Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Article(ISSN: 2638-6070) 
The Effects of Different Cooking Conditions on the Level of Acrylamide in Popular West African Foods
Volume 3 - Issue 5Timothy O Akinosun, Delia Ojinnaka* and Amar Aouzelleg
- School of Applied Sciences, London South Bank University, Borough Road, London UK.
Received: March 1, 2021 Published: March 15, 2021
*Corresponding author: Delia Ojinnaka, School of Applied Sciences, London South Bank University, Borough Road, London UK
DOI: 10.32474/SJFN.2021.03.000174
Abstract
Acrylamide (AA) is a carcinogenic contaminant found commonly in thermally processed carbohydrate-rich foods. The subject is topical and widely researched but information on acrylamide in West African (WA) foods is sparse, hence the need to investigate acrylamide formation in popular WA foods. Potentiometric analysis based on ammonium ion selective electrode and immobilised acrylamide amidohydrolase was used. 150 samples were subjected to varying frying and baking regimes; 150 to 210 °C for 5 to 15 minutes. Generally, an independent T-test for equality of sample means at α = 0.05 showed no statistical significant difference (p ˃ 0.05) in acrylamide produced by baking and frying at the same temperature. However, the ANOVA indicated that increasing the baking and frying temperatures effectively raised the amount of acrylamide (p < 0.05). The lowest (25±8μg/kg) and highest (706±13μg/kg) concentration of acrylamide were in buns baked at 150 °C and plantain chips fried at 210 °C, respectively.
Keywords: Acrylamide; Ammonium-Ion Selective Electrode; Cooking Methods; Process Contaminants West African Foods
Introduction
Several potentially hazardous compounds can be produced from Maillard reaction (MR) in thermally processed foods, and they include furans and heterocyclic amines [1]. The most recently identified MR derived process contaminant is acrylamide (AA), which is formed during baking, frying and roasting of carbohydrate rich foods [2]. AA is produced by the reaction of asparagine with the carbonyl groups of reducing sugars and bioactive compounds, when carbohydrate-rich foods are exposed to high temperatures. It is not usually found in non-thermally processed foods and highest levels have been found in fried potato products, bread and coffee. The presence of AA was also reported in foods such as hazelnuts, almonds, olives [3] and foods not subjected to severe thermal treatments, such as dried plums, pears, and apricots. Heat-treated foods derived from animal tissues such as meat and fish, generally exhibit low or negligible levels of AA [4].
AA has been reported to elicit genotoxic and multi-organ carcinogen effect in rats [5]. There is also another report of the neurotoxic symptoms in individuals exposed to high levels of AA through their occupation. For levels relevant to dietary exposure, AA is potentially, a carcinogenic and genotoxic compound. The average AA exposure for the general population (0.001 mg/kg bw per day) was estimated by JECFA - the Joint Expert Committee of FAO/WHO on Food Additives [6]. The committee noted that AA was a concern to human health as it was both genotoxic and carcinogenic. However, the FAO/WHO report indicated that adverse neurological effects were unlikely at the estimated acrylamide exposure [6]. AA was classed as “probably carcinogenic to humans” (Group 2A) by the international Agency for Research on Cancer [7] and, recently, the European Union set benchmark for dietary AA ranging from 40 mg/kg in processed cereal baby food to 850 mg /kg in coffee [8]. Reducing the consumption of certain products is difficult as staple food like bread constitute an important source of energy and more so for many poor communities in West Africa. Various approaches have been used to mitigate the level of AA produced in foods and these can be broadly classified as product control (e.g. agricultural practices such as cultivation of genetically modified crops), food processing (e.g. altering cooking conditions) and the use of additives (e.g. treatments with enzymes, polyphenols, and calcium) [1]. However, the easiest and cheapest method lies in controlling AA formation through lower temperature processing. This is specially so for countries such as those in WA which would rely on low technological solutions to prevent AA formation.
Generally, high levels of AA are directly correlated with severe heat processing for various foods. However, the processing of some foods above certain temperatures have been reported to result in the reduction of the contaminant. This is probably due to the interaction or bonding of the toxicant with other molecules [9]. According to Burch [10], the AA content of foods is determined by the processing time and temperature, and not the food preparation method itself. Romani, et al. [11] also associated an exponential increase in AA concentrations to longer period of thermal treatments of carbohydrate-rich foods. For WA countries, the processing and preservation of foods depends largely on thermal methods including baking, frying, roasting and smoking of foods. These heat-dependent methods of preservation are important since electric power supply is unreliable in this part of the world. Many studies conducted after the discovery of AA in potato fries and chips have focused on the influence of thermal methods and conditions on the presence, levels and mitigation of the toxicant [12-14] in several foods, but the study on heat processed WA food was almost non-existent. Consequently, this study assessed the effect of the application of different temperatures and common cooking methods on the AA content of quint-essential WA foods including akara (AK), bread (BR), buns (BN), chin-chin (CN), doughnut (DN), fish-roll (FR), meat-pie (MP), plantain-chips (PC), puff-puff (PF), and yam chips (YC).
Principle of Acrylamide Determination by A-ISE
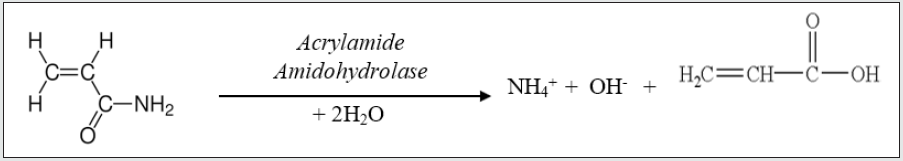
The quantitation depends on the hydrolysis of AA to acrylic acid and ammonium ion by acrylamide amidohydrolase produced by organisms such as Aspergillus nidulans, Bacillus clausii, Helicobacter pylori, Moraxella osloensis or Pseudomonas putida. The NH4+ selective electrode measures the concentration (in ppm (mg/L) and mol/L) of the ammonium ions produced from the enzymatic hydrolysis of the AA present in the samples.
The level of AA present in the sample is then determined from its stoichiometric relation to ammonium ion shown in the chemical equation in figure 1.
Materials and Methods
Chemical Reagents and Standards
Analytical grade reagents and solvents of sufficient purity obtained from Sigma-Aldrich were used to ensure high accuracy of the determination. These include AA (99%), ammonium chloride, hexane, Carrez I and II (potassium hexacyanoferrate (II) trihydrate solution, zinc sulfate heptahydrate solution), calcium chloride, and sodium alginate. NATE-0809 acylamide amidohydrolase (EC 3.5.1.4) derived from Pseudomonas aeruginosa was obtained from Creative Enzymes, U.S.A.
Preparation of reagents and standards
The 1000 ppm NH4+ recommended for the preconditioning of the A-ISE was prepared by dissolving 2.965 g anhydrous ammonium chloride (NH4Cl) in 1 litre de-ionised (DI) water. A stock solution of 0.1M AA was prepared by dissolving 7.11 g AA in 1 litre DI water. Consequently, tenfold serial dilution method was used to prepare standards including 0.01, 0.001, 0.0001 and 1x10-5M AA. Carrez I solution was prepared by dissolving 3.6 g of potassium hexacyanoferrate (II) trihydrate in 100ml de-ionised water, while Carrez II solution was prepared by dissolving 7.2 g of zinc sulphate heptahydrate in 100ml de-ionised water. Approximately 200 IU/ mg of Creative Enzymes NATE-0809 acrylamide amidohydrolase (AA-ase, EC 3.5.1.4) produced from Pseudomonas aeruginosa was added to 1000 μl Tris buffer of pH 7.2. The reconstituted enzyme was immobilized by methods similarly described by Sathesh and Thatheyus in 2007. The immobilization was done by adding 1ml of the enzyme-buffer mix to 9 ml of 2.5% sodium alginate solution, and transferring drops of the AA-ase -alginate mix into 100 ml of 2.0% CaCl solution, using a 10 ml syringe. The resulting AA-ase beads produced were washed with the Tris buffer solution and transferred into a 50 ml separating funnel. This served as the reaction vessel for the hydrolysis of any AA present in the sample. To remove the leftover analyte after each reaction the AA-ase beads were thoroughly washed with the buffer solution.
Food sample preparation
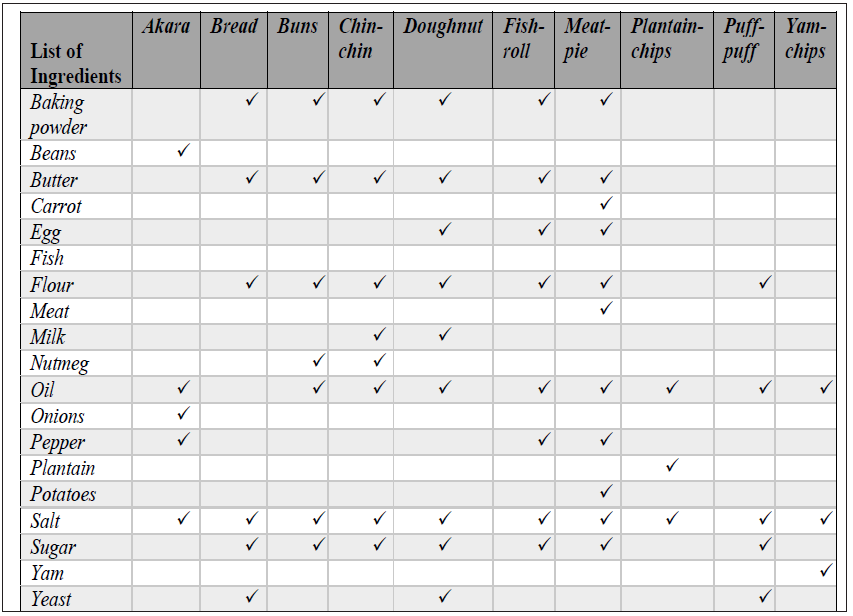
Local producers of WA foods were used for the preparation of the selected foods. The food materials processed at the pre-determined conditions include those listed in Table 1. Equal portions of each WA foods were prepared by baking and frying at 150 °C, 180 °C and 210 °C. Time of processing considered for the selected temperatures were 5 mins, 10 mins and 15 mins. For each cooking method studied under the different conditions, 150 samples of each WA food were used and all analysis were carried out in triplicate.
Extraction of acrylamide from samples and A-ISE Analysis
For the extraction of the analyte, 10 ml de-ionized water was added to 10 g portion of each WA food sample. Solid food samples with very low moisture content such as chin-chin were allowed to swell in the de-ionized water at 37 °C for 15 minutes. An air operated ultra-turrax T-25 high-speed homogenizer and analytical grade water were thereafter used for the blending and homogenization of the food samples. Approximately 100 ml of the homogenized food sample was subsequently mixed and stirred with 200 μl Carrez (I /II) reagents.
The fatty and oily portion of homogenized fat-rich samples such as meat-pie were separated through the addition of 3 ml hexane. The resulting top oily layer was then discarded. Approximately 10 ml of the treated samples were transferred into15 ml centrifuge tubes, which were centrifuged at 4000 rpm for 15 minutes. The resulting supernatant were further cleaned using a 10 ml syringe and 0.45 μm Fisher brand PTFE filter. For the enzymatic release of the analyte, the filtered sample was transferred into the separating funnel with the AA-ase beads and left for 5 mins. About 5 ml of the resulting sample was collected into a 15ml centrifuge tube and immediately treated with a 10% v/v of ionic strength adjustment buffer –ISAB (about 500 μl of 1M CuSO4 for 5 ml sample).
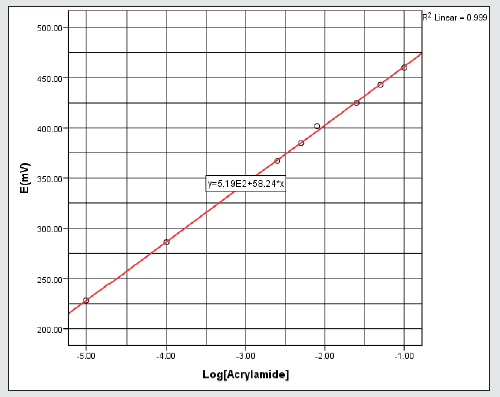
The treated sample was further filtered using a 10 ml syringe and 0.45 μm Fisher brand PTFE filter. An ammonium (NH4+) ISE which has been preconditioned for 5-10 minutes with 1000 ppm ammonium chloride (NH4Cl) standard solution was used to check the performance of the NH4+ ISE-system. The A-ISE system was calibrated using 1x10-5M– 0.1M of enzyme treated AA standard solutions to which 10% v/v of ISAB has been added. The molar concentrations of ammonium ion and AA present in the enzyme treated food sample were subsequently measured by using ELIT ion analyser (or pH/mV/Ion meter) connected to a personal computer (PC) data-recording interface. The level of AA present in the food sample was then determined using the NH4+ ISE system and the generated calibration curves. Overall, the calibration curve, figure 2, showed a good linear relationship between the logarithm of AA concentration and potentiometric values from the NH4+ released, with a correlation coefficient of 0.999. According to Nernst equation, the sensitivity of the instrument was defined by the slope of the curve which is 58.24 mV/log [AA]. In addition, the instrument response time was determined to be about 15 s.
Statistical Analysis:
For the WA food samples obtained through different cooking conditions, the mean AA and the standard deviation were calculated using IBM SPSS statistics 26 and MS Excel 2016 software. The independent T- test for equality of sample means and ANOVA tests for significant differences at α=0.05 were calculated for AA concentrations resulting from the use of two main cooking methods and the three temperature respectively. The effect of interaction between cooking methods and temperatures used for each WA food was also determined using 2-way ANOVA at α=0.05.
Results
Generally, independent T-test for equality of sample means at α=0.05 showed no statistical significant difference (p ˃ 0.05) in the AA produced by using baking and frying at the same temperature. However, ANOVA for the AA concentrations resulting from varying the cooking temperature indicated that increasing baking and frying temperatures effectively raised the amount of the process contaminants i.e. (p < 0.05).
Mean acrylamide concentration for West African foods processed by different cooking methods and temperatures
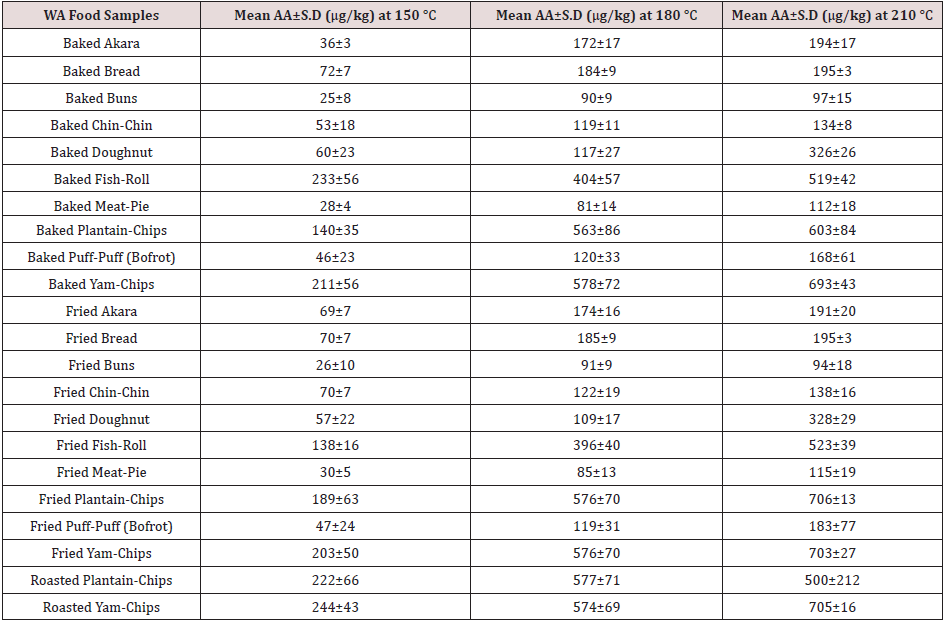
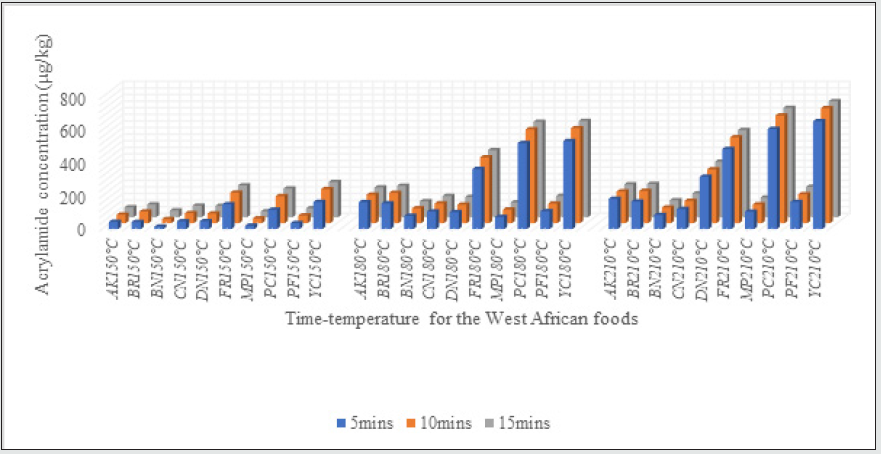
The acrylamide concentrations found in the WA foods processed under the different cooking conditions are shown in Table 2. The highest reading for the contaminant was noted for WA foods processed at 210 °C, while the lowest measurements were obtained for those processed at 150 °C. The lowest amounts of AA (25±8 mg/kg) was detected in buns baked at 150 °C, while the highest levels of the contaminant equal to 703±27 and 706±13 μg/kg, respectively, were noticed in yam and plantain chips fried at 210 °C. The contaminant was not detected in unheated formulations used as control.
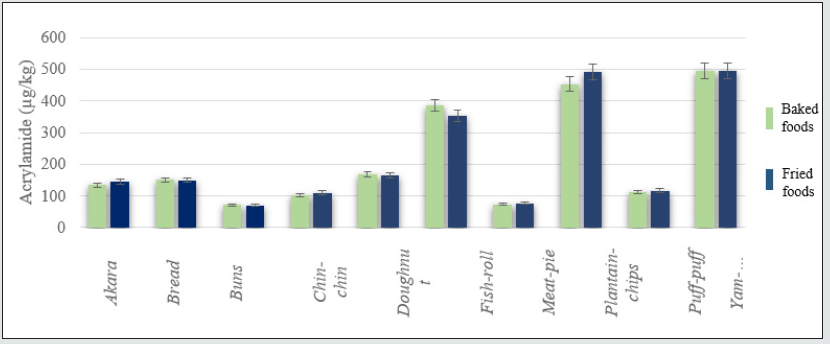
Overall effect of cooking methods on the acrylamide formed in the WA foods
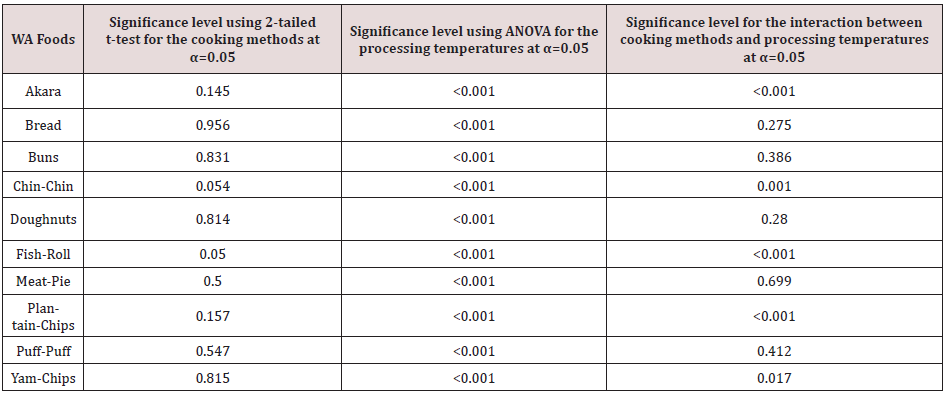
The main cooking methods used for the preparation of the WA foods were baking and frying. The overall effect of the two cooking methods on the production of AA in the WA foods is shown in Figure 3. There is a slight contribution of cooking methods to the formation of AA in WA foods such as chin-chin and fish roll. For most of the WA foods the effect of cooking method was insignificant i.e. p˃ 0.05, Table 3.
Table 3: Significance of the acrylamide contributed to each WA food by the cooking methods and processing temperatures.
Overall effect of processing temperatures on the acrylamide formed in the WA foods
The application of the different processing temperatures resulted in the formation of varying levels of the contaminant. Figure 4 shows the remarkable contribution of higher temperatures to the formation of AA in the WA foods. For all the WA foods studied, the effect of increasing the temperatures was significant i.e. p˂ 0.05, Table 3.
Discussion
Varying amounts of acrylamide were noticed in all the WA foods prepared under the different cooking conditions. For some of the WA foods, the contribution of frying compared to baking was slightly higher. The increment in acrylamide due to frying for akara, chin-chin, meat-pie, plantain-chips, and puff-puff were 8.09, 7.71, 3.97, 8.12, 4.40 % respectively. In comparison to frying, baking contributed 0.24, 1.21, 1.96, and 9.52% additional acrylamide for bread, buns, doughnut, fish-roll, and yam-chips respectively. Although there was a slight influence of the cooking methods on the acrylamide formed in the WA foods, generally, independent T-test for equality of mean showed no statistically significant effect between the contributions of baking and frying i.e. (p-value ˃ 0.05). Temperatures higher than 150 °C contributed remarkable amount of acrylamide to all the WA foods studied. The magnitude of thermal effect was particularly obvious as the cooking temperature changed from 150°C to 180°C. For doughnut (DN), the change in temperature from 180 °C to 210 °C raised the acrylamide level by 189.50%, while the change in temperature from 150 °C to 180 °C resulted in 93.59% increment in acrylamide formed. Apart from DN, the transition from 150 °C to 180 °C was more effective in raising the acrylamide levels in other WA foods studied. The switch in cooking temperature from 180 °C to 210 °C for akara, bread, buns, chin-chin, doughnut, fishroll, meat-pie, plantain-chips, puff-puff and yam-chips resulted in 11.2, 6.09,5.77, 12.82,30.29, 37.33,13.72,46.90, and 21.51% increment in acrylamide respectively. In contrast, the use of 180 °C instead of 150 °C for the preparation of WA akara, bread, buns, chin-chin, fish-roll, meat-pie, plantain-chips, puff-puff and yamchips resulted in 227.78, 160.80, 256.55, 96.24, 115.21, 185.79, 220.88, 157.63, 162.69 % increment in acrylamide respectively. The lowest and highest acrylamide concentrations depicted in figure 5, resulted from the use of the cooking temperatures of 150 °C for 5 mins and 210 °C for 15 mins respectively. Similar influence of baking and frying temperatures were obtained by Palazoğlu [15]. According to the study, an increase in frying temperature from 170 °C to 190 °C raised the acrylamide levels in potato chips. Another study by Granda, Moreira & Tichy [16] demonstrated that vacuum frying at a low temperature of 118 °C caused a 94% reduction in acrylamide content compared to traditional frying at 165 °C and normal atmospheric [17]. A drop in acrylamide formation by 68% and 88% was also observed by Palazoğlu & Gökmen [18] after the reduction in potato frying temperatures from 190 °C to 170 °C and 150 °C respectively [19]. ANOVA test of significant difference also showed that the three cooking temperatures were remarkably unequal in their contributions to the acrylamide content of the WA foods studied (p-value ˂ 0.05) i.e. increasing baking and frying temperatures effectively raised the amount of the process contaminants. Also, higher readings of AA were observed as the time of thermal processing increased [20]. The lowest amounts of acrylamide (25±8 μg/kg) was detected in buns baked at 150 °C, while the highest levels of the contaminant (703±27 and 706±13 μg/kg) were noticed in yam and plantain chips fried at 210 °C.
Conclusion
For the first time, the influence of parameters such as processing methods, temperatures and duration on the formation of acrylamide in popular WA foods was extensively studied. The application of temperatures and processing times more than 150 °C and 5mins respectively for the processing of the WA foods were shown to have high impact on the formation of acrylamide. Overall, the use of the different cooking methods under the same cooking conditions resulted in insignificant difference (p ˃ 0.05) in the formation of acrylamide. However, the use of frying compared to baking for WA foods including akara, chin-chin, meat-pie, plantainchips, and puff-puff increased the level of the contaminant by 8.09, 7.71, 3.97, 8.12, 4.40 % respectively. For bread, buns, doughnut, fish-roll, and yam-chips the use of baking in comparison to frying created 0.24, 1.21, 1.96, and 9.52% additional acrylamide respectively. Although cooking methods have minute effect on the AA produced, the temperature and time of cooking are the major determinant of the level of AA formed in heat-processed WA foods. Based on the findings from the study, mitigating the levels of acrylamide in popular WA foods such as akara and puff-puff requires the application of processing temperatures and times less than 150 °C and 5 mins. When it is impossible to use temperatures lower than 150 °C for domestic or industrial preparation of the WA foods, cooking times less than 15 mins should be used. More studies based on the use of a larger number of food samples and other analytical methods should be conducted to further evaluate the effect of the different cooking parameters on the acrylamide levels in the WA foods.
Conflicts of Interest
There is none as the research is not funded by any external body.
Acknowledgement
The research was carried out at London South Bank University as part of a PhD project. The authors would like to acknowledge the technical support of Mr Ken Unadkat and Mr William Cheung.
References
- Rannou C, Laroque D, Renault E, Prost C, Sérot T (2016) Mitigation strategies of acrylamide, furans, heterocyclic amines and browning during the Maillard reaction in foods. Food Research International 90: 154-176.
- Rosén J, Hellenäs KE (2002) Analysis of acrylamide in cooked foods by liquid chromatography tandem mass spectrometry. Analyst 127(7): 880-882.
- Amrein, TM, Andres L, Schönbächler B, Conde Petit B, Escher F, et al. (2005) Acrylamide in almond products. Eur Food Res Technol 221(1): 14-18.
- SNFA Swedish National Food Administration (2002) Swedish National Food Administration Analysis of Acrylamide in Food.
- Capuano E, Fogliano V (2011) Acrylamide and 5-Hydroxymethylfurfural, HMF: A Review on Metabolism, Toxicity, Occurrence in Food and Mitigation Strategies. Lebensm Wiss Technol 44: 793-810.
- WHO/FAO (2011) Safety evaluation of certain contaminants in food. Acrylamide. Seventy-second meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additives Series: 63 FAO JECFA Monographs 8 (JECFA).
- IARC (1994) Monographs on the Evaluation of the Carcinogenic Risk to Human, Some Industrial Chemicals, International Agency for Research on Cancer, Lyon, France 60: 389-433.
- European Commission (2017) Commission regulation (EU) 2017/2158 of 20 November 2017 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food.
- Claeys WL, DeVleeschouwer K, Hendrickx ME (2005) Quantifying the formation of carcinogens duringfood processing:acrylamide. Trends in Food Science & Technology 16(5): 181-193.
- Burch R (2007) Examination of the effect of domestic cooking on acrylamide levels in food. London Food Standards Agency.
- Romani S, Bacchiocca M, Rocculi P, Dalla Rosa M (2008) Effect of frying time on acrylamide content and quality aspects of French fries. European Food Research and Technology 226(3): 555-560.
- European Commission (2013) Commission Recommendation of 8 November 2013 on investigations into the levels of acrylamide in food (2013/647/EC).
- EFSA (2012) Scientific Report of EFSA Update on acrylamide levels in food from monitoring years 2007-2010 European Food Safety Authority. European Food Safety Authority (EFSA), Parma, Italy, EFSA Journal 10(10): 2938.
- Wang H, Lee AWM, Shuang S, Choi MMF (2008) SPE/HPLC/UV studies on acrylamide in deep-fried flour-based indigenous Chinese foods. Microchemical Journal 89(2): 90-97.
- Palazoğlu TK, Savran D, Gökmen V (2010) Effect of Cooking Method (Baking Compared with Frying) on Acrylamide Level of Potato Chips. Journal of Food Science 75(1): 25-29.
- Granda C, Moreira RG, Tichy SE (2004) Reduction of acrylamide formation in potato chips by low‐temperature vacuum frying. Journal of Food Science 69: 405-411.
- CIAA (2014) Acrylamide Toolbox.
- Palazoǧlu TK, Gökmen V (2008) Reduction of acrylamide level in French fries by employing a temperature program during frying. Journal of agricultural and food chemistry 56(15): 6162-6166.
- Pedreschi F, Kaack K Granby K (2004) Reduction of acrylamide formation in potato slices during frying. Food Sci Technol 37: 679-685.
- Svensson K, Abramsson L, Becker W, Glynn A, Hellena¨s KE et al. (2003) Dietary intake of acrylamide in Sweden. Food and Chemical Toxicology 41(11): 1581-1586.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...