Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Article(ISSN: 2638-6070) 
Potentials of Unconventional Liming Materials in Reducing Soil Acidity
Volume 3 - Issue 1Gregory Effiong and Dennis Edem*
- Department of Soil Science and Land Resources Management, Faculty of Agriculture, University of Uyo, Nigeria
Received: May 28, 2020; Published: June 12, 2020
*Corresponding author: Department of Soil Science and Land Resources Management, Faculty of Agriculture, University of Uyo, Nigeria
DOI: 10.32474/SJFN.2020.03.000152
Abstract
The shells of molluscs (oyster-Spondulusspinosus and snail - Achatina achatina), which are known to contain high amounts of calcium carbonate and which are abundant in the state were compared with commercial lime in the management of soils developed on acid sands in Akwa Ibom State. Results showed high neutralizing equivalent value of 65 and 75% for oyster and snail shells, respectively compared to 76% for CaC03. Chemical composition of mollusc shells indicated high mean Ca contents of 461.0 ± 28.4 and 441.0 ± 56.6gkg-1for oyster and snail shells while CaC03 contained 541.1 ± 41.7gkg Magnesium contents were higher in oyster (215.2 ± 5.1 gkg-1) than in snail (182.4 ± 17.2gkg-1) shells and CaC03 (91.2 ± 8.7gkg-1). Iron content was 796mgkg-1for oyster, 127mgkg-1for snail and 292mgkg-1for CaC03. The mollusc shells and CaC03, drastically reduced the exchange acidity and increased the soil pH, basic nutrients (Ca and Mg), effective cation exchange capacity and percent base saturation of the studied soil. Mollusc shells compared favorably with CaC03 and could serve as alternative liming materials for soil developed on acid sands in Akwa Ibom State.
Keywords: Mollusc shells; Liming equivalence; Acid sands; Chemical composition
Introduction
Soil acidity is a major problem in the production of arable crops
in the humid tropical soils. Yields of many crops are highly reduced
by soil acidity. Most of agricultural soils in Akwa Ibom State are
derived from Coastal plain sands and Beach ridge sands and are
generally referred to as ‘Acid sands’ [1], because they are strongly
weathered, sandy and highly acidic. Most arable crops give poor
yields and for the crops to do well the soils will have to be limed to
remove the adverse effects of high acidity. The source of limestone
close to Akwa Ibom State is M famosing in Cross River State and
this limestone is used mainly for the manufacture of cement and
a raw material in the iron and steel industry. The limestone in Ini
Local Government Area of Akwa Ibom State has not been exploited
so far for any purpose. There is therefore lack of limestone in the
area for agricultural purposes. There is also the problem of lack of
awareness of the farmers about the importance of lime in reducing
soil acidity and thus bringing about high yields of arable crops in
the high acidic soils of the area. The high cost and unavailability
of commercial lime underscores the need to look for cheap and
alternative sources of liming materials for management of soils
developed on acid sands in Akwa Ibom State. It was also to create
the awareness of the importance of liming to the farmers. The use
of mollusc shells may provide the solutions since the shells are
found to contain high percentage of calcium carbonate, which is the
active compound in liming materials. Mollusc sea foods are. sources
of animal protein to Akwa Ibom people and beyond, and for many
coastal inhabitants. Their shells are commonly found thrown away
in the market places and around homes. They include snail, slug,
periwinkle, clam, oyster and other shells.
The objectives of this study were
(i) To determine calcium carbonate equivalence of oyster
and snail shells.
(ii) Evaluate the chemical composition of snail and oyster
shells as liming materials for soils developed on acid sands in
Akwa Ibom State and
(iii) Examine the effects of these shells relative to commercial
lime on selected soil chemical properties.
Materials and Methods
Study area
Soil samples were taken from the University of IJyo Teaching and Research Farm while mollusc shells were collected at their dumping site near Etuk Market, along Aka Road in Oyo Metropolis. Uyo is situated at latitude 4030’ and 5030’N and longitude 7075’ and 7093’E. The area experiences two distinct seasons: the wet and dry seasons. The wet or rainy season begins from April and lasts till October. It is characterized by heavy rainfall of about 2500- 4000mm per annum. The rainfall is bimodal with peaks in July and September and a relatively moisture stressed period in August, known as “August break”. The dry season starts from November till March. It is characterized by high temperature with a mean annual temperature of 28 °C. The highest temperatures are experienced between January through March, the period described by Enwezor et al. [2] as overhead passage of the sun. Relative humidity is between 75% and 95%. The soil in the area is formed on coastal plain sands parent materials and has been described as Typic Paleudult [3].
Soil analysis
Composite soil samples were taken at (0-30cm) depth. The samples were air dried and sieved (< 2mm). The samples were processed for chemical analysis. The soils were analyzed using the procedures described in Il TA [4]. Soil pH was determined in 1:2.5 soil to water ratio using the glass electrode pH metre and organic carbon was determined using wet oxidation method. Total Nitrogen (N) was determined by the Kjeldahl digestion method, available phosphorus (P) was extracted with Bray P-1 method and P in the extract measured by the blue colour method. Exchange acidity (EA) was extracted with IN KCI and estimated in the extract by titration. Exchangeable bases were extracted using INNH40Ac. Potassium (K) and Sodium (Na) were determined by flame photometer while calcium (Ca) and magnesium (Mg) were determined by EDTA (ethylene diamine tetraacetic acid) titration using NaOH. Effective cation exchange capacity (ECEC) was taken as the sum of the exchangeable bases and exchangeable acidity.
Percentage base saturation was computed using the formula:
%BS = x 100
Collection and preparation of mollusc shells
Mollusc shells (snail and oyster) were collected from the dumping site near Etuk Market in Uyo metropolis. Commercial lime (calcium carbonate) was bought and used as control sample. The shells were washed in warm water and rinsed thoroughly with distilled water. They were placed in clean watch glasses, oven-dried at 80 °C for 48 hours, and were separately crushed to powder in a hammer mill and sieved to obtain particles less than 2mm. The samples and commercial lime were analyzed for Ca, Mg, K, Na, P, and organic carbon. The iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), boron (B), and molybdenum (MO) contents were also determined using the procedures recommended by the Association of Official Analytical Chemists [5]. One gram (lg) of each material was digested with a mixture of concentrated trioxonitrate (v), tetraoxochloroate (Vll) and hydrofluoric acids in the ratio of 1:1:1 in a fume cupboard at 130 °C, the digests were cooled and 20ml of distilled water added, filtered and made up to mark with distilled water. Na and K in the digest were measured using flame analyzer while Ca and Mg were determined by EDTA. Fe, Mn, Zn, Cu, B, and MO were measured with atomic absorption spectrophotometer (AAS) while P was determined by the molybdenum blue method.
Determination of liming equivalence of oyster and snail shells
Measured 0.5g of milled snail and oyster shells were placed in a 250ml flask, and 50ml of 0.5M HCI added, swirled gently and then boiled gently on a steam bath for 5 minutes. The flask was cooled, and 2-3 drops of phenolphthalein indicator added. The surplus acid was back titrated with 0.25M NaOH. The calcium carbonate equivalence was calculated as follows:
Effect of mollusc shells (snail and oyster) on pH and other chemical properties
The method adopted was described by Jacobs and Reed [6],
The soil sample was oven-dried at 105 °C, ground into powder and
sieved. One hundred grams (100 g) of sieved soil sample was placed
in each beaker thus. (i) Soil (100 g), (ii) CaC03 only (1g), (iii) Snail
only (1g), (iv) Oyster only (1g), (v) Snail (lg) + 100g of soil, (vi)
Oyster (lg) + 100g of soil, (vii) CaC03 (lg) + 100 g of soil.
Twenty (20)ml of distilled water was added and stirred to
mix. The mixture was allowed to stand for 1 hour with occasional
stirring. The pH was measured using glass electrode pH metre.
Similar experiment was set up thus:
a. snail (2g) + 100g soil
b. oyster (2g) + 100g soil
c. CaC03 (2g) + 100g soil
To assess the effect of liming materials on chemical properties
of the soil and the experiment was left for 21 days with occasional
stirring. The soil samples were taken and analyzed for exchangeable
bases, exchange acidity, available P, organic carbon and total
N. Effective cation exchange capacity (ECEC) and percent base
saturation were obtained by calculation. Other parameters were Fe,
Mn, Cu, Zn, B and MO, using atomic absorption spectrophotometer
after digestion.
Statistical analysis
The study used mean and standard deviation (X ± SD).
Results and Discussion
Mineral composition of oyster and snail shells and calcium carbonate
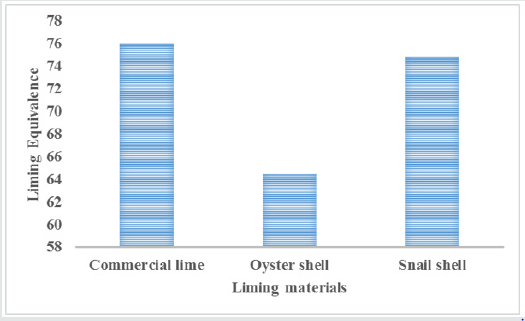
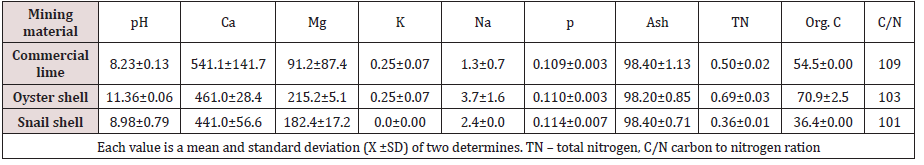
Liming equivalence of oyster and snail shellsLiming equivalence, which is the measurement of the relative value of the liming material in oyster and snail shells, .is presented in Figure 1. The shells have high neutralizing equivalent values, 65% for oyster shell and 75% for snail shell compared to calcium carbonate with 76.00%. These values are similar to those obtained by Tisdale et al. [7], implying that oyster and snail shells are good liming materials. Mean macronutrient contents of mollusc shells and commercial lime (CaC03) are presented in Table 1. Ca content of oyster (461.0gkg-1) and snail (441.0gkg-1) shells were lower than that of CaC03 (541.1gkg-1). Mg content of mollusc shells was higher in oyster (215.2gkg-1) than snail (182.4gkg-1) shells with commercial lime having the least (91.2gkg-1). In a similar study, Inyang (2006) obtained 373gkg-1 and 536gkg-1 Ca and 27.1gkg-1and 27.3gkg-1and Mg for oyster and snail shells, respectively, while commercial lime used advantage in 508gkg-1 and 208gkg-1 Mg. The relatively high contents of Mg in the shells are of significance because when made available to plants it improves plant growth especially through the synthesis of chlorophyll. All the liming materials were low in K (2.0-2.5gkg-1) while Na was moderate in oyster (3.7gkg-1) and snail (2.4gkg-1) shells but low in CaC03 (1.3gkg-1). The low Na content in commercial lime can be attributed to beneficiation while the shells had not been purified and sodium may be present as silicates.
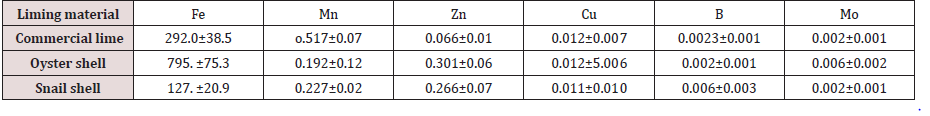
N and P were low in the shells and CaC03 (Table 1). However, since liming materials are usually applied in large quantities, (in mega grams per hectare), the low concentration of N and P in the shells may translate into significant level of addition to soils. The ash content, an index of mineral content in biota, was very high and values were the same for the liming materials (98%). This result implies that mollusc shells would be good sources of mineral elements to plants if properly ground and applied to soils. Organic carbon contents of the shells under study and commercial lime were high with the highest value obtained in oyster shell (70.9gkg- 1), followed by CaC03 (54.5gkg-1) and the least value was found in snail shell (36.4gkg-1). These values translate to high organic matter contents of 93.96gkg-1 for CaC03, 122.23gkg-1 for oyster shell and 62.75gkg-1 for snail shell, respectively. The carbon to nitrogen (C/N) ratio described as an indication of the type of organic matter present and in particular, the degree of humification [8], was very high (in favour of organic C) in mollusc shells and CaC03 studied. This confirms the fact that mollusc shells are poor sources of N. The micronutrient contents of mollusc shells and commercial lime are presented in Table 2. Fe concentration was highest for oyster shell (795.7mgkg-l) and least in snail shell (127.1mgkg-1) Mn was quite low with values of 0.517mgkg-1 , 0.227mgkg-1 and 0.192 mgkg-1 for CaC03, snail and oyster shells, respectively. The value of Zn was also low while Cu, B and MO were found in trace amounts in both the shells and commercial lime. The critical levels of 1.0, 0.5, 0.2 and 0.13mgkg-1 for Mn, Zn, Cu and B [9] and Oyinlola [10] show that the amount in these materials may not have any significant influence in the soil. However, since limes are usually applied in large quantities, the concentrations of these micronutrients may increase considerably, depending on the pH of the soil and quantity of liming materials supplied. Again, the very high concentration of Fe in the liming materials may not pose any threat to crops grown since the solubility of this element will decline with increase in the pH of the soil.
Soil properties
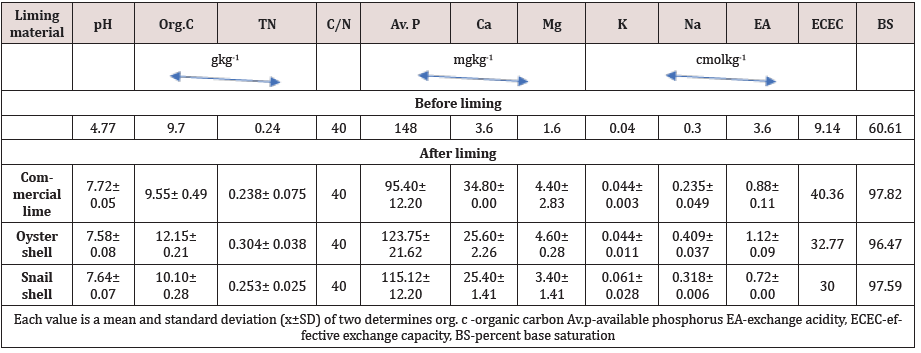
Some chemical properties of the soil studied before and after liming are presented in Table 3. The soil was strongly acidic with low total N (0.24gkg-1) and organic carbon content (9.70gkg-1). With a separating index of 25 between fertile and infertile soil [8], the C/N ratio of 40 obtained for this soil indicates that the soil is poor in N. Available P content of the soil was high and far above the (15-25mgkg-1) determined as critical level for this zone [11]. Calcium level was moderate (3.60 cmolkg-1) while K was lower than Na in the soil. Exchange acidity was high (3.6cmolkg-1) and effective cation exchange capacity low (9.14cmolkg-1) show that percent base saturation was high (60%) and within the >50% regarded as critical value for a fertile soil [8].
Effect of liming materials on soil chemical properties
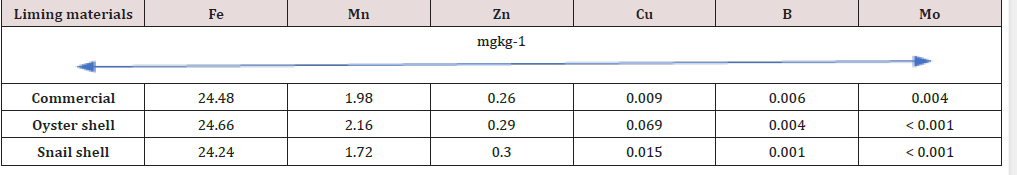
Results of incubating the soils with liming materials for 21 days indicate that all the liming materials significantly raised the soil pH to between 7.58 and 7.72, representing or 61.8, 58.9 and 00.2% by commercial lime respectively, oyster and snail shells. As shown in Table 1, snail shell and calcium carbonate were moderately alkaline (pH 8.98 and 8.23) while was very ‘strongly alkaline (pH 11.36) Such that oyster and snail shells could be used as suitable alternative liming materials for acid soils. Inyang [12] and Akpabio [13] noted that mollusc shells were as effective as calcium carbonate in reducing soil acidity. Liming soil with oyster and, snail shells increased organic C content from 9.70gkg-1 in the unlimed soil to 12.15 and 10.10gkg-1, respectively but decreased to 9.55gkg-1 in CaC03 treated soil. The liming materials had no effect on total N content of the soil. Available P content in the soil after treatment with mollusc shells was 123.75mgkg-1 for oyster and 115.12mgkg-1 for snail shells. Commercial lime had the least effect on the P value (95.-1) of the three liming materials. These values are many times above the critical level of 15-25mgkg-1 P for soils of this zone but lower than the amount of P before application of liming materials. Ibia et al. [14] and Effiong et al. [15] had observed increased availability of P when acid soils were limed, while a report by IlTA [16] indicated that available P decreased as the amount of various liming materials increased, in three Ultisols. The reduction of P content following liming might have resulted from phosphate fixation which is always a problem in alkaline soils due to the formation of complex insoluble calcium phosphates [17]. High pH value and available Ca content are also closely related to low availability of P especially at conditions of low Na content [18]. Liming acid soil with various liming materials increased exchangeable Ca and Mg, exchange acidity, ECEC and percent base saturation (Table 3). Exchangeable Ca increased appreciably from 3.60cmolkg-1 in the unlimed soil to 34.80, 25.60 and 25.40 cmolkg-1 in soil treated with lime, oyster and snail shells, respectively. Previous studies showed that exchangeable Ca increased with the quantity of lime applied [19,15]. Mg content also increased from 1.60 to 4.40, 3.40 and 4.60 cmol kg-1 in soil treated with lime, snail, and oyster shells. The various liming materials had no significant effect on K and Na, because of the very low concentrations of these two nutrients in the liming materials and soil. Brady and Weil [17] noted that the availability of K in soil may decrease or increase due to liming. The reduction of soil acidity is one of the most commonly mentioned specific effects of lime. As indicated in Table 4, exchange acidity was reduced by 80, 76 and 69%, respectively by snail, lime and oyster shells. This reduction raised soil pH and increased ECEC and percent base saturation, remarkably in the soil. Effective cation exchange capacity (ECEC) increased from 9.14cmolkg-1 in unlimed soil to 40.36, 31.79 and 30.00cmolkg-1 in the soil treated with lime, oyster, and snail shells, respectively. The micronutrient content of the soil after liming are presented in Table 4. Fe was 24.48, 24.66 and 24.24mgkg-1 in soils limed with lime, oyster, and snail shells, respectively. These values are many times lower than those found in the mollusc shells and commercial lime. The drastic reduction of Fe contents in the soil probably resulted from the alkaline nature of the soil following liming. This observation calls for proper calculation of the rate of liming to avoid over liming, which is probably responsible for the reduction in Fe content in the soil. Mn content was moderate at 1.98, 2.16 and 1.72mgkg-1 in the soil limed with CaC03, oyster and snail shells, respectively, which are slightly higher than the critical limit of 1.0mgkg-1 [8]; and also higher by 74, 91 and 87% than the values found in mollusc shells and CaC03. Soil Zn content was enhanced by liming though Cu, B and MO values were almost the same following liming with CaC03, oyster and snail.
Table 4: Values of micronutrient levels of the soil after liming with commercial and unconventional lime.
Conclusion
This study has revealed that mollusc shells (oyster and snail) have high neutralizing values, high contents of Ca and Mg, organic C and ash and Fe while micronutrients (Cu, B and MO) were low. Oyster and snail shells used as liming materials have drastically reduced exchange acidity and appreciably increased the soil pH, basic nutrients, ECEC and percent base saturation of the soil. Mollusc shells compared favorably with lime and so can be used in replacement for neutralizing acidity in soils developed on acid sands in Akwa Ibom State. Lime is unavailable and unaffordable at critical periods, whereas the mollusc shells are common place as wastes around homes and in market places.
References
- Obiora CH (1961) The Acid sands of the Eastern Nigeria. Part 1-extent. The Nigerian Scientists J Eastern Nigeria Science Association 1(1): 54 -64.
- Enwezor U0, Ohiri AC, Opuwaribo EE, Udo EJ (1990) A Review of Fertilizer use on crops in the South Eastern Zone of Nigeria. In Literature Review on Soil Fertility Investigation in Nigeria. Federal Ministry of Agriculture and Nat. Res, Lagos.
- Loganathan P, Sutton PM (1986) Phosphorus Fractions and Availability in Soils formed on different Geological deposits in the Niger Delta Area of'Nigeria. Soil Sciente143(1): 16-25.
- IlTA (1979) Selected methods for soils and plant analysis manual services No. 1 International Institute of Tropical Agriculture Ibadan, Nigeria.
- AOAC (1990) Official Method of Analysis of the Association of Analytical Chemists.15th(edn.), Association of Official Analytical Chemists, Washington DC, USA.
- Jacobs HS, ReedRM (1971) Soils laboratory exercise source book. AmeriSocArgonMadison Wisconson.
- Tisdale S L, Nelson WL, Beaton JD,Havlin JL (1993) Soil acidity and basicity. In: Soil fertility and fertilizers, Tisdale LS, Nelson WL (Eds.), 5th Edition, Macmillan Publishing Co. New York, USApp. 364-404.
- Landon JR (1984) Booker Tropical Soil Manual: A handbook for soil survey and agricultural land evaluation in the tropics and subtropics. Booker Agriculture International Limited United States of America, New York pp. 450.
- Viets FG, LindsayWL (1973) Testing soil for Zn, Cu, Mn, and Fe. In: Walsh LM, Beaton JD (eds.). "Soil Testing and Plant Analysis" Soil Sci. Soc. Am Madison. Wisconsin, USA, Pp. 193-172.
- Oyinola EY (1997) Boron requirement of tomato (Lycopersicum esculentum) in some selected soils of the Nigerian savanna. Unpublished PhD Thesis, Dept. of Soil Science, ABU, Zaria, pp.271.
- Ibia TO, Udo EJ (1993) Phosphorus Forms and fixation of representative soils In Akwa Ibom State of Nigeria. Geoderma 58:95-106.
- InyangND (2006) Use of mollusc shells as liming materials for acid soils of Akwa Ibom State. Unpublished MSc. Thesis, Dept of Soil Science, University of Uyo, Uyo, pp. 87
- AkpabioES (2007)Determination of the chemical properties and liming equivalence of mollusc Unpublished MSc. Thesis. Dept. of Chemistry, University of Uyo, Uyopp. 56.
- IbiaT0, EJUdo UOmoti (1997) Evaluation of slag by products from Delta steel plant,as liming materials. Global J Pure and Applied Sci3(3).
- Effiong GS,Isirimah NO, U Eshiet (2006)Influence of liming on extractable Pg. andyield of okra (Abelmoschus esculentåS (L.) Moench). N'JAFE 3 (1 & 2): 131-134
- IlTA (1975) Management of ultisols and oxisols in the tropics. International Institute of Tropical Agriculture, Ibadan, Nigeria pp. 12-14.
- Brady CN, WeilRR (2002)The Nature and Properties of Soils. Thirteenth Edition Pearson Education,Inc Delhi pp. 976.
- Tan K (1998) Principles of soil chemistry. Marcel Dekker New York Pp. 162-164.
- HA Mascarenhas, JR Crallo, BVan Raij, J Ique, OC Batalgia (1976) Effects of limingonchemical characteristics of soil and the nutrition of soybeans on a red latisol.pp. 30.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...