationship with HMSE score and level of inflammatory markers (IL-6, CRP & albumin), so the strength of relationship between age versus IL-6, age versus CRP, age versus albumin had explored by Pearson’s correlation coefficient along with 95% confidence interval. Similarly, the strength of relationship was explored between duration of illness with HMSE, IL6, CRP and serum albumin separately. It was also suspected that gender may have the significant contribution in strength of relationship, so the same analysis were conducted for male and female subjects separately apart from total study subjects. Table 3 shows that for total subject, age had significant but weak negative correlation (r = -0.21, 95% CI: -0.39, -0.05) with HMSE score. It was slightly more in male subjects (r = -0.27, 95% CI: -0.49, -0.05) as compared to total subjects and in female subjects (r = -0.12, 95% CI: -0.57, 0.21), it was much lower and statistically not significant. The IL 6, CRP and serum albumin levels had almost non-existent strength of relationship with age for total subjects as well as among male & female separately.
Table 3: Pearson’s Correlation of Age and Duration of illness with Inflammatory Markers and HMSE Score in Dementia Cases.
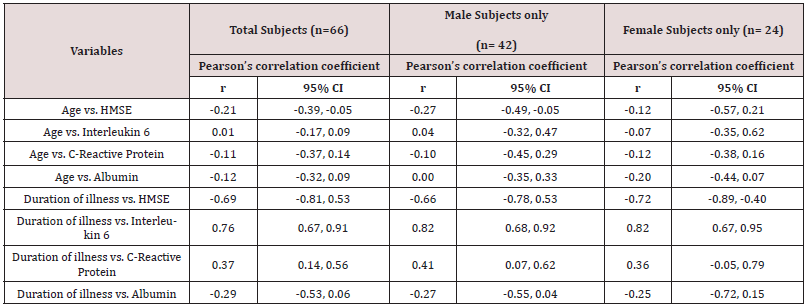
The duration of illness was strongly but inversely related with HMSE for all subjects (r = 0.69, 95% CI: -0.81, -0.53) and for both genders too separately whereas it was much stronger among females (r = -0.66, 95% CI: -0.78, -0.53) as compared to males (r = -0.72, 95% CI: -0.89, -0.40). The duration of illness had strong, positive and statistically significant relationship with IL 6 for total subjects (r = 0.76, 95% CI: 0.67, 0.91) as well as for male (r = 0.82, 95% CI: 0.68, 0.92) and female (r = 0.82, 95% CI: 0.67, 0.95) subjects separately.
The CRP is moderately but positively correlated with duration of illness among total subjects (r = 0.37, 95% CI: 0.14, 0.56) and similar strength & type of relationship were observed among males (r = 0.41, 95% CI: 0.07, 0.62) and female (r = 0.36, 95% CI: -0.05, 0.79) separately between these two variables. The correlation between duration of illness and serum albumin was very weak and statistically not significant. As per objective of the present study, relationship between HMSE score, a measure of cognitive decline, and level of inflammatory markers (IL-6, CRP and albumin) had been explored separately. As it is well known that two variable age and duration of illness were also correlated with HMSE, IL-6, CRP and albumin, so these two variables (age & duration of illness) may confound the strength of relationship between HMSE & IL- 6, HMSE & CRP and HMSE & albumin. To eliminate the effect of age and duration of illness, partial correlation method was used for examining the strength of relationship between HMSE & IL- 6, HMSE and CRP and HMSE and albumin. Table 4 shows that the partial correlation coefficient (strength of relationship) between HMSE and IL-6 was moderately negative but statistically significant among total subjects (r = -0.39, 95% CI: -0.68, -0.21) whereas it was slightly higher among males (r = -0.57, 95% CI; -0.73, -0.40) as compared to females (r = -0.51, 95% CI: -0.84, -0.31). The strength of relationship was observed almost non-existent between HMSE versus CRP and HMSE versus albumin.
Table 4: Pearson’s Correlation and Partial Correlation (Age & Duration of Illness Adjusted) between Inflammatory Markers and HMSE Score in Dementia Cases.

Discussion
This study adjusted both potential cofounders age of onset and duration of illness, while conducting the partial correlation between IL-6, CRP and albumin with HMSE scores to explore the strength of relationship between these three inflammatory markers and HMSE scores. This may help in establishing them as biomarkers for assessing the progress of dementia. The study results found that the strength of relationship between HMSE and IL-6 was moderately negative but statistically significant. IL-6, a non-specific pro-inflammatory biomarker, is an independent risk factor for cognitive decline, owing to its neurotoxic function. Wright et al, [11] examined IL-6 and CRP levels as independent variables in relation to MMSE scores as the dependent variable in stroke-free population. They showed inverse relation between IL-6 level and MMSE, but no relation was found between CRP and MMSE after adjusting for a history of vascular risk factors.
Our study results indicate that the strength of relationship was almost non-existent between HMSE versus CRP & albumin. Beydounet et al, [13] has reported increased levels of CRP in newly diagnosed AD cases regardless of age of onset suggesting that an amplified neuroinflammatory reaction plays an important role in the pathogenesis and progression of cognitive decline in AD [3]. Low serum albumin levels, acting as a negative acute phase reactant, reflect decreased liver function in the elderly and predispose to decreased antioxidant levels which may accelerate cognitive decline [22] . Inflammation due to acute and chronic events may have deleterious effect on cognition, which is further compounded by vascular risk factors like [22,23] diabetes and cardiovascular events. A meta-analysis performed by Darweeshet et al, [24] reported association of CRP with an increased risk of all-cause dementia, but HR of CRP for AD was lower as compared to it, but not significant. Very few studies have been taken up to investigate the association between CRP and VaD than with all cause dementia or AD alone. However, VaD patients are likely to have high prevalence of CVD, contributing to the association between inflammatory markers and incident of VaD [25]. In patients with AD, inflammation contributes to cognitive impairment through cerebrovascular pathology. This suggests that the association of CRP and VaD exist several years before onset of dementia. This further explains weak relationship between CRP and albumin with duration of illness in our study results.
Various studies have examined the association of serum albumin with cognitive function [26,27]. Beydoun et al, [13] demonstrated the protective effect of serum albumin on cognition. They reported higher baseline serum albumin to improved attention in the total population and a better baseline performance in psychomotor speed. No such relationship of serum albumin with duration of illness could be elicited in the present study.
In our study, weak relation was found between HMSE, a measure of cognitive decline and CRP, whereas no relation of albumin could be elicited with HMSE. There can be number of reasons for the same. Firstly, confirmed cases of all-cause dementia were included in the present study attending outpatient services at the institute regularly, thus it is difficult to say whether these findings are applicable to all stages of all-cause dementia and will be able to address the relationship between mid-life inflammatory markers and cognitive decline. Studies show that measurement of inflammatory markers at midlife reported more significant and consistent results [28] and may be a better indicator of future dementia risk. Secondly, out of total 66 dementia cases, 22 cases were of AD. As previous studies showed no significant risk of AD alone associated with increased levels of IL-6 and CRP [29] similar to our study results where robust relation among inflammatory markers and cognitive decline could not be established.
Our study shows that 80% subjects recruited were having low education. Berkman et al, [30] reported similar findings. They suggested that low education may increase the risk of cognitive disorders and vascular disease and act as an independent predictor of cognitive function. In present study, a strong and positive significant relation was found between IL-6 and duration of illness in both genders. CRP was positively, but moderately correlated with duration of illness among total subjects in the present study. Studies show [31-34] hat IL-6 crosses the blood brain barrier and also detected in blood representing the spill-over from brain. Bettchet et al, [15] also did not find any association of age with inflammatory markers, including IL-6. Neither education level nor family history of AD diagnosis was significantly associated with IL-6 and CRP in their study.
The study has its own limitations. Firstly, inflammatory markers i.e IL-6, CRP and albumin were measured only once in serum of subjects recruited. It would have been more effective if serial levels of these biomarkers should have been measured from early stage of dementia in mid-life, old and oldest subjects to differentiate the association between inflammation and dementia in different age group patients. Secondly, various medical conditions, which may affect the acute and inflammatory processes, not related to dementia and normal aging process and can affect the inflammatory marker levels have not been controlled while recruiting the subjects. Lastly, in this study peripheral markers were estimated in blood rather than within the brain, which may not explain intracerebral inflammatory processes. In view of these limitations, researchers may plan longitudinal serial measurements of these three inflammatory biomarkers in AD, VaD and other dementias in different age groups to establish relationship between IL-6, CRP and albumin and cognition. Further, it would be ideal to establish these relationships across APOE ɛ4 status.
Conclusion
IL-6 was found to be the most promising inflammatory marker showing strong positive correlation with duration of illness in dementia cases, whereas CRP had weak positive correlation with duration of illness. However, there was no relationship between albumin and dementia cases. Negative relationship was detected between HMSE and IL-6 only, on elimination of confounding variables, age and duration of illness.
References
- Iwashyna TJ, Ely EW, Smith DM, Langa KM (2010) Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304(16): 1787-1794.
- Holmes C (2013) Review: Systemic inflammation and Alzheimer’s disease. Neuropathol Appl Neurobiol 39(1): 51-68.
- Larson EB, Wang L, Bowen JD, Mc Cormick WC, Teri L, et al. (2006) Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Annals of Internal Medicine 144(2): 73-81.
- Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, et al. (2009) Physical activity, diet, and risk of Alzheimer disease. Jama 302(6): 627-637.
- Launer L (2003) Nonsteroidal anti-inflammatory drug use and the risk for Alzheimer's disease: Dissecting the epidemiological evidence. Drugs 63(8): 731-739.
- Reines SA, Block GA, Morris JC, Liu G, Nessly ML, et al. (2004) Rofecoxib: No effect on Alzheimer's disease in a 1-year, randomized, blinded, controlled study. Neurology 62(1): 66-71.
- Heneka MT, Sastre M, Dumitrescu Ozimek L, Hanke A, Dewachter I, et al. (2005) Acute treatment with the PPAR{gamma} agonist pioglitazone and ibuprofen reduces glial inflammation and A{beta}1-42 levels in APPV717I transgenic mice. Brain 128(6): 1442-1453.
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, et al. (2004) The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 292(18): 2237-2242.
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, et al. (2004) Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol 61(5): 668-672.
- Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, et al. (2002) Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology 59(3): 371-378.
- Wright CB, Sacco RL, Rundek TR, Delman JB, Rabbani LE, et al. (2006) Interleukin-6 is associated with cognitive function: The Northern Manhattan Study. Journal of Stroke and Cerebrovascular Diseases 15(1): 34-38.
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, et al. (2000) Inflammation and Alzheimer’s disease. Neurobiology of aging 21(3): 383-421.
- Beydoun MA, Dore GA, Canas JA, Liang H, Beydoun HA, et al. (2018) Systemic Inflammation Is Associated with Longitudinal Changes in Cognitive Performance Among Urban Adults. Frontiers in Aging Neuroscience 10(313): 1-12.
- Eikelenboom P, Hoozemans JJ, Veerhuis R, van Exel E, Rozemuller AJ, et al. (2012) Whether, when and how chronic inflammation increases the risk of developing late-onset Alzheimer's disease. Alzheimer's research & therapy 4(3): 15.
- Bettcher BM, Johnson SC, Fitch R, Casaletto KB, Heffernan KS, et al. (2018) CSF and Plasma Levels of Inflammation Differentially Relate to CNS Markers of Alzheimer’s Disease Pathology and Neuronal Damage. J Alzheimers Dis 62(1): 385-397.
- Hage FG, Szalai AJ (2007) C-reactive protein gene polymorphisms, C-reactive protein blood levels, and cardiovascular disease risk. Journal of the American College of Cardiology 50(12): 1115-1122.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34(7): 939-944.
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, et al. (1993) Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43(2): 250-260.
- Ganguli M, Ratcliff G, Chandra V, Sharma S, Gilby J, et al. (1995) A Hindi version of the MMSE: The development of a cognitive screening instrument for a largely illiterate rural elderly population in India. Int J Geriatr Psychitry 10(5): 367-377.
- Fillenbaum GG, Chandra M, Pandav R, Seaberg EC (1999) Development of an activities of daily living scale to screen for dementia in an illiterate rural older population in India. Age Ageing 28(2): 161-168.
- Morris JC (1993) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43(11): 2412-2414.
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286(3): 327-334.
- Ridker PM, Hennekens CH, Buring JE, Rifai N (2000) C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New England Journal of Medicine 342(12): 836-843.
- Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, et al. (2000) Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with alzheimer’s disease: Peripheral inflammation or signals from the brain?. Journal of Neuroimmunology 103(1): 97-102.
- Koyama A, O Brien J, Weuve J, Blacker D, Metti AL, et al. (2013) The Role of Peripheral Inflammatory Markers in Dementia and Alzheimer’s Disease: A Meta-Analysis. J Gerontol a Biol Sci Med Sci 68(4): 433-440.
- Song IU, Chung SW, Kim YD, Maeng LS (2015) Relationship between the hs-CRP as non-specific biomarker and Alzheimer’s disease according to aging process. Int J Med Sci 12(8): 613-617.
- Ravaglia G, Forti P, Maioli F (2007) Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiol Aging 28912): 1810-1820.
- Darweesh SKL, Wolters FJ, Ikram MA, de Wolf F, Bos D, et al. (2018) Inflammatory markers and the risk of dementia and Alzheimer’s disease: A meta-analysis. Alzheimer’s & Dementia 14(11): 1450-1459.
- Koyama T, Kuriyama N, Ozaki E, Matsui D, Watanabe I, et al. (2016) Serum albumin to globulin ratio is related to cognitive decline via reflection of homeostasis: A nested case-control study. BMC Neurol 16(1): 253.
- Berkman LF (1986) The association between educational attainment and mental status examinations: Of etiologic significance for senile dementias or not?. Journal of Chronic Diseases 39: 171-175.
- Galimberti D, Venturelli E, Fenoglio C, Guidi I, Villa C, et al. (2008) Intrathecal levels of IL-6, IL-11 and LIF in Alzheimer’s disease and frontotemporal lobar degeneration. J Neurol 255(4): 539-544.
- Llewellyn DJ, Langa KM, Friedland RP, Lang IA (2010) Serum albumin concentration and cognitive impairment. Curr Alzheimer Res 7(1): 91-96.
- Murayama H, Shinkai S, Nishi M, Taniguchi Y, Amano H, et al. (2017) Albumin, hemoglobin, and the trajectory of cognitive function in community-dwelling older Japanese: A 13-year longitudinal study. J Prev Alzheimers Dis 4(2): 93-99.
- Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, et al. (2002) Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Asia Aging Study. Ann Neurol 52: 168-174.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...