Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Article(ISSN: 2638-6070) 
Production and Selected Nutritional Analysis of Zobo Juice Preserved with Chitosan Flour
Volume 4 - Issue 2Okoronkwo Christopher U*
- Department of Food Science and Technology, Abia State University, Nigeria
Received: March 07, 2022 Published: March 17, 2022
*Corresponding author:Okoronkwo Christopher U, Department of food science and technology, Abia State University, Uturu, Nigeria
DOI: 10.32474/SJFN.2022.04.000184
Abstract
Zobo drink was prepared and preserved with varying concentrations of chitosan inclusion (0.5, 1.0, 1.5 and 2.0 w/v) over a period of 28 days at room temperature (28°C - 32°C.). Samples were analyzed on weekly basis alongside an unpreserved (control) of zobo drink for vitamin /mineral and microbial quality. Nutritional quality parameters were also affected by chitosan inclusion. Ash (1.09 -2.20⁒) and phosphorus (17.33 -20.42mg/100g) were retained in chitosan treated samples but lost considerably in the Ochito (control). Similarly, vitamins C (2.05 -16.43mg/100g), B1( 0.01 -1.007 mg/100g), B2 (0.012 - 0.036mg/100g) and B3 ( 0.16 - 6.79mg/100g) were preserved during storage in the chitosan treated samples but were lost significantly (P<0.05) in the control. The microbial load of the control increased from 4.3 x 104 to 266.3 x 104 cfu/ml after storage but reduced significantly at 2% chitosan inclusion (2.7 x 104 - 122.7 x 104cfu/ml). We, therefore, conclude that chitosan inclusion preserved the zobo drinks significantly (P<0.05).
Keywords:Roselle; Shelf-life; Zobo; Chitosan; Juice; Preservative
Introduction
The edible Roselle (Hibiscus sabdariffa) is a member of the family malvacecae which belongs to the family of okra, cotton and kernat. It is much like the kernat but can be distinguished by the size of the flour and the shape of the seed [1] . The flour of Roselle is generally smaller and are kidney shaped while those of kenat are bigger and triangular in shape [2]. Hibiscus sabdariffa is a vegetable plant of West Africa, Asia, Austria, and many tropical countries. It is the most widely acceptable of the roselle producing area of Nigerian savannah region where it is grown as vegetable crops [3]. The different parts of the roselle are the seeds, leaves and calyces and these have been used for different purposes as vegetables, sauces, sources of oil, refreshing drinks and food preservatives [4]. The calyx is rich in vitamin C and other antioxidants such as flavonoids [5] as well as in minerals [6]. The vitamins and antioxidants are essential as healthy foods in the building up of the immune system and prevention of diseases [7]. Roselle contains succinic and oxalic acids, vitamins A, riboflavin, niacin, calcium and iron [5,6]. The oil extracted from the seed is a substrate for castor seed oil while the residue is used in fermented form as soup or cake [4]. Research have shown that Indians utilizes the calyces of roselle to produce refreshing beverage, jelly, yams sources and food preserves [8]. In Nigeria, the dried roselle calyces are prepared into a refreshing drink called ZOBO. The name is derived from the local Hausa (Northern Nigeria) name for roselle plant which is called “Zoborodo”. “Zobo” is an indigenous non-alcoholic drink made from a hot water extract of roselle calyx. It is popular in northern Nigeria with a wide patronage at various social gathering. It is popularly spreading across the entire country, because of reports of the medical value. The popularity could be attributed to its ease of processing at home and the income generation by the local market women. The spread of zobo could also be linked to its non-alcoholic nature, a situation which makes it favorable to people of different religions to consume the drink. Despite the popularity of zobo, much interest has not been channeled on the shelf -life extension of the local beverage. This work is designed to further improve the shelf-life of zobo drink at ambient temperature using chitosan flour as a preservative.
Material and Methods
Source of Materials
The sorrel flower (calyx) was obtained from Umuahia main market, Abia State Nigeria. The shells of freshwater snail used for the production of chitosan was obtained from mile 3 market in Port-Harcourt, Rivers State. The laboratory work was conducted in Microbiology laboratory of Abia State University, Uturu.
Production of Zobo Drink
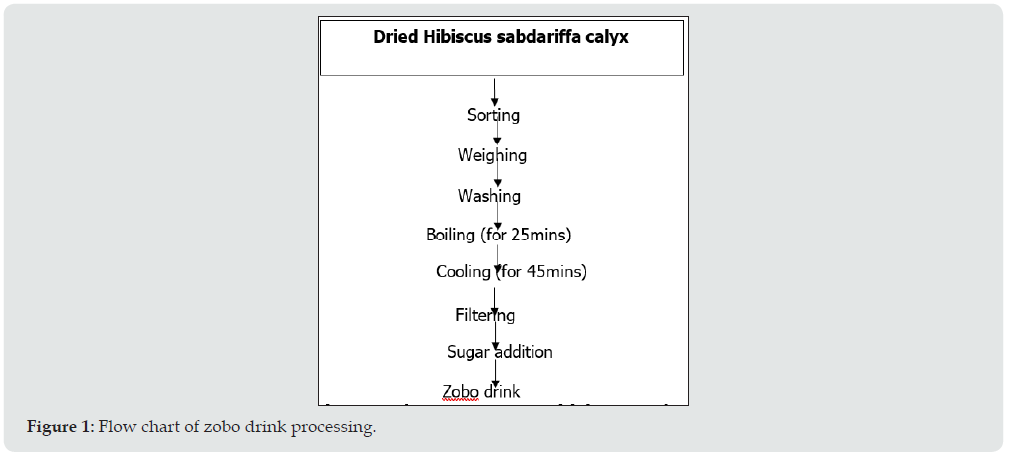
The method of producing zobo drink according to Babajide [2] was used in the production. One hundred grams (100g) of dried Hibiscus sabdariffa was sorted, weighed and washed in a very large bowel of potable water. After washing, the sample was boiled with 4 litres of distilled water for 25 minutes after which it was allowed to cool for 45 minutes before filtration. The extracted juice was filtered through muslin cloth and 0.3 kg of sugar was added to enhance the sweetness Figure 1.
Preparation of Sample
Fifty-four (54) numbers of fresh water snails of the species pila-ovata were used. The snail meat was extracted by breaking the coiled region with the help of the stainless-steel knife. The shells were soaked in running tap water and allowed to stand for 30 minutes before being washed carefully to remove the slimmy substances. Handglove was used during all the handling and washing because of snails have been implicated to host nematodes. The shells were then rinsed twice in distilled water and kept to drain dry at room temperature. They were later placed in the carbolite electronic oven (model PF 200) at 65 °C for 2hours, cooled in a dessicator and weighed. The samples were milled separately in an Arthur Thomas mill (model 0224), the milled samples were passed through a 1mm test sieve to obtain a flour like product used in chitin-chitosan production.
Production of Chitosan
Production of chitin-chitosan from fresh water snail shell was carried out following the method of Anderson et al., [9]. The chitosan was obtained by chemical deacetylation of chitin after deproteinization and demineralization of the ground snail shell. A weight of 50g of each snail shell flour samples was placed separately in a conical flask and mixed with 500 cm3 of 1N NaoH solution in a ratio of 1/10 (W/v). The mixture was boiled for 10 minutes in a GFL 1083 electronic water bath and was centrifuged at 5000xg for ten minutes. The deproteinized residue was washed with hot water and treated with excess 6NHCL solution for 10minutes in an electronic water bath to demineralize it. Thereafter, it was washed with distilled water and filtered with a whatman No 42-grade filter paper. The residue obtained was the crude chitin. The crude chitin was deacetylated by treating with 50% sodium hydroxide solution and boiled for 4 hours at 100 °C. The chitosan obtained was filtered and washed with several portions of hot distilled water until the washed water tested negative to phenolphtalein alkaline test. The chitosan recovered was oven dried to constant weight and cooled in a desicator. It was finally placed in opaque bottles and stored at ambient temperature until needed for analysis Figure 2.
Sample Treatments of Ratios
Treatment of the prepared zobo samples for shelf-life assessment study involved the direct addition of the chitosan to the prepared zobo drink at four levels of concentration as shown below:
a. Sample 1: Control = this sample has no chitosan addition
b. Sample 2: Contains 0.5% chitosan for 100ml of zobo
c. Sample 3: Contains 1.0% chitosan per 100ml of zobo
d. Sample 4: Contains 1.5% chitosan per 100ml of zobo
e. Sample 5: contains 2% chitosan per 100ml of zobo
All the treated samples were left in a screw capped plastic bottle at ambient temperature while sub samples were collected at weekly intervals and analyzed for quality parameters.
Determination of Vitamins
Vitamin A (Carotene) determination
Vitamin A was determined by the method of Association of vitamin chemist described in Kirk and Sawyer [10].
Determination of vitamin C
The titrametric method of vitamin C determination described in Barakat [11] was adopted.
Determination of thiamin, riboflavin and niacin
Thiamin, riboflavin and niacin was determined by the spectrophotometric methods described in A.O.A.C, [12]
Ash content determination
The ash content was determined by the furnace incineration gravimetric method of AOAC,[12].
Determination of phosphorus content
Phosphorus content of the sample was determined by the vanadomolybdate (yellow) spectrophotometry described by James, [13].
Microbial analysis
Determination of microbial load
The method of International Commission on Microbiological Specification for Foods (ICMSF)[14] was used. 1ml of each sample was aseptically mixed with 9 ml of sterile distilled water in a test tube. After mixing, 1 ml of the aliquot of the mixture was aseptically transferred to another tube containing 9 ml of sterile distilled water and mixed. The dilutions were repeated to (10-4). The innocular (a loopful) were taken from each of the first and 3rd diluents of each sample mixture and cultivated by spread plate techniques on SDA and NA respectively.
The inoculation was aseptically placed on the surface of the sterile medium in a petri – dish with the flamed glass hockey, the inoculums was spread evenly over the surface of the medium. The inoculated plates were incubated (upside down for 24-48 hours). They were observed for growth and number of colonies were counted with the aid of an electronic colony counter.
Statistical analysis
The data generated were analyzed using the analysis of variance. Least significant different (LSD) test was used to determine if there was a significant difference between means. Significant difference was accepted at P<0.05.
Results and Discussion
Vitamins
Tables 1 to 5 shows the results of changes in some selected vitamins of zobo drinks at different chitosan inclusion during the four weeks of storage. There was losses of vitamins C (16.43 - 2.35mg/100g), A (29.4 -21.1mg/100g), thiamine (0.02 –ND), Riboflavin (0.032 –ND), Niacin (6.79 -0.16mg/100g) in the drink samples during storage. The loss in Vitamin C in the control was 87.83% (16.43-2.05 mg/100g) after storage while the corresponding loss in 2% chitosan treated sample was 20.04% (14.67- 11.73%). These significant losses were recorded for all the water-soluble vitamins. Thiamin loss in the control was too high after two weeks (14 days) of storage while a loss of 65% was recorded in the chitosan treated samples (Table 3). The loss of riboflavin was significantly higher (0.042- ND) at 0.5 chitosan treatment. A total loss (100%) was recorded in the second week for the control and 0.5% level of chitosan treatment. The concentration of 1% inclusion of chitosan resulted to total loss after three weeks while vitamin reduced from 0.03mg/100g to 0.01/6mg/g (46.7%) at 2% chitosan concentration. Rameen, [15] stated the same vitamins losses during food processing operations. Dandago, [16] recorded that the most susceptible Vitamin are the B1 and B2. Hurst [17] explained that stored milk can lose substantial amount of Vitamin B2 and C within few hours if stored in a clear bottle in sunlights. Niacin reductions were recorded from 6.79mg/100g to 0.16mg/100g) in the control (Figures 1 & 2) and (6.76 to 4.54) mg/100g (32.84%) in the 2% chitosan inclusion. The reduction was significantly lower (P<0.05) in the treated samples than in the control. This was implicated as the ability of the chitosan to preserve the vitamin content of foods during storage. Angelica [18] proved that chitosan and its derivatives have been applied in beverage either as natural preservatives or active packaging agent due to their antimicrobial and antioxidant properties.
Table 1: Results of changes in vitamin C of zobo drink at different chitosan concentration during four weeks of storage.
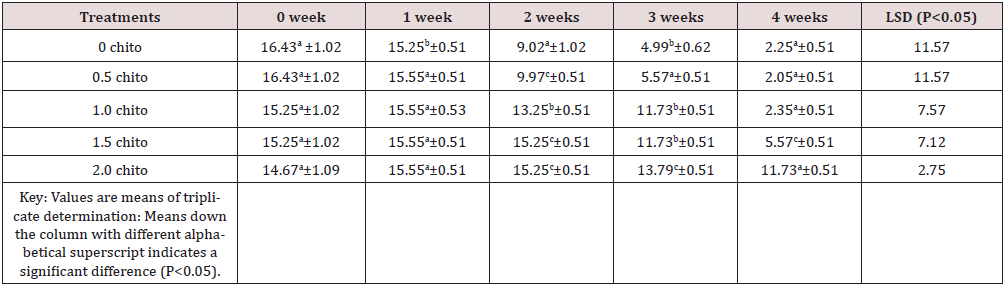
Vitamin C (mg/100g).
Table 2: It shows changes in vitamin A of zobo drink at different chitosan concentration during four weeks of storage ( mg/100g).
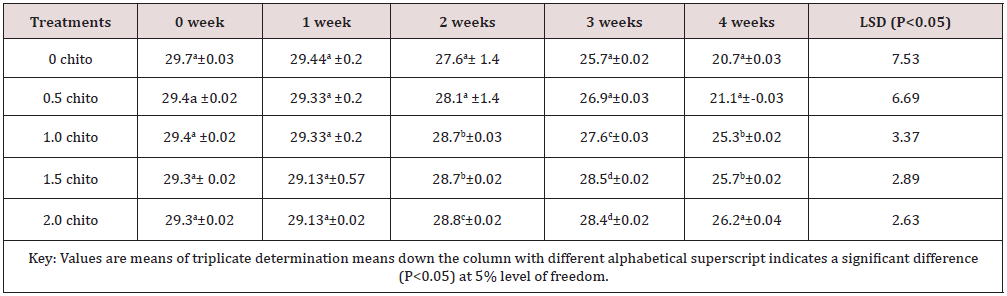
Table 3: Results of changes in vitamin B1 (Thiamine) of zobo drink at different chitosan concentration during four weeks of storage.
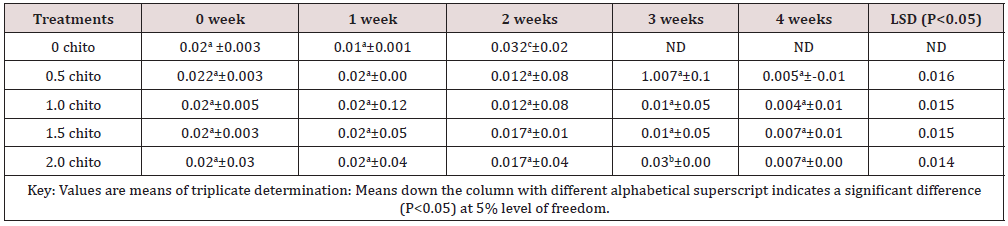
Thiamine (B1) (mg/100g).
Table 4: Results of the changes in Riboflavin (B2) of zobo drink at different chitosan concentration during four weeks of storage.
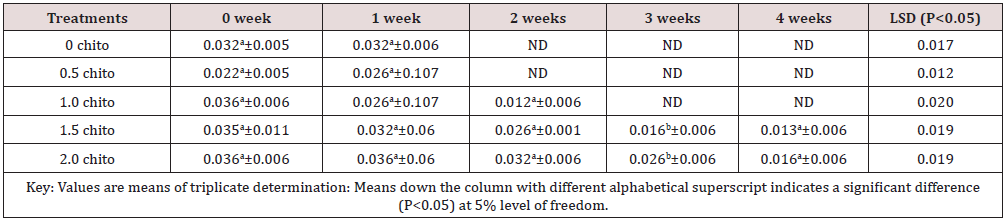
Riboflavin (B2) (mg/100g).
Table 5: Results of changes in niacin (B3) of zobo drink at different chitosan concentration during four weeks of storage (mg/100g).

Tables 6 & 7 shows changes in Ash and Phosphorus of zobo drink at different chitosan concentration during four weeks of storage. Zobo drink was found to contain (1.28% - 2.20) Ash on different level of chitosan treatment (Table 6). The ash content increased from (1.28% to 2.20%) but reduced significantly along the weeks of storage at 5% level of freedom (1.09% to 1.82%). The Phosphorus content of the samples was reduced to 10.35% (19.35 - 17.33)mg/100g in the control whereas a reduction of 5.34% (20.42-19.33)mg/100g were recorded in the 2% chitosan inclusion. Phosphorus is crucial as antioxidant and anti-nutritional substances in diet and fruits [19] freedom.
Table 6: Results of changes in ash content of zobo drink at different chitosan concentration during four weeks of storage.
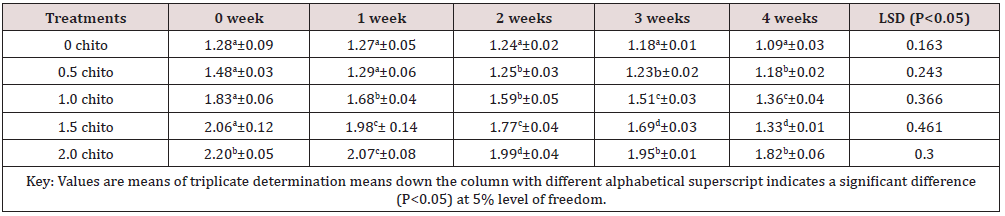
Ash (%)
Table 7: Results of changes in phosphorus of zobo drink at different chitosan concentration during four weeks of storage.
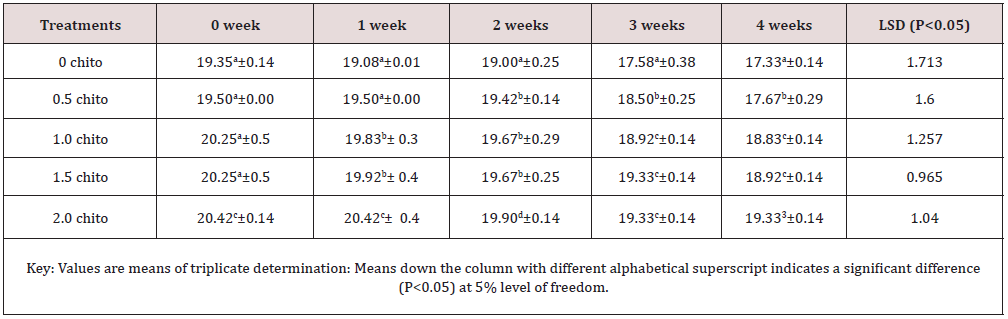
Phosphorus (mg/100g).
Table 8: Shows the changes in Total Microbial load of zobo drink at different chitosan concentration during four weeks of storage
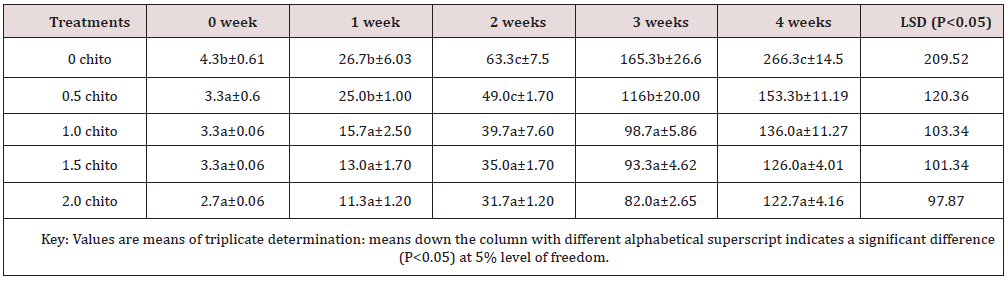
Table 8 presented the result of the total microbial count of zobo drink treated with different concentrations of chitosan flour. The microbial load of the drink recorded values 4.3 x 10 4cfu/ml at the point of zobo preparation without chitosan inclusion, but the load increased to 266.3 x 104 cfu/ml which indicated high level of microbial multiplication. The increase was significantly different (P<0.05) in the chitosan treated sample which recorded 122.7 x 104 cfu /ml during 2% chitosan inclusion. The increase of microbial load of untreated zobo drink is in accordance with high bacterial count in untreated zobo (4.9 x 105 - 4.6 x 105) cfu/ml recorded in Udensi [20]. Diriba [21] implicated the increase of microbes in fruit juice as a result of inappropriate storage and over dilution of water during processing. Poor sanitation habit, collection of samples and transportation may contribute to increase in microbial load especially when the juice is not preserved [22]. It was observed that treated zobo drinks with chitosan inclusion up to 2% (w/v) resulted in reduction of microbial load as well as increasing its nutritional and shelf- life extension. The results show significant difference between the treated samples and the control (0 chito inclusion) at (P<0.05).
Conclusion
It may be concluded that chitosan inclusion in zobo drinks can offer protection to the juice against some spoilage microbes, but this depends on the concentration of the chitosan. It was observed that nutritional losses during storage was higher in vitamins than other parameters studied. The high level of microbial load multiplication was observed in 0 chitosan inclusion (control) which also resulted to some nutrient loss owing to the nutrient utilization by the microbes. The nutrient losses were observed to reduce during the 2% chitosan inclusion. I hereby conclude that chitosan helped in the preservation of prepared zobo drinks.
References
- Atta S, Diallo AB, Sarr B, Bakasson Y, Saadou M, et al. (2010) Variation in macro-element and proteins of Roselle (Hibiscus sabdariffa L.) from Niger. Afri J Food Agric Nutri Dev 10: 2707-2718.
- Babajide JM, Bodunde YG, Salami AA (2004) Quality and Sensory evaluation of processed calyces of six varieties of Rosellle (Hibiscus sabdariffa L.) Nig J Horticul Sci 9: 110-115.
- William WA (1998) Significance of fruit production in the region and constraints to expansion. Paper presented for the regional tropical fruit meeting, Moumea, New Caledonia pp: 21-24.
- Sahar YA, Adel GA, Shaimaa EM, Mohamoud EO (2017) Rosselle seed as a potential new source of healthy edible oil. Journal of Biological Sciences 17: 267-277
- Wong PS, Yuoaf HM, Ghazah, Cheman JE (2002) Physicochemical characteristics of roselle (Hibiscus sabdariffa L.). Nutrition & Food Science 32: 68-73.
- Babalola SO, Aworh OC (2001) Compositional attributes of roselle (Hibiscus sabdariffa L.) Journal of Food Technology in Africa 6: 133-140.
- CTA (2000) The fruits we eat. Technical centre for agricultural and rural cooperation, Wageningen, the Netherlands pp: 9-20.
- Chydescale FM, Main JH, Francis FJ (1997) Roselle (Hibiscus sabdariffa L.). Antocyanins as colourants for beverages and gelatin deserts. J Food Prote 42(3): 204-267.
- Anderson LO (2002) Methods for the physical and chemical analysis of chitin – chitosan. 5th edition pp: 100-105.
- Kirk RS, Sawyer R (1998) Pearson’s composition and Analysis of Foods. 9th edn Longman Singapore pp: 238-242.
- Khan MM, Rahman MM, Murad ATM, Begum SA (2005) Determination of vitamin C content in various fruits and vegetables by UV spectrophotometric method at Sylhet area, Bangladesh. J Environ Sci 11: 190-193.
- AOAC (1995) Official method of analysis of the association of analytical chemist. 4th Edition Washington DC, USA pp: 805-865.
- James CS (1995) Experimental procedure in analytical chemistry of food, Chapman and Hall, New York, USA.
- ICMSF (1978) International Commission on Microbiological Standard for Foods. Microorganisms in food sampling for microbiological analysis. Principles and specific application, University of Toronto Press, Toronto, USA.
- Rameen D (2015) Food processing and impact on nutrient. Scholars Journal of Agriculture and Veterinary Sciences 2(4A): 304-311.
- Dandago MA (2009) Changes in Nutrients during storage and processing of foods. A Review. Technol Sci Africana Journal 3(1): 23-27.
- Hurst WC, Reynolds AE, Schuler GA, Christian JA (1993) Maintaining Food Quantity in Storage. University Of Georgia Cooperative Extension Service Bulletin pp. 914.
- Angelica M, Rocha M, Manuel AC, Claudia N (2017) Application of chitosan and new derivatives in beverage. A critical review. Current Opinion in Food Science 15: 61-69.
- John NA, Lott I, Irene O, Victor R, Graeme DB (2007) Phytic and Phosphorus in crop seed and fruits: A global estimate. Accessed online by Cambridge University press. Seed Sci Resear 10(1): 11-33.
- Udensi CG, Nwankpa UD, Amanze EK, Nwokafor CV, Udekwu CE, et al. (2020) Microbiological Analysis of zobo drink preserved with scent leaves (Ocimum gratissimum). South Asian Journal of Research in Microbiology 8(2): 1-10.
- Diriba LW (2017) Assessment on Bacterial Load of Ready to Use Fruit Juices Served in Cafes and Juice Bars in Hossana Town Southern Ethiopia. Inter J Adv Technol Innov Reseac 9(9): 1426-1430.
- Katema T, Gaddisa T, Bacha K (2008) Microbiological safety of fruit juices served in cafes/Restaurants in Jimma town, southwest Ethiopia, Ethiop. J Health Sci 18(3): 98-100.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...