Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-6921
Review Article(ISSN: 2641-6921) 
Memory Metals or Shaping Alloys Volume 3 - Issue 4
Bahman Zohuri1,2*, Farahnaz Behgounia1, and Masoud J Moghaddam2
- 1Golden Gate University, Ageno School of Business, USA
- 2Galaxy Advanced Engineering, Albuquerque, USA
Received: February 10, 2021; Published: February 19, 2021
*Corresponding author: Bahman Zohuri, Golden Gate University, Ageno School of Business, San Francisco, California 94105, USA
DOI: 10.32474/MAMS.2021.03.000170
Abstract
Past few decades of research around nanomaterials has brought up a new interest of research by engineers and scientists in an area known as Memory Metals or Shaping Alloys and this is the subject of this A short Review as an article presented here. Shape Memory Alloys that often called ‘Memory Metals’ are a class of metal alloys that “remember” their original physical forms and shapes. When the metals are bent or twisted from their original form or shape, they can retain their initial form, when heated to a certain level of temperature.
Keywords: Physical form; original shape; material, alloy; physical and chemical properties; alloy; memory; industrial applications
Introduction
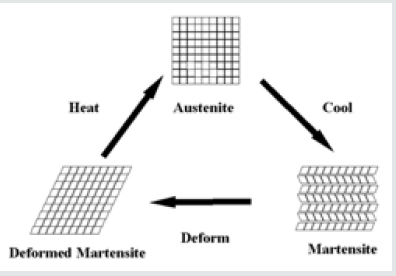
In 1965, the first of a series of metal alloys of nickel and titanium was produced by the Naval Ordnance Laboratory. These alloys are called Nitinol, for Nickel Titanium Naval Ordnance Laboratory. Many of the alloys have a rather remarkable property: they remember their shape. This “smart” property is the result of the substance’s ability to undergo a phase change-a kind of atomic ballet in which atoms in the solid subtly shift their positions in response to a stimulus like a change in temperature or application of mechanical stress. A simple demonstration involves bending a sample, then exposing it to a source of heat like hot air or hot water. The sample recovers its original shape as its temperature is raised above the temperature corresponding to the phase change. This temperature may be tuned by varying the ratio of nickel to titanium atoms in the solid by a few percent relative to a 1:1 ratio. Shape Memory Alloys (SMAs) present two distinct crystal structure or phases and they are type of metals that after being shaped they can come back and reverse themselves to their original form. Temperature and internal stresses (which play a part in super-elasticity) determine the phase that the SMA will be at. Martensite exists at lower temperatures, and austenite exists at higher temperatures.
When a SMA is in martensite form at lower temperatures, the metal can easily be deformed into any shape. When the alloy is heated, it goes through transformation from martensite to austenite. In the austenite phase, the memory metal “remembers” the shape it had before it was deformed. From the stress vs. temperature graph below, one can see that at low stress and low temperature, martensite exists. At higher temperature and higher stress, austenite exists. See (Figure 1) Memory alloys also demonstrate great rates of super-elasticity. For example, eyeglass frames are in a martensite phase. Bending the arms in half (at room temperature) introduces a phase change at the bend to austenite. Austenite is not stable at room temperature, and because systems always seek lower energy states, the austenite will change back to the martensite phase, and to do this, the arm must bend back. The most common memory metal is called NiTinol, consisting of equal parts of nickel and titanium. The Table 1 below displays having shape memory effects. Note that: The memory transfer temperature is the temperature that the memory metal or alloy changes back to the original shape that it was before deformation. This temperature can be very precise, within 1 or 2 degrees of the desired temperature.
Heating is the only way that most memory metals retain their original shape. Since heat is the property that determines the shape of the metal, heat is the first property used for manipulation for formation. If an alloy is subjected to the same heating and deformation, the alloy will begin to acquire two-way training. The treatment for a NiTinol wire is, for example:
- The wire is hot/cold worked (stretched) by 3% when it is in the martensite phase.
- The wire is then heated to austenite finish (AF) to recover its shape.
- The wire is then cooled to martensite.
Memory transfer temperatures can be altered by slight changes in composition, and by slight changes in heat treatment. As noted above, Nitinol is an alloy of nearly equal numbers of nickel and titanium atoms, with the exact amounts varied to match the temperature of the phase change to the application. The alloy can exist in either of two structures (phases) at room temperature, depending on the exact ratio of nickel to titanium atoms. The structure found above the temperature of the phase change possesses the high symmetry of a cube and is called austenite; the structure found below the temperature of the phase change is much less symmetric and is called martensite. In the martensite phase the material is very elastic, while in the austenite phase the material is comparatively rigid.
Nitinol can be “trained” to have a new shape while in the austenite phase by deforming it into the desired shape. As it then cools to below the phase transition temperature, the material enters the martensite phase. In the martensite phase the shape can then be changed by mechanical stress: groups of atoms that were “leaning” in one direction will accommodate the mechanical stress by “leaning” in another direction, as allowed by the less symmetric structure. The sample will revert to the shape enforced upon it while it was in the austenite phase by returning it to the austenite phase through an increase in its temperature. The thermal energy acquired by the shape through heating it provides the energy the atoms need to return to their original positions and the sample to its original shape. See (Figure 2) for presentation of Memory metal behavior. The transformation from austenite to martensite can be accomplished in 24 different ways. These 24 ways of producing martensite from austenite are the result of the symmetric CsCl structure having 6 equivalent face diagonal planes, each of which can shift in one of two directions and can distort (shear) in one of two directions, 6 x 2 x 2 = 24. See (Figure 3), where it shows the crystallization structure of Martensite and Austenite for CsCI [1].
Metal Memory Applications
Shape Memory metals that were developed by NASA for the space industry and have been used for increasing applications down on earth. The following is a list of just some of the applications that shape memory alloys have been used for. With progress in nanoscience and nanotechnology research progress in past decade, more attention has been paid to science to memory metal as well for purpose of medical applications and specifically in area of Artificial Intelligence (AI), which also has tremendous influence in the field of medication as well along with its Machine Learning functionality [2].
One of the application of applications for shape metal is in field of bioengineering from orthopedic medical perspective such as:
- Bones: broken bones, where shape or memory metal can be mended with shape memory alloys. The alloy plate has a memory transfer temperature that is close to body temperature and is attached to both ends of the broken bone. From body heat, the plate wants to contract and retain its original shape, therefore exerting a compression force on the broken bone at the place of fracture. After the bone has healed, the plate continues exerting the compressive force, and aids in strengthening during rehabilitation. Memory metals also apply to hip replacements, considering the high level of super-elasticity. The photo above shows a hip replacement (Figure 4).
- Reinforcement for Arteries and Veins: For clogged blood vessels, an alloy tube is crushed and inserted into the clogged veins. The memory metal has a memory transfer temperature close to body heat, so the memory metal expands to open the clogged arteries.
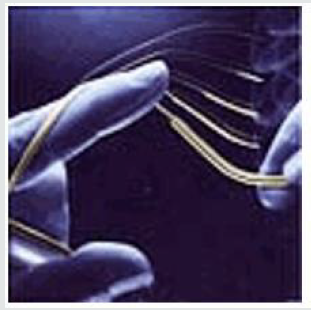
- Dental Wires: used for braces and dental arch wires, memory alloys maintain their shape since they are at a constant temperature, and because of the super elasticity of the memory metal, the wires retain their original shape after stress has been applied and removed.
- Anti-scalding protection: Temperature selection and control system for baths and showers. Memory metals can be designed to restrict water flow by reacting at different temperatures, which is important to prevent scalding. Memory metals will also let the water flow resume when it has cooled down to a certain temperature.
- Fire security and Protection systems: Lines that carry highly flammable and toxic fluids and gases must have a great amount of control to prevent catastrophic events. Systems can be programmed with memory metals to immediately shut down in the presence of increased heat. This can greatly decrease devastating problems in industries that involve petrochemicals, semiconductors, pharmaceuticals, and large oil and gas boilers.
- Golf Clubs: a new line of golf putters and wedges has been developed using shape memory alloys are inserted into the golf clubs. These inserts are super elastic, which keep the ball on the clubface longer. As the ball meets the clubface, the insert experiences a change in metallurgical structure. The elasticity increases the spin on the ball and gives the ball more "bite" as it hits the green.
- Helicopter blades: Performance for helicopter blades depend on vibrations; with memory metals in micro processing control tabs for the trailing ends of the blades, pilots can fly with increased precision. See (Figure 5)
- Tubes, Wires, and Ribbons: For many applications that deal with a heated fluid flowing through tubes, or wire and ribbon applications where it is crucial for the alloys to maintain their shape during a heated environment, memory metals are ideal. See (Figure 6).
In area of energy and Thermonuclear Fusion Confinement (TFC) [3] driving energy as a source of generating electricity as area of interest for the scientists to be able to apply such memorial metals to build their reactors so the internal walls of these types of reactors in Magnetic Confinement Fusion (MCF) [4] of Inertial Confinement Fusion (ICF) [5] can survive the plasma temperature generated in these reactors for their fusion reactions. Historically, once the physics of fusion devices is understood, which is expected to be achieved in the early1980’s one or more Experimental Power Reactor (EPR) are planned, which will produce net electrical power. The structure material for the device will probably be a mod-fraction of an austenitic stainless steel. Unlike fission reactors, whose pressure bound-arise are subjected to no or only light irradiation, the pressure boundary of a fusion reactor is subjected to high atomic displacement-damage and high production rates of trans-mutation products, e.g., helium and hydrogen. Hence, the design data base must include irradiated materials. Since in situ testing to obtain tensile, fatigue, creep, crack-growth, stress-rupture, and swelling data is currently impossible for fusion reactor conditions, a program of service-temperature irradiations in fission reactors followed by post irradiation testing, simulation of fusion conditions, and low-fluence 14 MeV neutron-irradiation tests are planned.
For the Demonstration Reactor (DEMO) expected to be built within ten years after the EPR, higher heat fluxes may require the use of refractory metals, at least for the first 20 cm. A partial data base may be provided by high-flux 14 MeV neutron sources being planned. Many materials other than those for structural components will be required in the EPR and DEMO. These include superconducting magnets, insulators, neutron reflectors and shields, and breeding materials. The rest of the device should utilize conventional materials except that portion involved in tritium confinement and recovery. With fourth generation Small Modular Reactors (SMRs) in form of Advanced High Temperature, where they are supposed to operate within range of 800˚C-1000 ˚C, in order to take advantage of Nuclear Air Combined Cycle (NACC) [6,7] in order to drive their thermal output efficiency near to gas or coal burn turbine generated energy for producing electricity for companies that own them and providing electricity into national grid. Small Modular Reactors and Nuclear Micro Reactors (NMRs) [8] under serious consideration by electrical companies to own them for almost %60 thermal efficiency output and their mobility for transportation as well as their modulization capabilities.
Anyway, these types of advanced high temperature reactors need such shape metal or memory metal to prevent any erosion and corrosion due to high temperature involved in their operation day-in and day-out, while in line in support of national grid. Furthermore, Very High Temperature Reactors (VHTR’s) have high priority among the U.S. reactor concepts of Next Generation Nuclear Power Plants (NGNP), since they can operate at very high temperatures (above 850 ˚C) producing 600-2400 megawatts of thermal power [9]. The materials used in the VHTR must withstand very high temperature, intense neutron radiation and corrosive environments. For high-temperature intermediate heat transport liquid salts are a desirable heat transfer fluid due to their high volumetric heat capacity. For this purpose, there will be a need for a long pipe of some 100 meters or more for the transport of this liquid-salt coolant from the nuclear power plant to the Intermediate Heat Exchanger (IHX) or thermo-chemical plant. For this purpose, efficient heat transfer fluids are required. Besides helium, primary molten (or liquid) fluoride and chloride salt coolants are the first choice and therefore under investigation [10]. The material screening focuses on salt compositions with high chemical stability for T > 800 ˚C, melting points T < 525 ˚C, low vapor pressure, and compatibility with alloys, graphite and ceramics as needed for the heat transfer loop. Impurities, temperature gradients and activity gradients might increase liquid salt corrosion problems. In addition to the LiF/NaF/KF salt flinak, future high temperature corrosion tests will also study LiCl-KCl-MgCl2 salts since they also have the potential to meet these basic requirements and are very inexpensive [11]. them for almost %60 thermal efficiency output and their mobility for transportation as well as their modulization capabilities. Anyway, these types of advanced high temperature reactors are in need of such shape metal or memory metal to prevent any erosion and corrosion due to high temperature involved in their operation day-in and day-out, while in line in support of national grid.
Furthermore, Very High Temperature Reactors (VHTR’s) have high priority among the U.S. reactor concepts of Next Generation Nuclear Power Plants (NGNP), since they can operate at very high temperatures (above 850 ˚C) producing 600-2400 megawatts of thermal power [9]. The materials used in the VHTR must withstand very high temperature, intense neutron radiation and corrosive environments. For high-temperature intermediate heat transport liquid salts are a desirable heat transfer fluid due to their high volumetric heat capacity. For this purpose, there will be a need for a long pipe of some 100 meters or more for the transport of this liquid-salt coolant from the nuclear power plant to the Intermediate Heat Exchanger (IHX) or thermo-chemical plant. For this purpose, efficient heat transfer fluids are required. Besides helium, primary molten (or liquid) fluoride and chloride salt coolants are the first choice and therefore under investigation [10]. The material screening focuses on salt compositions with high chemical stability for T > 800 ˚C, melting points T < 525 ˚C, low vapor pressure, and compatibility with alloys, graphite and ceramics as needed for the heat transfer loop. Impurities, temperature gradients and activity gradients might increase liquid salt corrosion problems. In addition to the LiF/NaF/KF salt flinak, future high temperature corrosion tests will also study LiCl-KCl-MgCl2 salts since they also have the potential to meet these basic requirements and are very inexpensive [11].
Development of high temperature/high strength materials, corrosion resistant coatings, and improved cooling technology have led to increases in gas turbine firing temperatures. This increase in firing temperature is the primary development that has led to increases in CCGT thermal efficiencies. The improvements in combined-cycle thermal efficiencies and the commercial development of combined-cycle power plants have proceeded in parallel with advances in gas turbine technologies [12]. Typical gas turbine exhaust gas temperatures of 1000-1100˚F are well suited to efficient combine-cycle operation. The exhaust gas temperature enables the heat transfer from exhaust gas to steam cycle to occur with minimal temperature difference. This small difference ensures maximum in thermodynamic availability while operating with the highest steam cycle efficiency. Gas turbine materials, coatings, and cooling systems enable reliable high firing temperatures. This achieves high gas turbine specific power and high efficiency of combined-cycle operation. A combined cycle gas turbine power plant is essentially an electrical power plant in which a gas turbine and a steam turbine are used in combination to achieve greater efficiency than would be possible independently. The gas turbine drives an electrical generator while the gas turbine exhaust is used to produce steam in a heat exchanger (called a Heat Recovery Steam Generator, HRSG) to supply a steam turbine whose output provides the means to generate more electricity. If the steam is used for heat (e.g., heating buildings) then the plant would be referred to as a cogeneration plant [13].
The Generation IV (GEN-IV) materials fundamental issues are listed as follows. The co-evolution of all components of the microstructure, and their roles in the macroscopic response in terms of swelling, anisotropic growth, irradiation creep, and radiation-induced phase transformations should be studied within the science of complex systems. See (Figure 7) In summary, we can conclude that.
- Six concepts have been identified with the potential to meet the Generation IV Goals
- Concepts operate in more challenging environments than current LWRs and significant material development challenges must be met for any of the Generation IV systems to be viable.
- Experimental programs cannot cover the breadth of materials and irradiation conditions for the proposed Gen IV reactor designs.
- Modeling and microstructural analysis can provide the basis for a material selection that is performed based on an incomplete experimental database and that requires considerable judgment to carry out the necessary interpolation and extrapolation.
Conclusions
These days, some metal implants in biomedical applications are getting replaced by ceramics and polymers due to their excellent biocompatibility and bifunctionality. However, for implants, which requires high strength, toughness, and durability, are still made of metals. On the other hand, clinical use of the promising research in using bioactive polymers and ceramics in regenerative medicine is still far away from practice. With further innovative approach to technique of metallurgy, further improvement can be observed on novel bifunctionalities and revolutionary use of metal such as for biodegradable implants and with confidence we can claim that metals will continue to be used as biomaterials in the future and commercialization of memory metal and its application in medical and other fields a new path will be taken as well. In nuclear industry with technology both fission and fusion a demand for shape alloy or memory metal is on horizon as well, to deal with erosion and corrosion due the range of heat these advanced reactors need to operate. In summary, last but not list memory metal has found its way too many industrial applications as we saw it in this short review article.
References
- University of Wisconsin-Madison, MRSEC Education Group, USA.
- Behgounia F, Zohuri B (2020) Artificial Intelligence Integration with Nanotechnology. Open Access Journal of Biogeneric Science and Research p. 1-7.
- Zohuri B (2017) Plasma Physics and Controlled Thermonuclear Reactions Driven Fusion Energy Springer Publishing Company.
- Zohuri B (2017) Magnetic Confinement Fusion Driven Thermonuclear Energy. 1st Edn, Springer Publishing Company.
- Zohuri B (2017) “Inertial Confinement Fusion Driven Thermonuclear Energy. 1st Edn, Springer Publishing Company.
- Zohuri B, McDaniel P (2020) Advanced Smaller Modular Reactors: An Innovative Approach to Nuclear Power. 1st Edn Springer Publishing Company.
- Zohuri B, McDaniel P (2017) Combined Cycle Driven Efficiency for Next Generation Nuclear Power Plants: An Innovative Design Approach. Springer Publishing Company.
- Zohuri B (2020) Nuclear Micro Reactors. 1st 2020 Edition, Springer Publishing Company.
- Future Reactor Materials - A Revolutionary Reactor concept-ORNL Review 37(1).
- Williams DF (2006) Assessment of candidate molten salt coolants for the NGNP/NHI heat transfer loop, ORNTL-report/TM-2006/69.
- Olson LC (2006) Evaluation of material corrosion in molten fluoride salt, Presentation on AIChE conference, San-Francisco 12-17.
- Chase DL, Kehoe PT GE Power Systems, Schenectady, NY: GE Combined-Cycle Product Line and Performance.
- Jim Causey ‘Combined Cycle Systems for the Utility Industry. Universal Silencer, Noise Control and Air Filtration Solutions.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...