
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Article(ISSN: 2638-6070) 
Vitamin D Levels and Immune System Cells and Hematology in Saudi and Non-Saudi Males
Volume 4 - Issue 2Sawsan Hassan Mahassni1* and Fatimah Saleh Bafarhan1,2
- 1Department of Biochemistry, Faculty of Science, King Abdualziz University, Jeddah, Saudi Arabia
- 2Department of Laboratories, Blood Donation Services, King Abdulaziz University Hospital, Jeddah, Saudi Arabia
Received: January 17, 2022 Published: January 25, 2022
*Corresponding author:Sawsan Hassan Mahassni, Department of Biochemistry, Faculty of Science, King Abdualziz University, Jeddah, Saudi Arabia
DOI: 10.32474/SJFN.2022.04.000183
Abstract
Vitamin D has many important physiological functions in the body and is essential for the immune system. Vitamin D deficiency is a major widespread global public health issue. The aim of this study was to determine the relationship between serum vitamin D levels and the counts of immune system cells and other hematological parameters in healthy Saudi and non-Saudi males and the differences between the two groups of subjects for the parameters. Fifty-four Saudi and 41 non-Saudi male subjects, with an age range of 35-45 years, were randomly chosen. Each group of subjects was divided into insufficient (levels at 30-70 nmol/l), deficient (levels < 30 nmol/l), and normal (control) (levels > 70 nmol/l) vitamin D3 (25-OH) levels. Results showed that RBC counts for Saudis were significantly higher for lower vitamin D levels, while for non-Saudis there was no dependence on vitamin D levels. Neutrophil counts for both Saudis and non-Saudis were lower for lower vitamin D levels. Saudis had lower RBC counts compared with non-Saudis for the normal and insufficient groups. On the other hand, neutrophil counts were not different between Saudis and non-Saudis. In conclusion, low levels of vitamin D (insufficient and deficient) did not lead to major changes in the hematology of both groups of subjects and the existent changes were alike in both groups. Thus, it may be concluded that the subject’s immune system was not affected considerably by the sub-normal levels of vitamin D and that both Saudis and non-Saudis were affected in the same way.
Keywords:Vitamin D; Immunity; Immune system cells; Hematology; Saudi males; Non-Saudi males
Introduction
Vitamin D is a fat-soluble vitamin that is essential in small amounts for human health and proper function in the body. Vitamin D deficiency is a major widespread global public health issue. It is estimated that around 1 billion people suffer from hypovitaminosis D [1]. Hypovitaminosis D is very common among Saudi Arabian adults where the prevalence was 17.7-100% for all healthy and nonhealthy age groups from the year 1984 to 2015 [2-8]. It is estimated that in 2015 the prevalence of hypovitaminosis D was 40.6% for men and about 63% for women in Saudi Arabia [8]. Vitamin D has many important physiological functions in the body, such as maintaining normal serum calcium and phosphate concentrations and enabling proper mineralization and growth of bones for strong healthy bones. Vitamin D in the human body is found in two forms, termed D2 and D3, which differ slightly in their structures and source. Vitamin D3, commonly known as cholecalciferol, is derived from the diet and is produced in the skin upon sun exposure, while vitamin D2 is termed ergocalciferol and is found in plants. Vitamin D deficiency leads to many conditions and diseases, with some being skeletal and others non-skeletal, such as increased fracture risk, tooth loss, rickets in children, osteomalacia and osteoporosis in adults [9-12], body aches, fatigue, and myopathy [13] In addition, hypovitaminosis D is associated with an increased risk for infectious diseases [14], diabetes mellitus [15], metabolic syndrome [16], dementia [17], cardiovascular diseases [18], and certain types of cancers [19-21], rheumatoid arthritis [22], multiple sclerosis [23], depression [24], schizophrenia [25], and obesity [16- 27]. On the other hand, some diseases lead to hypovitaminosis D, such as liver disease, renal failure, and any condition or disease that involves or effects organs involved in vitamin D metabolism [28-30]. Hypovitaminosis D is usually termed as insufficiency or deficiency with the deficient levels being lower than the insufficient levels. The ranges and cutoff points for these two categories vary widely and none are approved worldwide. Worldwide, vitamin D levels [31-33] used for insufficient vitamin D vary from 30 up to 75nmol/L while deficient levels range from < 20 to < 50 nmol/L. Likewise, the vitamin D levels considered normal or healthy vary from > 70 to > 75 nmol/L. Finally, hypertoxicity of vitamin D is considered to be around 250 nmol/L. Worldwide, around 50% of the world population suffer from vitamin D insufficiency [34], while around 37% suffer from deficiency [35]. Lower than normal levels of vitamin D are mainly found in the elderly and is more prevalent in females compared to males [1,11].
The major causes of hypovitaminosis D are a diet lacking in vitamin D, low or no exposure to the sunlight, and problems with absorbing vitamin D in the body [36]. Other factors implicated in low vitamin D levels are such as more melanin in the skin (darker skin), overweight and obesity, older age, remaining indoors most of the time, using sunscreen wearing clothing that covers most of the body, low intake of vitamin D-containing foods, and some disease that lead to low vitamin D levels [36-39]. Sunny countries are not immune from hypovitaminosis and in fact many such countries have a high prevalence of hypovitaminosis [40,41]. It has been observed that the prevalence of hypovitaminosis D among Saudis has been increasing for the last four decades [4-43]. The proposed reasons for this are the major changes in the lifestyle for Saudis towards a more relaxed sedentary lifestyle and the greater availability of different types of foods including Western-type foods leading to more overweight and obesity and their subsequent related diseases and conditions [2]. It is known that increased body weight leads to lower vitamin D levels since vitamin D is sequestered in body fat due to it being a fat-soluble vitamin [44]. The prevalence rates of hypovitaminosis D vary widely between countries [40-46] and between different ethnic groups [40,41] with white Caucasians having lower vitamin D levels than nonwhite individuals. Some studies found Saudis have a higher prevalence of hypovitaminosis D compared to no Saudis living in Saudi Arabia and even in the same areas [47]. An explanation for this is the genetic differences between different ethnic groups [48-51] and the genetic influence on vitamin D levels in the body [49,51]. Vitamin D is important for many systems and organs in the body as suggested by the presence of the vitamin D receptor (VDR) on many cells of the body including cells of the bone marrow, colon, breast, brain and cells of the immune system including lymphocytes [52]. Thus, vitamin D has an important role in the immune system and response [53,54]. Vitamin D has been shown to enhance both the innate and acquired immune systems through different mechanisms [55-59]. Most cells of the immune system, except B lymphocytes, have the VDR on their membrane [60], making vitamin D essential for a strong immune response and explaining the fact that people with low vitamin D levels are more prone to infections and to be infected by infectious diseases [61]. The immune system is one of the most important systems in the body. Its main function is to protect against infections while at the same time it is sensitive to changes and conditions of the body [62]. The two types of the immune response mediate their actions through cells, tissues and molecules. The major white blood cells of the innate immune response are the neutrophils, monocytes, eosinophils and basophils, while for the acquired immune response it is the lymphocytes along with other cells and components of the immune system. Vitamin D deficiency is linked to an increased risk for serious diseases and has numerous effects on cells within the immune system. This study determined vitamin D levels and the counts of white blood cells and other hematological parameters (platelets and red blood cell counts, and hemoglobin concentration) in healthy male adult Saudis and non- Saudis. This was done to determine the relationship between the vitamin D levels and the state of the immune system cells and other hematological parameters and to ascertain if there are differences between Saudis and non-Saudis in these effects. This is the first study to determine the differences in the vitamin D levels and the differential complete blood count (CBC) between both Saudi and non-Saudi subjects living in Saudi Arabia (Figure 1).
Materials and Methods
Subjects and categorizations
The subjects were 95 randomly chosen male blood donors at King Abdulaziz University, with an age range of 35-45 years and living in Jeddah, Saudi Arabia. Of the subjects, 54 were Saudis and 41 were non-Saudis. None of the subjects had any chronic, genetic, or immune-related diseases nor were they taking vitamin D supplements. As for the presence of other diseases, one subject had eczema and was taking medications for it, and 5 subjects had asthma, hey fear, or dust allergies but only one of these subjects was taking medications for his condition. The only other subjects taking medications were two subjects taking medications for acid reflux, one taking iron supplements and one taking prostate medications. This study was reviewed and approved by the Unit of Biomedical Ethics Research Committee at King Abdulaziz University, Jeddah, Saudi Arabia. All subjects gave written informed consent and filled a questionnaire on health and lifestyle factors that may influence the parameters. Subjects were categorized as Saudi and non-Saudi, and subsequently each group of subjects was divided into three groups based on the subject’s vitamin D3 (25-OH) level as follows: insufficient (levels equal 30-70 nmol/l), deficient (levels less than 30 nmol/l), and normal vitamin D levels (control) (levels more than 70 nmol/l).
Blood Collection
Venous blood samples were collected from each subject into ethylenediaminetetraacetic acid (EDTA) vacutainer tubes for the CBC analysis. Whole blood was collected into gel separator vacutainer tubes for the determination of vitamin D. Serum samples were obtained by centrifugation at 5,000 RPM for 10 minutes. Serum and plasma samples was stored at -40 °C until use.
Determination of vitamin D concentrations
The serum concentrations of vitamin D3 were measured on a Cobas e 601 Systems instrument (Modular analytics E170, Roche Company, Frankfurt, Germany) using the Reagent Rackpack and the pretreatment reagents (VITD-T) (Roche Diagnostics GmbH Company, Mannheim, Germany).
Determination of the differential complete blood counts
The CBC for each blood sample was done on an XN-9000 Hematology System instrument (Sysmex Europe GmbH, Kobe, Japan), using Cellpack, Sulfolyzer, Fluorocell and Lysercell Reagents (Sysmex Corporation, Kobe, Japan).
Statistical analysis
Data were statistically analyzed by using the Megastat statistical program (version 10.3) and the range (minimum and maximum) mean, standard deviation (± SD), and the standard error of the mean (± SE) were determined for all results. After testing for the homogeneity and normality of the parameters, the ANOVA oneway test was used to test for the presence of significant differences between the different groups of subjects for the normally distributed parameters. As for the homogeneous non-normally distributed parameters, the Kruskal-Walli’s test was used to test for the significance in the differences between the groups. The T-test was used to test for the presence of significant differences for the normally distributed parameters between the normal and deficient groups. Additionally, the Mann-Whitney U test was used to test for the significance in the comparison between the normal and deficient groups for the non-normally distributed parameters. Subsequently, for the post hoc comparisons for the differences between the groups, the Mann-Whitney U test was used for the age and neutrophil counts for non-Saudis, while the T-test was used for the vitamin D levels for non-Saudi subjects. The resulting P values demonstrate significance or lack thereof as follows: P > 0.05 is a non-significant (NS) difference, 0.01 ≤ P ≤ 0.05 is a significant (S) difference, P < 0.01 is a highly significant (HS) difference.
Results
Subjects’ characteristics, categorizations, and lifestyle questionnaire
All subjects filled a lifestyle questionnaire to find any lifestyle differences within the groups and between Saudis and non-Saudi (Table 1). The number of Saudi subjects (Table 2) in the vitamin D levels groups were three (5.56%) for the normal, 18 (33.33%) for the insufficient, and 33 (61.11%) for the deficient vitamin D levels. The normal groups were not included in the comparisons of the parameters between the different vitamin D level groups due to the very low number of subjects. As for the non-Saudis groups, the number of subjects for the normal group was five (12.20%), for the insufficient they were 23 (56.10%), and, finally, for the deficient they were 13 (31.71%). In addition, another categorization was done where the normal and insufficient subjects were combined into one group in addition to the deficient group. For Saudis the normal/insufficient group had 21 (22.11%) subjects, while for non- Saudis the combined group had 28 (29.47) subjects. The other group (deficient) remains unchanged. Both groups of subjects (Saudi and non-Saudi) had an age range of 35-45 years. The mean age, weight, height, and heart rate for the Saudi and non-Saudi subjects are shown in Tables 3-5. These parameters were compared between the insufficient and deficient Saudi subjects using the T-test (Tables 3) and no significant differences were found. The normal Saudi subjects were not compared with the other vitamin D groups since the number of subjects (3) is too low. The comparison between the mean ages for the normal, insufficient and deficient groups for the non-Saudi subjects, using the Kruskal-Wallis test (Table 3), showed a significant difference. As for the post hoc comparisons between groups, using the Mann-Whitney U test (Table 4), the mean age for the normal group was significantly higher than for the deficient groups while there were no significant differences for the other group comparisons. The mean weight, height and heart rate for the three vitamin D groups for non-Saudis, using the one-way ANOVA test (Table 3), were not significantly different. The normal and insufficient groups were combined into one group termed normal/ insufficient group. Comparing between the normal/insufficient and deficient groups, using the T-test (Table 5), showed no significant differences for the mean age for Saudis and the mean weight, height and heart rate for Saudis and non-Saudis, separately. The mean age for non-Saudis was significantly higher for the normal/ insufficient group compared to the deficient group, using the T-test (Table 5). Statistical comparisons between the Saudi and non-Saudi subjects for the normal/insufficient, insufficient and deficient groups are shown in Table 6. Comparing the same groups between Saudi and non-Saudi subjects, the mean age for the non-Saudis was significantly higher between the normal/insufficient groups, using the T-test, and the insufficient groups, using the Mann-Whitney U Test, while it was not significantly different between the deficient groups, using the T-test. As for the mean weight, height and heart rate, using the T-test, there were no significant differences between the Saudis and non-Saudis for each of the three vitamin D groups.
Table 2: The number and percent of subjects in each group of vitamin D level among Saudis and non-Saudis.
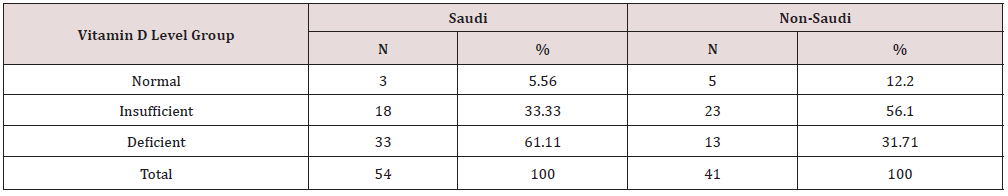
Table 3: Statistical analysis of the mean age, weight, height, and heart rate for Saudi and non-Saudi subjects and the P-values for the differences between the vitamin D levels groups.

T: T-test, O: One way ANOVA test, K: Kruskal-Walli’s test were used for the significance testing
NS: Not significant (P > 0.05), S: Significant (P ≤ 0.05)
Table 4: Post hoc statistical analysis for the statistical differences between the groups in Table 3 and 7.
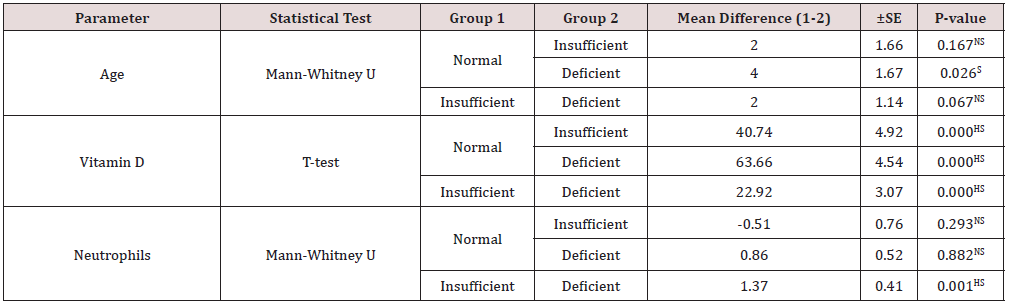
NS: Not significant (P > 0.05), HS: High significant (P < 0.01), S: Significant (P ≤ 0.05)
Table 5: Statistical analysis of mean age, weight, height, and heart rate for Saudi and non-Saudi subjects and the P-values for the differences between the two groups of the vitamin D levels.

T-test was used for the significance testing
NS: Not significant (P > 0.05), S: Significant (P ≤ 0.05)
Table 6: Statistical analysis of mean age, weight, height, and heart rate for the vitamin D level groups and the P-values for the differences between Saudi and non-Saudi subjects.

T-test and Mann-Whitney U test were used for the significance testing
NS: Not significant (P > 0.05), S: Significant (P ≤ 0.05)
Determination of vitamin D concentrations and CBC
Statistical analysis for the two vitamin D groups for the Saudis, using the T-test (Table 7), showed a highly significantly lower mean vitamin D level for the deficient group compared to the mean level for the insufficient group. The mean vitamin D concentrations for the vitamin D groups for non-Saudis, using the ANOVA one-way test (Table 7), showed a significant difference. The between group comparisons, using the T-test (Table 4), showed highly significantly lower mean vitamin D levels for the insufficient and deficient groups compared with the normal vitamin D group and for the deficient group compared with the insufficient group. The other parameters that showed a significant difference between the groups were the mean RBC counts for Saudis and the mean neutrophil counts for both Saudis and non-Saudis. The mean RBC counts for the Saudi deficient group was significantly higher, using the T-test, compared to that of the insufficient. The mean neutrophil count for the deficient Saudis, using the T-test, was significantly lower compared with count for the insufficient group. The mean neutrophil counts for the non-Saudis, using the Kruskal-Wallis test, was significantly different between the three vitamin D groups. As for the post hoc comparisons, using the Mann-Whitney U test (Table 4), the mean neutrophil counts were not significantly different between both the insufficient and deficient groups compared with the normal group, while the counts for the deficient group was highly significantly lower than for the insufficient group. All other cell counts and comparisons did not show significant differences. The statistical comparisons for the parameters between the normal/insufficient and deficient groups for the Saudi and non-Saudi subjects are shown in Table 8. The mean vitamin D levels were highly significantly lower for the deficient groups for the Saudi and non- Saudi subjects, using the T-test. The mean RBC count for the Saudis deficient group was significantly higher, using the Mann-Whitney U test, compared to the normal/insufficient group mean counts. On the other hand, the mean RBC counts for the non-Saudis were not significantly different, using the T-test, between the groups. The mean neutrophil counts for the Saudis and non-Saudi, using the T-test, were highly significantly lower for each deficient group compared to the respective normal/insufficient group. All other parameters for the Saudi and non-Saudi subjects did not show any significant differences. Comparing the vitamin D concentrations and differential CBC between Saudi and non-Saudi subjects for the normal/insufficient, insufficient, and deficient groups (Table 9), there were no significant differences for all parameters except for the mean RBC counts. The mean RBC counts for the normal/ insufficient and insufficient, using the Mann-Whitney U test, were significantly higher for non-Saudi subjects compared to the respective level for Saudi subjects.
Table 7: Statistical analysis of the mean hematological parameters and vitamin D concentrations for Saudi and non-Saudi subjects and the P-values for the differences between the vitamin D level groups.

T-test, One way ANOVA test, Kruskal-Wallis test and Mann-Whitney U test were used for the significance testing
NS: Not significant (P > 0.05), HS: High significant (P < 0.01)
Max: Maximum, Min: Minimum
Table 8: Statistical analysis for the mean hematological parameters and vitamin D concentrations for Saudi and non-Saudi subjects and the P-values for the differences between two groups of the vitamin D levels.

T-test and Mann-Whitney U test were used for the significance testing
NS: Not significant (P > 0.05), HS: High significant (P < 0.01), S: Significant (P ≤ 0.05)
Table 9: Statistical analysis for the mean hematological parameters and vitamin D concentrations for the vitamin D level groups and the P-values for the differences between Saudi and non-Saudi subjects.
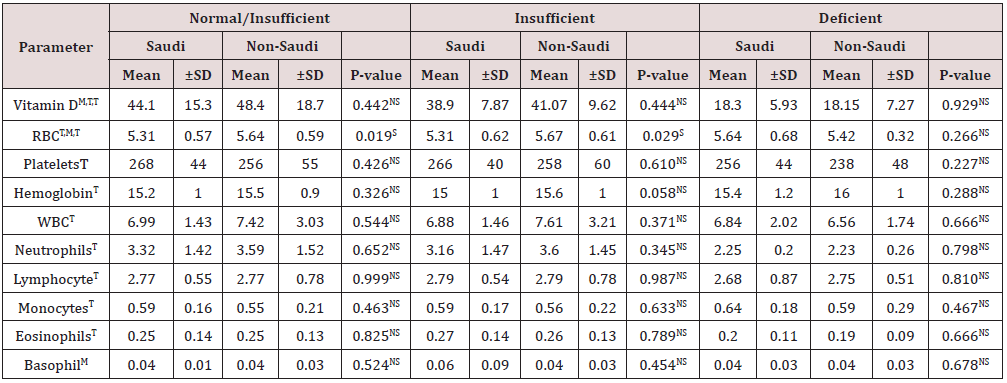
T-test and Mann-Whitney U test were used for the significance testing
NS: Not significant (P > 0.05), S: Significant (P ≤ 0.05)
Discussion
There are no studies on the effects of vitamin D levels on the CBC in healthy Saudi and non-Saudi subjects living in Saudi Arabia and the differences between them. Most available studies are statistical in nature and mainly focus on lifestyle. Therefore, this study is the first to determine the CBC for both healthy Saudi and non-Saudi subjects residing in Saudi Arabia and to determine the differences between the CBC for the two groups. In addition, anthropometric measurements were taken, and a lifestyle questionnaire was used and compared between the two groups of subjects.
For the number of subjects in the groups of the study, most Saudi subjects had deficient vitamin D levels followed by the insufficient subjects. On the other hand, the non-Saudi subjects were the opposite with most subjects being insufficient followed by deficient. The Saudi and non-Saudi subjects with normal vitamin D levels, in general, had lower levels of education compared to the Saudi and non-Saudi subjects with insufficient and deficient vitamin D levels. This is contradictory to the findings of a previous study [5- 65] that found a lack of education in non-Saudi subjects with low levels of vitamin D. Most subjects with lower-than-normal vitamin D levels did not know that they had low levels or thought they did not. Concerning sun exposure, most subjects (Saudi and none) were exposed daily. Most Saudis in the deficient group consumed many foods rich in vitamin D, such as milk, cooked liver, salmon, eggs, tuna, mushrooms and sardine, while the Saudis in the insufficient group and non-Saudis in both low vitamin D groups consumed few foods rich in vitamin D, and all consumed these foods 2-3 days per week. As for foods that enhance immunity, such as garlic, honey, vitamin C, green tea and vegetables (tomato, broccoli, colliflower, cabbage, and turnip), most subjects consumed them often or few. As for exercise, most of the non-Saudis with normal vitamin D levels never exercised. Most of the Saudis in the insufficient group exercised daily while the ones in the deficient group rarely exercised. As for the non-Saudis, most of them never exercised. Most subjects drank coffee and/or tea. Most of the Saudi and non-Saudi subjects were non-smokers except for the Saudi deficient subjects that were mostly smokers. Most of the Saudi and non-Saudi insufficient vitamin D level subjects were not exposed to second-hand smoke while most of the Saudi and non-Saudi deficient subjects were exposed to second-hand smoke. Subjects that were exposed to second-hand smoke were exposed to it daily and mainly at work. Most subjects slept most of their sleeping hours at night. The comparisons between the vitamin D level groups among Saudi and non-Saudi subjects separately, and for each vitamin D level group between Saudi and non-Saudi subjects for the mean age, weight, height, and heart rate, showed that only the age showed significant differences. The mean ages for the three vitamin D level groups for the non-Saudi subjects showed a significantly lower mean age for the deficient group compared with the mean age for the normal group. Similarly, the mean ages for the two vitamin D groups for non-Saudis showed a significantly lower mean age for the deficient group compared with the normal/insufficient group. As for the comparison between Saudi and non-Saudi subjects, the mean age for the non-Saudi subjects was significantly higher than for the Saudi subjects for both the normal/insufficient and insufficient groups. As for the other parameters, there were no significant differences for all comparisons. These findings agree with the findings of [65] that vitamin D prevalence was less in older people. While the findings disagree with the previous studies [3,66] that found higher ages for people with lower vitamin D levels. The nonsignificant difference in the weight of the subjects disagree with the previous finding [44,67] of lower vitamin D levels in people with higher body weight or obesity. The no effect of vitamin D on the heart rates of the subjects agrees with previous findings [68].
The findings of this study for the two vitamin D groups for the Saudis showed a highly significantly lower mean vitamin D level for the deficient group compared with the mean vitamin D level for the insufficient group. Likewise, the mean vitamin D levels for the insufficient and deficient groups were highly significantly lower than for the normal vitamin D group. The deficient group had highly significantly lower vitamin D levels compared to the normal/ insufficient groups in Saudi and non-Saudi subjects separately. These above findings are expected since the vitamin D level groups were designed to categorize the patients and differentiate between them according to their vitamin D levels. On the other hand, no significant differences were found between Saudi and non-Saudi subjects in the mean vitamin D levels for each of the normal/ insufficient, insufficient and deficient vitamin D groups. This last finding disagrees with the previous studies [47,64] that found a significant increase in vitamin D deficiency in Saudi compared to non-Saudi subjects. As for the mean RBC counts in Saudi subjects, they were significantly higher for the deficient group compared with each of the insufficient and normal/insufficient groups, while for the non-Saudi subjects there were no significant differences between the groups. As for the comparison between Saudi and non-Saudi subjects, non-Saudi subjects had significantly higher mean RBC counts compared with the non-Saudis for the normal/ insufficient and insufficient groups, separately, while the deficient groups were not significantly different. Mean neutrophil counts for the Saudi deficient subjects was highly significantly lower compared to the insufficient and normal/insufficient groups separately. As for the non-Saudi subjects, the mean neutrophil counts were highly significantly lower for the deficient group compared to the insufficient and normal/insufficient groups separately. All other cell counts and comparisons did not show significant differences.
Vitamin D is known to affect cellular differentiation and growth in the bone marrow and cell development [69 71]. Therefore, the lower neutrophil counts for the lower vitamin D levels found in the current study for both Saudi and non-Saudi subjects comply with the above finding [69,71] of enhanced cellular differentiation and development with higher vitamin D levels. On the other hand, the higher RBC counts found in the present study for the lower vitamin D levels does not comply with these findings [69,71] since the RBC counts for Saudi, but not non-Saudi, subjects were significantly higher for the lower vitamin D levels. Nor do the current findings agree with the previous study [68] that did not find a relationship between the vitamin D levels and RBC counts. On the other hand, these findings agree with the previous finding Doudin, Becker, Rothenberger, and Meyer [72] of lower RBC counts for higher vitamin D levels. In addition, the RBC counts were higher for non-Saudis compared to Saudis for the vitamin D groups, which may mean that there is a difference due to ethnic differences. Hemoglobin concentrations were not different for the different vitamin D groups nor between Saudi and non-Saudi subjects. A previous study Doudin et al. [72] found that higher vitamin D levels led to lower hemoglobin concentrations. Other previous studies found associations between vitamin D levels and hemoglobin although these studies were on patients with renal disease and chronic kidney disease [73], on hemodialysis [74], and requiring cardiac surgery [75] or other diseases or conditions, thus it is not possible to compare these findings with the current ones. A previous study found differences in the presence or lack of the relationship between vitamin D and hemoglobin in black and white children Atkinson et al. [76]. Platelet counts did not show any significant differences between the vitamin D groups nor between Saudi and non-Saudi subjects. This is contradictory with the previous studies [77,78] that found a negative correlation between platelet counts and vitamin D levels.
The counts for WBC and the types of white blood cells were all not significantly different between the vitamin D groups except for the neutrophil counts in Saudi and non-Saudi subjects while not between Saudis and non-Saudis for the counts for each vitamin D level group. These results agree with the findings of other studies [68-80] of no significant effects of vitamin D levels on the total and differential counts of WBC except for the neutrophil counts which disagree. Other studies found an association between the vitamin D levels and WBC counts [81]. In contradiction with the current findings, other studies [82] found a negative association between vitamin D levels and neutrophil and monocyte counts, and higher eosinophil counts with lower vitamin D levels [83, 84]. A study found that the counts of basophils and neutrophils are inversely correlated with the vitamin D levels [84] in contradiction to the present findings. Most other studies found no significant associations between vitamin D levels and eosinophils [85-91] which is in agreement with the present findings. In summary, the lifestyle questionnaire displayed several differences between the Saudi and non-Saudi subjects. The major differences were that Saudi vitamin A deficient subjects was the group that had the greatest number of subjects that got sun exposure daily and ate more immunity enhancing foods and foods containing vitamin D. In addition, RBC counts for the Saudi subjects were significantly higher for lower vitamin D levels, while for non-Saudi subjects there was no dependence on vitamin D levels. Saudi subjects had lower RBC counts compared with non-Saudi subjects for the normal and insufficient vitamin D groups. Neutrophil counts for both Saudi and non-Saudi subjects were lower for lower vitamin D levels. On the other hand, neutrophil counts were not different between Saudi and non-Saudi subjects. In conclusion, low levels of vitamin D (insufficient and deficient) did not cause major changes in the hematology of both groups of subjects and the existent changes were alike in both groups. Thus, it may be concluded that the subject’s immune system was not affected considerably by the sub-normal levels of vitamin D and that both Saudi and non-Saudi subjects were affected in the same way. This may be partially due to the fact that the subjects were healthy and middle-aged not old and with diseases. We recommend that further studies be done to determine the concentrations of different inflammatory markers using a larger number of subjects and to determine the vitamin D levels in samples other than blood.
Funding
There was no funding provided for this research study.
Conflicts of Interest
There were no conflicts of interest.
References
- Sahota O (2014) Understanding vitamin D deficiency. Age ageing 43(5): 589-591.
- Al Daghri NM, Al Attas OS, Wani K, Alnaami AM, Sabico S, et al. (2015) Sensitivity of various adiposity indices in identifying cardiometabolic diseases in Arab adults. Cardiovascular diabetology 14(1): 1-8.
- Alfawaz H, Tamim H, Alharbi S, Aljaser S, Tamimi W (2014) Vitamin D status among patients visiting a tertiary care center in Riyadh, Saudi Arabia: a retrospective review of 3475 cases. BMC public health 14(1): 1-6.
- Ardawi MS, Qari M, Rouzi A, Maimani A, Raddadi R (2011) Vitamin D status in relation to obesity, bone mineral density, bone turnover markers and vitamin D receptor genotypes in healthy Saudi pre-and postmenopausal women. Osteoporosis international 22(2): 463-475.
- Ardawi MS, Sibiany A, Bakhsh T, Qari M, Maimani A (2012) High prevalence of vitamin D deficiency among healthy Saudi Arabian men: relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporosis international 23(2): 675-686.
- Fonseca V, Tongia, R, El Hazmi M, Abu Aisha H (1984) Exposure to sunlight and vitamin D deficiency in Saudi Arabian women. Postgraduate medical journal 60(707): 589-591.
- Nabi G, Hobani Y, Sarwat M (2015) High prevalence of vitamin D deficiency and cancer in Saudi Arabian populations: Can we hypothesize a link? Med hypotheses 85(2): 117-119.
- Tuffaha M, El Bcheraoui C, Daoud F, Al Hussaini HA, Alamri F, et al. (2015) Deficiencies under plenty of sun: Vitamin D status among adults in the kingdom of Saudi Arabia, 2013. N Am J Med Sci 7(10): 467.
- Hill TR, Aspray TJ, Francis RM (2013) Vitamin D and bone health outcomes in older age. Proceedings of the Nutrition Society 72(4): 372-380.
- Holick MF (2007) Vitamin D deficiency. New England journal of medicine 357(3): 266-281.
- Palacios C, Gonzalez L (2014) Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol 144: 138-145.
- Sheikh A, Saeed Z, Jafri S A D, Yazdani I, Hussain SA (2012) Vitamin D levels in asymptomatic adults-a population survey in Karachi, Pakistan. PloS one 7(3): e33452.
- Tulchinsky TH (2010) Micronutrient deficiency conditions: global health issues. Public health reviews 32(1): 243-255.
- Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V (2009) Vitamin D for Treatment and Prevention of Infectious Diseases; A Systematic Review of Randomized Controlled Trials. Endocr Pract 15(5): 438-449.
- Berridge MJ (2017) Vitamin D deficiency and diabetes. Biochemical Journal 474(8): 1321-1332.
- Belenchia AM, Tosh AK, Hillman LS, Peterson CA (2013) Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. The American journal of clinical nutrition 97(4): 774-781.
- Buell J, Dawson Hughes B, Scott T, Weiner D, Dallal G, et al. (2010) 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology 74(1): 18-26.
- Gunta SS, Thadhani RI, Mak RH (2013) The effect of vitamin D status on risk factors for cardiovascular disease. Nature Reviews Nephrology 9(6): 337-347.
- Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, et al. (2006) The role of vitamin D in cancer prevention. American journal of public health 96(2): 252-261.
- Grant WB (2002) An estimate of premature cancer mortality in the US due to inadequate doses of solar ultraviolet‐B radiation. Cancer 94(6): 1867-1875.
- Lappe, JM, Travers Gustafson D, Davies KM, Recker RR, Heaney RP (2007) Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 85(6): 1586-1591.
- Zwerina K, Baum W, Axmann R, Heiland GR, Distler JH, et al. (2011) Vitamin D receptor regulates TNF-mediated arthritis. Ann Rheum Dis 70(6): 1122-1129.
- Holick MF (2006) High prevalence of vitamin D inadequacy and implications for health. Paper presented at the Mayo Clinic Proceedings.
- Ju SY, Lee YJ, Jeong SN (2013) Serum 25-hydroxyvitamin D levels and the risk of depression: a systematic review and meta-analysis. The journal of nutrition, health and aging 17(5): 447-455.
- Murri MB, Respino M, Masotti M, Innamorati M, Mondelli V, et al. (2013) Vitamin D and psychosis: mini meta-analysis. Schizophrenia research 150(1): 235-239.
- González Molero I, Rojo Martínez G, Morcillo S, Gutierrez C, Rubio E, et al. (2013) Hypovitaminosis D and incidence of obesity: a prospective study. European journal of clinical nutrition 67(6): 680-682.
- Khan AH, Iqbal R, Naureen G, Dar FJ, Ahmed FN (2012) Prevalence of vitamin D deficiency and its correlates: results of a community-based study conducted in Karachi, Pakistan. Archives of osteoporosis 7(1): 275-282.
- Courbebaisse M, Alberti C, Colas S, Prié D, Souberbielle JC, et al. (2014) VITamin D supplementation in renAL transplant recipients (VITALE): a prospective, multicentre, double-blind, randomized trial of vitamin D estimating the benefit and safety of vitamin D 3 treatment at a dose of 100,000 UI compared with a dose of 12,000 UI in renal transplant recipients: study protocol for a double-blind, randomized, controlled trial. Trials 15(1): 1-14.
- Vos R, Ruttens D, Verleden SE, Vandermeulen E, Bellon H, et al. (2017) High-dose vitamin D after lung transplantation: a randomized trial. Randomized Controlled Trial 36(8): 897-905.
- Zhou Q, Li L, Chen Y, Zhang J, Zhong L, et al. (2019) Vitamin D supplementation could reduce the risk of acute cellular rejection and infection in vitamin D deficient liver allograft recipients. Int immunopharmacol 75: 105811.
- Hanley DA, Cranney A, Jones G, Whiting SJ, Leslie WD, et al. (2010) Vitamin D in adult health and disease: a review and guideline statement from Osteoporosis Canada. Cmaj 182(12): E610-E618.
- LeBlanc ES, Zakher B, Daeges M, Pappas M, Chou R (2015) Screening for vitamin D deficiency: a systematic review for the US Preventive Services Task Force. Ann Intern Med 162(2): 109-122.
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, et al. (2011) The 2011 Dietary Reference Intakes for Calcium and Vitamin D: what dietetics practitioners need to know. J Am Diet Assoc 111(4): 524-527.
- Nair R, Maseeh A (2012) Vitamin D: The “sunshine” vitamin. J Pharmacol Pharmacother 3(2): 118-126.
- Hilger J, Friedel A, Herr R, Rausch T, Roos F, et al. (2014) A systematic review of vitamin D status in populations worldwide. British journal of nutrition 111(1): 23-45.
- Thacher TD, Clarke BL (2011) Vitamin D insufficiency. Mayo Clin Proc 86(1): 50-60.
- Holick MF (2003) Vitamin D: A millenium perspective. Journal of cellular biochemistry 88(2): 296-307.
- Iqbal R, Khan AH (2010) Possible causes of vitamin D deficiency (VDD) in Pakistani population residing in Pakistan. J Pak Med Assoc 60(1): 1-2.
- Pereira Santos M, Costa Pd F, Assis Ad, Santos C d S, Santos D d (2015) Obesity and vitamin D deficiency: a systematic review and meta‐analysis. Obes rev 16(4): 341-349.
- Cashman KD (2020) Vitamin D deficiency: defining, prevalence, causes, and strategies of addressing. Calcified tissue international: 1-16.
- Cashman KD, Dowling KG, Škrabáková Z, Gonzalez Gross M, Valtueña, J, et al. (2016) Vitamin D deficiency in Europe: pandemic? The American journal of clinical nutrition 103(4): 1033-1044.
- Al Alyani H, Al Turki HA, Al Essa ON, Alani FM, Sadat Ali M (2018) Vitamin D deficiency in Saudi Arabians: a reality or simply hype: a meta-analysis (2008–2015). Journal of family & community medicine 25(1): 1-4.
- Bassil D, Rahme M, Hoteit M, Fuleihan GEH (2013) Hypovitaminosis D in the Middle East and North Africa: prevalence, risk factors and impact on outcomes. Dermato-endocrinology 5(2): 274-298.
- Vanlint S (2013) Vitamin D and obesity. Nutrients 5(3): 949-956.
- Sarafin K, Durazo Arvizu R, Tian L, Phinney KW, Tai S, et al. (2015) Standardizing 25-hydroxyvitamin D values from the Canadian health measures survey. Am J Clin Nutr 102(5): 1044-1050.
- Schleicher RL, Sternberg MR, Looker AC, Yetley EA, Lacher DA, et al. (2016) National estimates of serum total 25-hydroxyvitamin D and metabolite concentrations measured by liquid chromatography–tandem mass spectrometry in the US population during 2007–2010. J Nutr 146(5): 1051-1061.
- Hussain AN, Alkhenizan AH, El Shaker M, Raef H, Gabr A (2014) Increasing trends and significance of hypovitaminosis D: a population-based study in the Kingdom of Saudi Arabia. Archives of osteoporosis 9(1): 1-5.
- Chaplin G, Jablonski NG (2009) Vitamin D and the evolution of human depigmentation. American Journal of Physical Anthropology: The Official Publication of the American Association of Physical Anthropologists 139(4): 451-461.
- Coşkun S, Şimşek Ş, Camkurt MA, Çim A, Çelik SB (2016) Association of polymorphisms in the vitamin D receptor gene and serum 25-hydroxyvitamin D levels in children with autism spectrum disorder. Gene 588(2): 109-114.
- Ginde AA, Liu MC, Camargo CA (2009) Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Archives of internal medicine 169(6): 626-632.
- Pike JW, Meyer MB, Benkusky NA, Lee SM, John HS, et al. (2016) Genomic determinants of vitamin D-regulated gene expression. Vitam Horm 100: 21-44.
- Bikle D (2009) Nonclassic actions of vitamin D. The Journal of Clinical Endocrinology & Metabolism 94(1): 26-34.
- Hossein nezhad A, Holick MF (2013) Vitamin D for health: a global perspective. Paper presented at the Mayo clinic proceedings.
- Khaw KT, Luben R, Wareham N (2014) Serum 25-hydroxyvitamin D, mortality, and incident cardiovascular disease, respiratory disease, cancers, and fractures: a 13-y prospective population study. The American journal of clinical nutrition 100(5): 1361-1370.
- Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, et al. (2009) Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. The Journal of Immunology 182(7): 4289-4295.
- Cantorna MT (2010) Mechanisms underlying the effect of vitamin D on the immune system. Proceedings of the Nutrition Society 69(3): 286-289.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet 395(10223): 497-506.
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, et al. (2006) Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311(5768): 1770-1773.
- Rondanelli M, Miccono A, Lamburghini S, Avanzato I, Riva A, et al. (2018) Self-care for common colds: the pivotal role of vitamin D, vitamin C, zinc, and echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds-practical advice on dosages and on the time to take these nutrients/botanicals in order to prevent or treat common colds. Evid Based Complement Alternat Med 2018.
- Cannell J, Vieth R, Umhau J, Holick M, Grant W, et al. (2006) Epidemic influenza and vitamin D. Epidemiology and Infection 134(6): 1129-1140.
- Bikle DD (2011) Vitamin D regulation of immune function. Vitamins & Hormones 86: 1-21.
- De Castro LN, Castro LN, Timmis J (2002) Artificial immune systems: a new computational intelligence approach: Springer Science & Business Media.
- Jääskeläinen T, Knekt P, Marniemi J, Sares Jäske L, Männistö S, et al. (2013) Vitamin D status is associated with sociodemographic factors, lifestyle and metabolic health. European journal of nutrition 52(2): 513-525.
- Nasr MH, Othman N, Hassan BA, Karoppannan M, Abdulaziz NB, et al. (2019) The prevalence of vitamin D deficiency between Saudis and non-Saudis in Al-Madinah Al-Munawarah a cross-sectional study. bioRxiv 613729.
- Naugler C, Zhang J, Henne D, Woods P, Hemmelgarn BR (2013) Association of vitamin D status with socio-demographic factors in Calgary, Alberta: an ecological study using Census Canada data. BMC public health 13(1): 1-10.
- Chen W, Zhang X, Wang, H, Zhang W, Xu Y, et al. (2015) The epidemic investigation of serum 25-hydroxy vitamin D levels in the adults in Qujing area of Yunnan province in China. International journal of clinical and experimental pathology 8(8): 9597-9601.
- Parva NR, Tadepalli S, Singh P, Qian A, Joshi R, et al. (2018) Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus 10(6).
- Soliman AT, Eldabbagh M, Elawwa A, Saleem W (2012) Does Vitamin D therapy affect hematological indices in adolescents with vitamin D deficiency? Indian J Endocrinol Metab 16(4): 659-660.
- Bellido T, Girasole G, Passeri G, Yu XP, Mocharla H, et al. (1993) Demonstration of estrogen and vitamin D receptors in bone marrow-derived stromal cells: up-regulation of the estrogen receptor by 1, 25-dihydroxyvitamin-D3. Endocrinology 133(2): 553-562.
- Yetgin S, Ozsoylu S (1982) Myeloid metaplasia in vitamin D deficiency rickets. Scand J Haematol 28(2): 180-185.
- Yetgin S, Yalçin SS (2004) The effect of vitamin D3 on CD34 progenitor cells in vitamin D deficiency rickets. Turk J Pediatr 46(2): 164-166.
- Doudin A, Becker A, Rothenberger A, Meyer T (2018) Relationship between serum 25 hydroxyvitamin D and red blood cell indices in German adolescents. European journal of pediatrics 177(4): 583-591.
- Kendrick J, Targher G, Smits G, Chonchol M (2009) 25-Hydroxyvitamin D deficiency and inflammation and their association with hemoglobin levels in chronic kidney disease. American journal of nephrology 30(1): 64-72.
- Kiss Z, Ambrus C, Almasi C, Berta K, Deák G, et al. (2011) Serum 25 (OH)-cholecalciferol concentration is associated with hemoglobin level and erythropoietin resistance in patients on maintenance hemodialysis. Nephron Clinical Practice 117(4): 373-378.
- Ernst JB, Becker T, Kuhn J, Gummert JF, Zittermann A (2015) Independent association of circulating vitamin D metabolites with anemia risk in patients scheduled for cardiac surgery. PloS one 10(4): e0124751.
- Atkinson MA, Melamed ML, Kumar J, Roy CN, Miller III ER, et al. (2014) Vitamin D, race, and risk for anemia in children. The Journal of pediatrics 164(1): 153-158.
- Alanlı R, Küçükay MB, Yalçın KS (2020) Relationship between vitamin D levels and platelet count: A retrospective study.
- Park YC, Kim J, Seo MS, Hong SW, Cho ES, et al. (2017) Inverse relationship between vitamin D levels and platelet indices in Korean adults. Hematology 22(10): 623-629.
- Souto Filho JTD, de Andrade AS, Ribeiro FM, Alves P d A S, Simonini VRF (2018) Impact of vitamin D deficiency on increased blood eosinophil counts. Hematol Oncol Stem Cell Ther 11(1): 25-29.
- Yildirim I, Hur E, Kokturk F (2013) Inflammatory markers: C-reactive protein, erythrocyte sedimentation rate, and leukocyte count in vitamin D deficient patients with and without chronic kidney disease. International journal of endocrinology 2013.
- Mellenthin L, Wallaschofski H, Grotevendt A, Völzke H, Nauck M, et al. (2014) Association between serum vitamin D concentrations and inflammatory markers in the general adult population. Metabolism 63(8): 1056-1062.
- Titmarsh HF, Gow AG, Kilpatrick S, Cartwright JA, Milne EM, et al. (2015) Low vitamin D status is associated with systemic and gastrointestinal inflammation in dogs with a chronic enteropathy. PloS one 10(9): e0137377.
- Brehm JM, Celedón JC, Soto Quiros ME, Avila L, Hunninghake GM, Forno E, et al. (2009) Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. American journal of respiratory and critical care medicine 179(9): 765-771.
- Hollams E, Hart P, Holt B, Serralha M, Parsons F, et al. (2011) Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. European Respiratory Journal 38(6): 1320-1327.
- Alyasin S, Momen T, Kashef S, Alipour A, Amin R (2011) The relationship between serum 25 hydroxy vitamin d levels and asthma in children. Allergy, asthma & immunology research 3(4): 251-255.
- De Groot JC, van Roon, EN, Storm H, Veeger NJ, Zwinderman AH, et al. (2015) Vitamin D reduces eosinophilic airway inflammation in nonatopic asthma. Journal of Allergy and Clinical Immunology 135(3): 670-675. e673.
- Dogru M, Kirmizibekmez H, Mutlu RGY, Aktas A, Ozturkmen S (2014) Clinical effects of vitamin D in children with asthma. International archives of allergy and immunology 164(4): 319-325.
- Krobtrakulchai W, Praikanahok J, Visitsunthorn N, Vichyanond P, Manonukul K, et al. (2013) The effect of vitamin d status on pediatric asthma at a university hospital, Thailand. Allergy asthma & immunol res 5(5): 289-294.
- Li F, Peng M, Jiang L, Sun Q, Zhang K, et al. (2011) Vitamin D deficiency is associated with decreased lung function in Chinese adults with asthma. Respiration 81(6): 469-475.
- Montero Arias F, Sedó Mejía G, Ramos Esquivel A (2013) Vitamin D insufficiency and asthma severity in adults from Costa Rica. Allergy Asthma Immunol Res 5(5): 283-288.
- Al Daghri NM, Al Saleh Y, Aljohani N, Alokail M, Al Attas O, et al. (2015) Vitamin D deficiency and cardiometabolic risks: a juxtaposition of Arab adolescents and adults. PloS one 10(7): e0131315.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...






