Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Artcle(ISSN: 2638-6070) 
Staphylococcus Aureus Health Risk from Ready-To-Eat Raw Beef Meat and Associated Risk Factors in North West Ethiopia
Volume 3 - Issue 2Birhan Agmas1*,Bizuneh Tsehayneh2 and Taddesse Yayeh1
- 1Department of Veterinary Science, Bahir Dar University, Bahir Dar, Ethiopia
- 2Department of Veterinary Science, Deber Tabor University, Ethiopia
Received: August 09, 2020; Published: August 26, 2020
*Corresponding author: Birhan Agmas,Department of Veterinary Science, College of Agriculture and Environmental Science, Bahir Dar University, Bahir Dar, Ethiopia
DOI: 10.32474/SJFN.2020.03.000159
Abstract
Assessment of the food safety status is a pro-active measure to minimize the risks foodborne intoxications resulted from foodborne pathogens like S. aureus. This study was conducted from October 2018 to April 2019 on S. aureus with the objective of assessing prevalence and associated beef contamination risk factors from ready-to-eat raw beef in Bahir Dar city with cross-sectional study design. About 101 raw beef samples were collected from butcheries using simple random sampling method for prevalence estimation of S. aureus using conventional culture method. Potential risk factors related to meat contamination were evaluated by using personal observation and questionnaire survey. The data were analyzed by using STATA software version 12.0 for descriptive statistics and logistic regression model. The prevalence of S. aureus in ready-to-eat raw beef was 54.45%. Of all respondents, 80.59% showed positive attitude towards food safety; 75.39% had good overall food safety knowledge and 61.32% proper food safety practice. The main contamination risk factors for this prevalence were worker’s inadequate food safety training ( AOR=5(2.13-9.17)), low knowledge level of foodborne diseases (AOR=2.96, (1.37-2.65)), poor attitudes to the necessity of workers medical evaluation before employment (AOR=5.18, (2.03-8.12)), poor washing practice of working clothes (AOR= 3.16(1.31-3.65)) and use of rings, necklaces or watches while serving food (AOR= 8.63, (5.23-10.17)). In conclusion, S. aureus is a major meat contaminant in butcheries of Bahir Dar city due to inadequate food safety training and low level of hygienic practices. Therefore, improving the food safety knowledge and handling practice of meat handlers is important to prevent S. aureus foodborne intoxications.
Keywords: Bahir dar city; Butcher shops; Contamination; Prevalence; Staphylococcus aureus
Background
Staphylococcus aureus belongs to the genus Staphylococcus under the family of Staphylococcaceae. It is one of the more formidable disease-causing bacteria affecting humans and livestock with low DNA G + C content (32-36%) [1,2]. Staphylococcus aureus is a Gram-positive, facultative anaerobic bacterium with size 0.5-1.0μm in diameter which grows individually, in pairs, short chains or grape-like clusters [3]. The bacterium is catalase and coagulase positive, oxidase-negative, non-motile microorganism that does not form spores. On agar medium it creates smooth, convex, glistening, circular colonies with entire margins reaching a size of 6-8mm in diameter [4,5].
Billions of people are at risk every year and thousands die as a result of consuming unsafe
food. Many outbreaks of FBD are due to cross contamination that occurs during food preparation within food service establishments [6]. Staphylococcus aureus is a foodborne pathogen which is responsible for contamination of different food products and results food spoilage, reduction of food safety and shelf life and cause foodborne poisoning via production of deadly enterotoxins. From all major foods and food products, meat is the most perishable because of the presence of all required nutrients for the growth of bacteria, yeast and molds [7,8]. Many factors could be involved in food contaminations and intoxication at any point along the food chain, including the environment and animal handling procedures during slaughtering and processing practices [9]. The prevalence and burden of S. aureus is growing worldwide and it is a major concern of public health programs [10]. However, the true incidence of staphylococcal food poisoning is underestimated due to misdiagnosis, under-reporting, improper sample collection and laboratory examination [11]. Due to poor hygienic practices and low level of awareness, this problem is worse in developing countries. The global prevalence of S. aureus ranges from 23.3% to 73% [12]. Staphylococcal foodborne intoxication causes an estimated 1,513,000 cases of illnesses and 1,210 deaths annually in the United States with an estimated cost of $6.8 billion [13]. In Africa, there is scarce data regarding to the public health impact and burden of S. aureus. Considering the poor hygienic conditions during food production and processing, coupled with poor cooling facilities [14], there is a likely of high impact of staphylococcal food poisoning in African countries like Ethiopia. The prevalence varies from 16.0% in Tunisia to 52.0% in Egypt [15] and up to 57.8% in Ethiopia [16]. In addition, the prevalence in Ethiopia from meat reaches up to 40% reported form butcher shops of Mekelle city [17]. The epidemiology of S. aureus bacteria, the widespread habit of raw meat (locally called “Kurt”) consumption in the population and the availability of raw beef in open-air local butchers without the cold-chain process are suggestive of the risk of acquiring S. aureus related foodborne illnesses in Ethiopia [14-20]. Despite S. aureus prevalence (45%) reported from milk sample in Bahir Dar city [21], there is a paucity of data from raw beef in the city. Therefore, this study was focused on assessing the prevalence of S. aureus from ready-to-eat raw beef and its risk factors of beef contamination in Bahir Dar city.
Methods
Description of the study area
The study was conducted in Bahir Dar city, North West Ethiopia. Bahir Dar city is the capital city of North West Ethiopia. Geographically Bahir Dar city is located between 11.29° to 11.38° North latitude and 37.23° to 37.36° East longitude. Its average elevation is estimated to be 1810 meter above sea level. The city’s mean annual temperature ranges from 7.1 °C to 29.7 °C, whereas annual mean temperature was 20.85 °C [22]. Bahir Dar city is one of the leading tourist destinations in Ethiopia, with a variety of attractions in the nearby Lake Tana and Blue Nile River. In Bahir Dar city, there are about 137 butcher shops which prepare raw meat for human consumption [23]. Consumption of raw and partially cooked meat is commonly practiced in Bahir Dar city like other parts of Ethiopia.
Study design: A cross-sectional study was conducted from October 2018 to April 2019 in Bahir Dar city. The sanitary conditions of butcher shops and the meat handler’s food safety knowledge, attitudes and practices were assessed by using personal observation and structured questionnaire survey.
Sample size determination and sampling procedures
In Bahir Dar city, about 137 licensed butcher shops were operating on meat and meat products and all the butcheries were included in the sampling procedure. The butcher shops obtained legal license and supervised by the nearby health centers under the Bahir Dar city administration health department. The sample size was determined by using 95% confidence interval and 5% desired level of precision. Because there were no previous studies conducted in ready-to-eat raw beef products in the study area, the expected prevalence of S. aureus was taken as 50% and the size was determined by the formula for infinite population given below [24].

Where:- n = required sample size,
Pexp = expected prevalence,
d = desired absolute precision.
Based on the above given formula, the total sample size was
expected to be 384. However, in relatively small populations, the
required sample sizes need to be adjusted ( nadj) by the following
formula for the same degree of precision [24].

Where:- n = the sample size based on an infinite population
(384)
N = the size of the study population (137).
Based on the above calculation, the total sample size for this
study was 101.
In the city, there were six health centers that supervise
surrounding butcher shops. The lists of all 137 butcher shops were
obtained from the health centers out of a total 101 retailers were
selected based on simple random sampling.
Raw beef sample collection
From randomly selected butcher shops, about 250 gram of readyto- eat raw beef (Kurt) samples were collected in sterile stomacher plastic bags and kept in icebox containing ice. The collected samples were immediately taken to Bahir Dar University, Institute of Technology food microbiology laboratory for homogenization and the homogenate were transported to the Amhara Public Health Institute (APHI) microbiology laboratory unit within 4 hours by keeping the cold chain for bacteriological analysis.
Questioner survey and observation
Personal observation and structured questionnaire survey were used to assess the food safety knowledge, attitudes and practices of beef handlers and sanitary conditions of butcher shops of Bahir Dar city. The questionnaire was also prepared to include issues addressing demographic characteristics of respondents, health status and personal hygiene of beef handlers in retailers. The questionnaire was pre-tested and modified as needed before the formal study begins. The questionnaire was filled by face to face interview with one representative beef handler from each 101 retailers. Respondents were selected based on major contribution on food processing and handling. This questionnaire and observational checklists were developed based on comprehensive food safety literature reviews of several published questionnaires [25-29] and managed in-accordance with the standard guidelines of Codex Alimentarius Commission of Food and Agriculture Organization [30] and rearranged to suit target objectives of this research.
Bacteriological investigation
Isolation and identification of S. aureus from ready-to-eat raw beef was done according the methods described by ISO 6888- 3 [8,31]. Briefly 25 gram of raw beef sample was transferred aseptically into a sterile stomacher bag containing 225ml of peptone water and homogenized for 3 minute using a stomacher. From the original homogenate, a loopful aliquot was inoculated on blood agar plates (5% difibrinated sheep blood) and mannitol salt agar (MSA). Pure cultures of presumptive colonies were streaked on nutrient agar and incubated for 24-36h at 37 °C for Gram stain and further biochemical tests (catalase, coagulase test and oxidationfermentation test).
Data quality assurance
The data quality and the reliability of the study findings were assured by following standard operating procedures and the routine use of control bacterial strains. The sterility of prepared media was checked by incubating some randomly selected plates for 24- 48 hours at 37 °C. Uninoculated media was incubated as negative control to check for sterility. The quality of the culture media and test procedures were thoroughly checked using standard American Type Culture Collection (ATCC) strain of S. aureus (ATCC25923) as a positive control for screening tests, confirmatory tests and disk diffusion antibiotic susceptibility tests. Escherichia coli ATCC- 25922 was used as a negative control for culture on mannitol salt agar.
Ethical approval
The study was conducted after the protocol was ethically reviewed and approved by Institutional Review Board of Bahir Dar University, College of Agriculture and Environmental Sciences. A letter of support from Bahir Dar University was written to APHI. Then, ethical clearance was obtained from APHI and official permission was received from Bahir Dar city Administration Health Bureau. Retailer’s owner permission and interviewee’s willingness were asked to participate in the research with full consent by explaining the objectives of the study, their right and their participation is fully voluntarily. They were also being informed about their right during participation that refusing to participate or discontinue the interview at any time will not affect them and their family in anyway and their responses were kept confidential. To ensure confidentiality, any personal identifying information on participants was not collected and it was managed with unique code. A consensus was made that information collected for this study was not be used for any other study without their permission.
Data management and statistical analysis
Raw data and laboratory findings were encoded into Microsoft Excel exported into STATA software version 12.0 and analyzed using descriptive statistics such as frequency, percentages, mean and standard deviation (SD). The answers for respondents for the questions of knowledge, attitudes and practices of beef handlers were analyzed by giving ‘one’ point for each yes/correct reply and ‘zero’ point was awarded for each no/incorrect and I have no idea answers. Binary logistic regression model was employed and variables having p-value of < 0.2 were exported to multi-variable logistic regression model to assess the effect of confounders. The degrees of associations of risk factors with the occurrence of S. aureus in beef sample were quantified using adjusted prevalence odds ratio. In all the analyses, confidence level was held at 95% and used to determine association.
Results and Discussion
Prevalence of Staphylococcus aureus
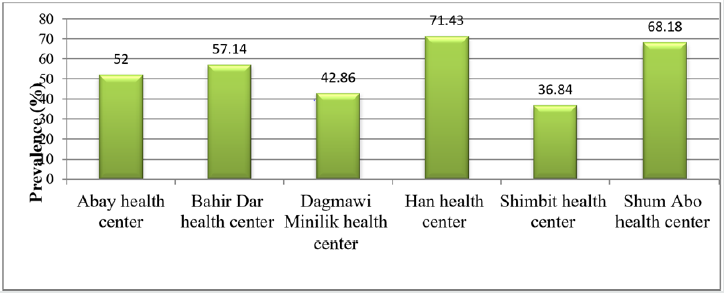
From a total of 101 ready-to-eat raw beef samples subjected for cultural and biochemical isolation, 55(54.45%) were positive for S. aureus. This high prevalence suggests the bacteria could be a major food contaminant in butcher’s shops and the higher rate of contamination of meat with S. aureus organism might be due to poor hygienic and sanitary practices employed right from the slaughtering house, transportation and processing at the butcher shops [32]. The present result was higher than the previous findings of 29.17% from butcher shops of Addis Ababa [33]; 32.22% from retail houses of Jigjiga town [34]; 40% from hand and knife in butcher shops of Mekelle city [17] and 36.5% from ready-to-eat meat in Debre-Zeit [35]. These differences in prevalence may reflect the level of meat contamination during their food handling practices, level of environmental hygiene and the degree of awareness related to microbial contamination. This notion was supported by the previous report stated that high level of food contamination with S. aureus has been related to improper personal hygiene of employees during food handling and processing [33]. The distribution of S. aureus varied between Bahir Dar city health centers in accordance with butcher shops they supervised. The highest prevalence was observed in butcher shops around Han health center (71.43%) followed by Shum Abo health center (68.18%) whereas the lowest prevalence occurred in butcher shops supervised by Shimbit health center (36.84%) (Figure 1). The probable reason for high prevalence around Han health center could be due to the presence of bus station and animal marketing centers which might be the main factor for contamination. Likewise, the butcher shops supervised by Shum Abo health center were vulnerable for contamination due to their location along the main road of the city with high human trafficking. This finding was supported by Fasanmi and his colleagues who stated that contamination of meat could be higher at meat establishments surrounded with high population density due to the presence of contaminated air in form of bioaerosol which is loaded with common microbial contaminants [36].
Figure 1: Prevalence of S. aureus within health centers with respect to butcher shops of Bahir Dar city.
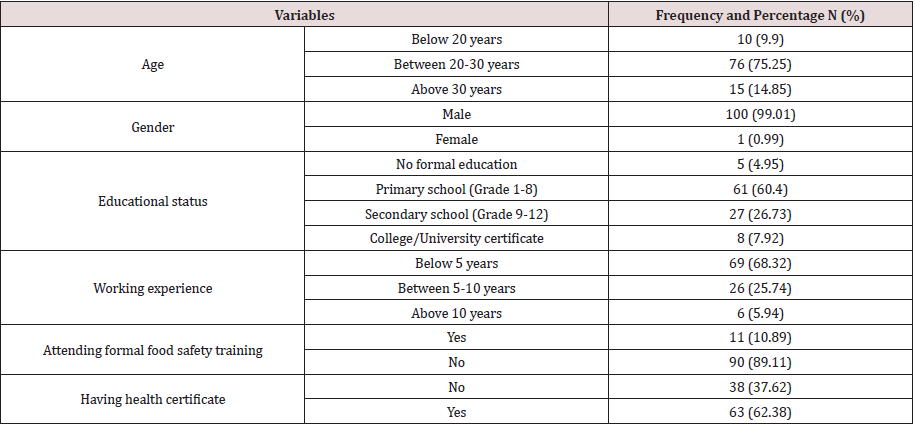
Demographic characteristics of respondents
From total number of 101 food handlers participated in this study (with 100% response rate), 99.01% were male; 75.25% of the respondents had age range between 20-30 years and 14.85% were above 30 years (Table 1). This result was in harmony with of the reports of males were most likely involved in meat processing than females [29] and butchering activity requires much physical strength and it is more dominated by more energetic youth and middle aged (18-40years) men [37]. Based on education level, 60.4% of respondents were attended primary school (Grade 1-8) whereas 5 (4.95%) did not have formal education. Approximately 68.32% of the participants have been working below 5 years in this sector while only 6 people (5.94%) have worked for over 10 years. It was found that 89.11% of respondents did not attend formal food safety training which contradicts and violets the principle laid by the Codex Alimentarius, which states that all individuals coming into direct or indirect contact with food must be qualified and must recognize their role and responsibility in protecting food against contamination and deterioration. It further determines that every food producing area should provide a training program that is revised and updated whenever needed [38]. Additionally, training should include procedures to prevent food contamination, risks of foodborne pathogens, perception of good handling practices and personal hygiene, proper sanitation of food, utensils and the environment and ensure that food handlers are kept updated about the required procedures in maintaining the quality and safety of the food produced [39]. Despite that 62.38% of the participants passed through medical evaluation and certified before employment, no food handler had renewed the certificate, and this was on the contrary that the food handlers should be examined clinically and bacteriologically before they are employed and at regular intervals afterwards to avoid meat contamination [40].
Food safety knowledge of respondents
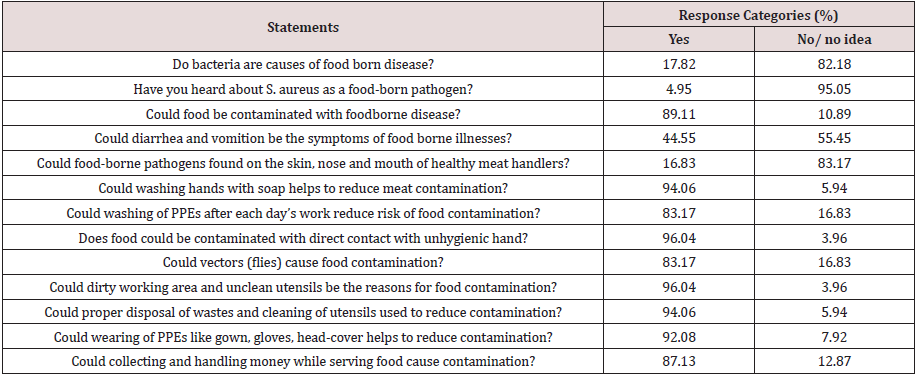
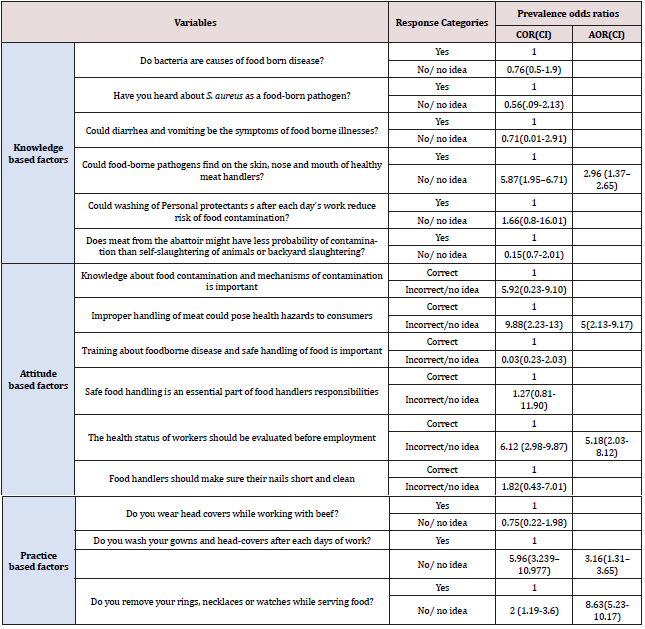
Respondents were asked to assess their knowledge on food safety related to foodborne disease, personal hygiene of food handler and level of cross contamination. The respondent’s overall knowledge level in this study was 75.39% and 96.04% of respondents were aware that meat can be contaminated with dirty working area and unclean utensils and people with skin injury and other diseases. However, only 4.95% of food handler’s had knowledge about S. aureus as source of foodborne intoxication, although 17.82% respondents knew that bacteria could cause foodborne disease (Table 2). Knowledge regarding to S. aureus as source of foodborne intoxication (4.95%) in this study was also highly different from the previous findings (71.4%) in Malaysia [25]. This variation might be due to differences in educational status of respondents and the acquisition of training about foodborne disease because 77% of the respondents attended secondary school and above in Malaysia. This claim was strengthened by the idea that as education level increases, food handler’s knowledge and attitude towards good food handling practice would have improved [41]. It was found that (89.11%) of the respondents had information about food contamination with foodborne pathogens which was fairly compatible with the finding (83.5%) [42]. A very large proportion (94.06%) of respondents knew that proper hand washing and cleaning of storage area before storing new product reduces the risk of meat contamination. The increased food safety knowledge of respondents in this finding was supported by previous reports indicated that knowing the importance of proper handling of meat, proper hand washing and other important hygienic procedures is very important since meat handlers can serve as vehicles for cross contamination and spread of foodborne pathogens [43]. In this study, only 16.83% of the respondents had knowledge about existence of foodborne pathogens on the skin, nose and mouth of healthy individuals. Those respondents who did not have this knowledge could contaminate meat with S. aureus by 2.96 times more likely than those who aware this (AOR=2.96 (1.37-2.65)) after holding other factors constant (Table 5).
Food safety attitude of respondents
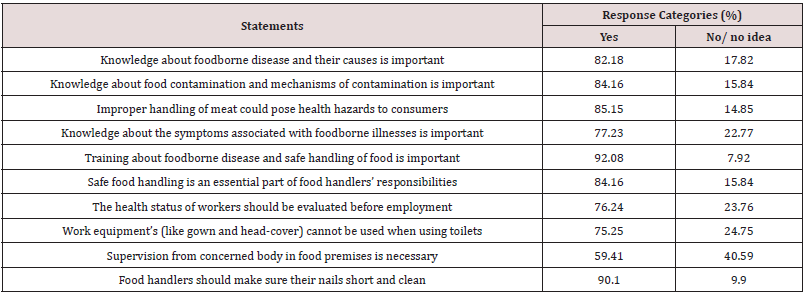
Apart from the knowledge, attitude is also a crucial factor that may influence food safety practice. Of all respondents participated in this study, 80.59% had positive attitude towards food safety (Table 3). Majority of respondents (92.08%) believed that giving training for food handlers regarding to safe handling of food can prevent foodborne infection and intoxication and this finding was in agreement with [44], who found that 96.7% of participants believe on training. In addition, 90.1% of the respondent’s advised that food handlers should make sure their nails short and clean. On the other hand, 85.15% of participants believed that improper handling of meat may pose public health hazard to the meat consuming community.
This result was indicated that the odds of respondents who didn’t believe the health status of workers should be evaluated before employment had a probability of 5.18 times more contribution in beef contamination as compared to their counterparts (AOR=5.18 (2.03-8.12)). In this study, it was observed that food handlers who did not attend food safety training had the probability of contaminating meat with S. aureus 5 times more likely than those who have attended the training (AOR=5(2.13-9.17)) Table 5.
Food safety practices of respondents
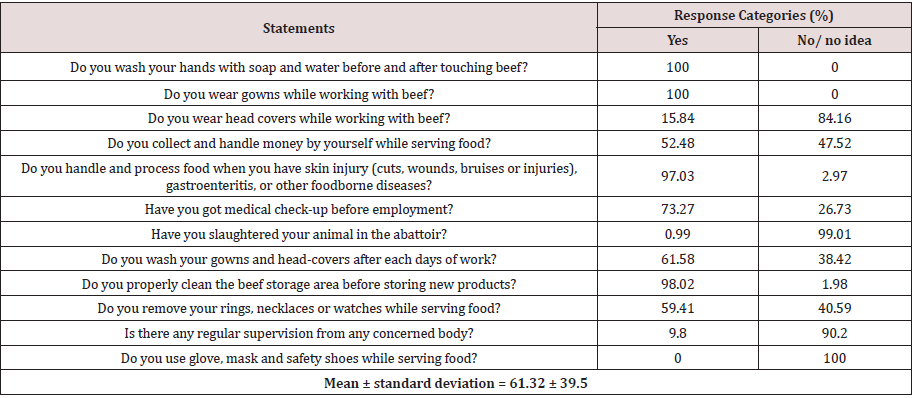
The average good food safety practice level of this finding was 67.64% with a maximum score percentage that all the respondents (100%) were always clean their hands with soap and water before and after touching meat (Table 4). In addition, all respondents were wearing protective gown. However, only 15.84% of the participants had wearing head cover and none (0%) of they were using face mask and glove. This practice was in contrary with the food hygiene principles of food handlers must wear suitable protective clothing to prevent contamination because, bacteria like S. aureus could live on the skin, nose, mouth, throat, ears and hair of humans and be transferred to food, work-surfaces and equipment by the food handler [45]. The overall practices of wearing protective clothes in the study area were poor and contradict with the recommendations of Ethiopian Ministry of Agriculture recommends that protective clothes (like gowns and hair cover) should be worn at all times when handling meat [46]. In this study, about 59.41% of the meat handlers were using jewelries like watches, earrings and rings which was against the principle that jewelry should not be worn in foodservice areas because bacteria and food could gather within them and the area underneath the jewelry warms up thus further encourage the growth and spread of bacteria [45,47]. In this research, the overall food safety knowledge level (75.39%) of respondents was better than the food safety practice of 67.64%, which supports the idea that although food handlers had good knowledge of food safety, they seldom applied this knowledge when handling foods [48]. This discrepancy between knowledge and practice may be explained by a few factors including reluctance to practice what they know due to negligence, lack of encouragement and the weak enforcement of sanitary provisions. Nearly half (48.52%) of the respondents had a practice of collecting and handling money while serving food. Money in hard currency is most widely and highly exchanged for goods and services for every trade in Ethiopia. This makes it as a prime transmission medium for various microorganisms and it may pose a major health hazard particularly, when meat and money were grasped together without washing hands [49]. Food safety practices respondents revealed that meat handlers who did not have every day gowns and head-covers washing practice had the likelihood of providing S. aureus contamination of their beef 3.16 times more than those handlers who had washing practice after each days of work (AOR; 3.16(1. 31–3.65). In addition, meat handlers who use rings, necklaces or watches while serving meat had 8.63 times higher probability of contaminating meat with S. aureus as compared to their counterparts (AOR; 8.63(5.23-10.17)) after considering other variables constant (Table 5).
Table 5: Multi-variable logistic regression analysis of factors associated with prevalence of S. aureus in butcheries (N=101).
Conclusion and Recommendations
This study revealed that S. aureus was a major contaminant of ready-to-eat raw meat in Northwest Ethiopia. This was due to inadequate food safety training and low level of knowledge regarding to foodborne diseases. This necessitates education and training regarding to safe food handling and source of foodborne pathogens should be delivered for meat handlers. Awareness creation and strict follow-up with regular medical checkup should be installed for meat handlers. Poor habit of washing gowns and head-covers after each working day and in ability to remove jewelries like rings, necklaces or watches should be discouraged while serving food. This requires monitoring of cleanliness of food premises and food safety on a regular and more active basis, then carrying out a more comprehensive and effective law enforcement activities by the sanitarian authorities. Future epidemiological investigations should be conducted with type of strain and larger number of samples.
Availability of Data and Materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that they have no competing interests.
Funding
The fund for this study was obtained from Mizan Agricultural Technical Vocational and Educational Training College and Bahir Dar University; College of Agriculture and Environmental Science
Authors’ Contributions
BT developed research idea and conducted the main study. TA and BA supervised, commented and corrected the research protocol, data analysis and all the write-up processes. All authors read and approved the final manuscript.
Acknowledgements
As per their contribution to this study, stuff members of Mizan Agricultural Technical Vocational and Educational Training College and Bahir Dar University are acknowledged. The support of laboratory technicians of Amhara Public Health Institute and Bahir Dar University Technology Institute, and butcher shop owners of Bahir Dar city is highly appreciated.
References
- Götz F, Bannerman T, Schleifer KH (2006) The genera staphylococcus and macrococcu The Prokaryotes 4: 5-75.
- Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, et al. (2011) Bergey's manual of systematic bacteriology 3.
- Schleifer KH, Bell JA (2009) Family VIII Staphylococcaceae fam nov Bergey's Manual of Systematic Bacteriology 3: 392.
- Le Loir Y, Baron F, Gautier M (2003) Staphylococcus aureus and food poisoning. Genet Mol Res 2(1): 63-76.
- Medveďová A, Valík Ľ (2012) Staphylococcus aureus: characterisation and quantitative growth description in milk and artisanal raw milk cheese production.
- Sibanyoni JJ, Tshabalala PA, Tabit FT (2017) Food safety knowledge and awareness of food handlers in school feeding programmes in Mpumalanga, South Africa. Food control 73: 1397-1406.
- Tafesse F, Desse G, Bacha K, Alemayehu H (2014) Microbiological quality and safety of street vended raw meat in Jijiga town of Somali Regional State, southeast Ethiopia. African Journal of Microbiology Research 8(48): 3867-3874.
- Beyene T, Hayishe H, Gizaw F, Beyi AF, Abunna F, et al. (2017) Prevalence and antimicrobial resistance profile of Staphylococcus in dairy farms, abattoir and humans in Addis Ababa, Ethiopia BMC research notes 10(1): 171.
- Sofos JN (2008) Challenges to meat safety in the 21st Meat science 78(1-2): 3-13.
- Kadariya J, Smith TC, Thapaliya D (2014) Staphylococcus aureus and staphylococcal food-borne disease: an ongoing challenge in public health. BioMed research international 2014: 1-9.
- Argudín MÁ, Mendoza MC, Rodicio MR (2010) Food poisoning and Staphylococcus aureus Toxins 2(7): 1751-1773.
- Reygaert W (2013) Antimicrobial resistance mechanisms of Staphylococcus aureus. Microb Pathog Strateg Combat them Sci Technol Educ 1: 297-305.
- Behling RG, Eifert J, Erickson MC, Gurtler JB, Kornacki JL, (2010) Selected pathogens of concern to industrial food processors: infectious, toxigenic, toxico-infectious, selected emerging pathogenic bacteria. Principles of microbiological troubleshooting in the industrial food processing environment.
- Tarekgne E, Skeie S, Rudi K, SkjerdaL T, Narvhus JA (2015) Staphylococcus aureus and other Staphylococcus species in milk and milk products from Tigray region, Northern Ethiopia. African Journal of Food Science 9(12): 567-576.
- Falagas ME, Karageorgopoulos DE, Leptidis J, Korbila IP (2013) MRSA in Africa: filling the global map of antimicrobial resistance PloS one 8(7): e68024.
- Alebachew T, Yismaw G, Derabe A, Sisay Z (2012) Staphylococcus aureus burn wound infection among patients attending Yekatit 12 hospital burn unit, Addis Ababa, Ethiopia. Ethiopian journal of health sciences 22(3): 209-213.
- Endale BG, Hailay G (2013) Assessment of bacteriological quality of meat contact surfaces in selected butcher shops of Mekelle city, Ethiopia. Journal of Environmental and Occupational Science 2(2): 61-66.
- Ejeta G, Molla B, Alemayehu D, Muckle C (2004) Salmonella serotypes isolated from minced meat beef, mutton and pork in Addis Ababa, Ethiopia. Revue de médecine vétérinaire 155: 547-551.
- Tassew H, Abdissa A, Beyene G, Gebre-Selassie S (2010) Microbial flora and food borne pathogens on minced meat and their susceptibility to antimicrobial agents. Ethiopian journal of health sciences 20(3): 137-43.
- Wabeto W, Abraham Y, Anjulo AA (2017) Detection and identification of antimicrobial-resistant Salmonella in raw beef at Wolaita Sodo municipal abattoir, Southern Ethiopia. Journal of Health Population and Nutrition 36(1): 52.
- Shiferaw S, Ahmad M (2016) Prevalence and antibiotic susceptibility of Staphylococcus aureus from lactating cow’s milk in Bahir Dar dairy farms. African Journal of Microbiology Research 10(35): 1444-1454.
- Kasim OF, Abshare MW, Mukuna TE, Wahab B (2018) Land Use and Ambient Air Quality in Bahir Dar and Hawassa, Ethiopia. Air, Soil and Water Research 11: 1178622117752138.
- Getnet S (2018) RE: Butcher shops in Bahir Dar city administration Type to TSEHAYNEH B.
- Thrusfield M (2005) Veterinary epidemiology. 3rd. Cambridgck e, USA: Blak Well Science Ltd 225-8.
- Sani NA, Siow ON (2014) Knowledge, attitudes and practices of food handlers on food safety in food service operations at the Universiti Kebangsaan Malaysia. Food control 37: 210-217.
- VO TH, LE NH, LE ATN, MINH NNT, NUORTI JP (2015) Knowledge, attitudes, practices and training needs of food-handlers in large canteens in Southern Vietnam. Food control 57: 190-194.
- Kunadu APH, Ofosu DB, Aboagye E, Tano-Debrah K (2016) Food safety knowledge, attitudes and self-reported practices of food handlers in institutional foodservice in Accra, Ghana. Food control 69: 324-330.
- Baser F, Ture H, Abubakirova A, Sanlier N, CIL B (2017) Structural modeling of the relationship among food safety knowledge, attitude and behavior of hotel staff in Turkey. Food control 73: 438-444.
- Tegegne HA, Phyo H (2017) Food safety knowledge, attitude and practices of meat handler in abattoir and retail meat shops of Jigjiga Town, Ethiopia. Journal of preventive medicine and hygiene 58(4): E320-E327.
- FAO AND WHO (2009) Food hygiene basic texts. Rome Italy.
- Kompanikova J, Neuschlová M, Sadloňová V (2016) Special Bacteriology-Basic Laboratory Tests Slovakia.
- Haileselassie M, Taddele H, Adhana K, Kalayou S (2013) Food safety knowledge and practices of abattoir and butchery shops and the microbial profile of meat in Mekelle City, Ethiopia. Asian Pacific journal of tropical biomedicine 3(5): 407-412.
- Zerabruk K (2017) Microbial Safety and Quality of Fresh Beef Supplied to Gullele Sub-City market, Addis Ababa. Master of Science in Food Science & Nutrition Addis Ababa University Ethiopia.
- Ayalew H, Berhanu A, Sibhat B, Serda B (2015) Microbiological assessment of meat contact surfaces at abattoir and retail houses in Jigjiga town, Somali National Regional State of Ethiopia. ISABB Journal of Food and Agricultural Sciences 5(3): 21-26.
- Senait G, Moorty PA (2016) Isolation and identification of Staphylococcus species from ready-to-eat meat products in and around Debre-Zeit, Ethiopia. International Journal of Research 3(4): 6-16.
- Fasanmi O, Makinde G, Popoola M, Fasina O, Matere J, et al. (2018) Potential risk factors associated with carcass contamination in slaughterhouse operations and hygiene in Oyo state, Nigeria. International Journal of Livestock Production 9(8): 211-220.
- Adzitey F, Teye G, Dinko M (2011) Pre and post-slaughter animal handling by butchers in the Bawku Municipality of the Upper East Region of Ghana. Livestock Research for Rural Development 23(2).
- Codex Alimentarius Commission (2003) Recommended international code of practice general principles of food hygiene. CAC/RCP 1-1969.
- Campos AKC, Cardonha ÂMS, Pinheiro LBG, Ferreira NR, DE Azevedo PRM, et al (2009) Assessment of personal hygiene and practices of food handlers in municipal public schools of Natal, Brazil. Food control 20: 807-810.
- Bersisa A, Tulu D, Negera C (2019) Investigation of Bacteriological Quality of Meat from Abattoir and Butcher Shops in Bishoftu, Central Ethiopia. International journal of microbiology 2019(2): 1-8.
- Meleko A, Henok A, Tefera W, Lamaro T (2015) Assessment of the sanitary conditions of catering establishments and food safety knowledge and practices of food handlers in Addis Ababa University Students’ Cafeteria. Science 3: 733-43.
- Kumie A, Zeru K (2007) Sanitary conditions of food establishments in Mekelle town, Tigray, north Ethiopia. Ethiopian Journal of Health Development 21(1): 3-11.
- Ansari-Lari M, Soodbakhsh S, Lakzadeh L (2010) Knowledge, attitudes and practices of workers on food hygienic practices in meat processing plants in Fars, Iran. Food control 21(3): 260-263.
- Stratev D, Odeyemi OA, Pavlov A, Kyuchukova R, Fatehi F, et al. (2017) Food safety knowledge and hygiene practices among veterinary medicine students at Trakia University, Bulgaria. Journal of infection and public health 10(6): 778-782.
- FSSAI (2010) Essentials Of Food Hygiene-III. India: Food Safety and Standards Authority of India.
- MINISTRY OF AGRICULTURE (2010) Meat Handlers Personal Hygiene Guideline for Abattoir and Airport Cargo Terminal Workers. Addis Ababa, Ethiopia.
- Marriott NG, Gravani RB (2006) Foodservice sanitation. Principles of Food Sanitation pp. 371-391.
- Clayton DA, Griffith CJ, Price P, Peters AC (2002) Food handlers' beliefs and self-reported practices. International journal of environmental health research 12(1): 25-39.
- Girma G, Ketema T, Bacha K (2014) Microbial load and safety of paper currencies from some food vendors in Jimma Town, Southwest Ethiopia. BMC research notes 7: 843.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...