Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Article(ISSN: 2638-6070) 
Influence of Cultivation Conditions on the Nutritional Composition and Bioactive Components of two Undervalued Edible Plants (Porophyllum ruderale and Portulaca oleracea)
Volume 4 - Issue 3Tamara Fukalova Fukalova1*, María Dolores García Martínez2 and Maria Dolores RaigónJiménez2
- 1Facultad de Ciencias Químicas, Universidad Central del Ecuador (UCE), av. Universitaria, Quito-Ecuador
- 2Instituto de Conservación y Mejora de la Agrodiversidad Valenciana (COMAV), Universitat Politècnica de València, Valencia, Spain
Received: May 31, 2022 Published: June 10, 2022
*Corresponding author:Tamara Fukalova Fukalova, Facultad de Ciencias Químicas, Universidad Central del Ecuador (UCE), av. Universitaria, 170521 Quito, Ecuador
DOI: 10.32474/SJFN.2022.04.000187
Abstract
Wild plants have received considerable attention regarding ethnobotanical and pharmacological aspects. However, the potential of wild edible plants in terms of their nutritional and bioactive benefits has been investigated in only a few cases, despite being an important food source. This study aims to be a reference to promote the inclusion of two undervalued food plants as a nutritional alternative for balanced and healthy diets. The nutritional composition and bioactive components of Poriphyllum ruderale and Portulaca oleracea, two Mediterranean species, have been evaluated under wild and organic farming conditions. The quantification of nutrients and bioactive compounds was carried out with leaves and small stems of fresh plants. The proximal and mineral composition was determined with the official methods. The analysis of antioxidants was carried out with the DPPH technique and of total phenolic compounds by Folin-Ciocalteu. Other chemical components such as nitrates, pH and total acidity were determined. The most representative nutritional components were crude fiber and carbohydrates under wild conditions for P. ruderale and under cultivated conditions for P. oleracea. The most abundant mineral macroelements were calcium and magnesium in cultivated P. ruderale and wild P. oleracea. For both species, the outstanding microelement was iron in wild conditions. In addition, both species stood out for a high content of antioxidants and chlorophyll in wild conditions. Finally, these undervalued plants showed significant nutrient content and are a good source of antioxidants and phenolic compounds for a healthy diet. The results suggest that both undervalued plants have a considerable nutritional potential and high content of bioactive compounds, highlighting antioxidants, they also have the potential to diversify production and as an attractive ingredient in healthy diets.
Keywords:Undervalued Species; Nutritional Composition; Bioactive Compounds; P. ruderale; P.oleracea
Introduction
Among the seventeen Sustainable Development Goals (SDGs), the third is focused on health and wellness [1]. Undervalued wild species could be a means of sustainable use of local resources and diversify healthy diets, contributing to this SDG. The Mediterranean diet as a world heritage [2] stands out for the consumption of wild vegetables that are still part of traditional diets. It is especially worth remembering the health benefits provided by the Mediterranean diet and on which several nutritional studies corroborate [3-6]. Many of these wild species are appreciated for their organoleptic and nutritional-medicinal properties. However, globally its uses have often been relegated to a local environment. The abandonment of species that at some points were an important component of food, gives them the status of undervalued or forgotten, increasing food poverty and loss of agrobiodiversity heritage. At the same time, the loss of these resources and the absence of adequate links between conservation and their use are a major danger for future food security [7]. Today, agricultural crops have displaced many previously known and appreciated wild species. However, traditions have made it possible for some wild plant resources to continue to be present in the diet of many people and to form part of traditional gastronomy [8]. In this context, preserving the selection of traditional local products, the transmission of knowledge, traditional culinary activities are part of the resilience to the current globalized and changing world. In addition, wild plants contain phytochemical compounds that, in combination with nutritional compounds, act synergistically, improving the effects for the prevention of many chronic diseases [9,10]. As Medina [11] points out, the food heritage (Mediterranean diet in this case), must be constantly recreated, and this within cultural frameworks in continuous evolution, which demands adaptation capacity. In this way, all the studies that advance in the knowledge of traditional foods consumed locally are very valuable from the cultural and nutritional point of view. Thus, the influence of the cultivation conditions of two undervalued food plants inherent to the springsummer period in Mediterranean conditions was studied: purslane (Portulaca oleracea L.) and quirquiña (Porophyllum ruderale (Jacq.) Cass). According to the previous ethnobotanical review, these two species have been rooted in the traditional gastronomy of the Valencian coast (Spain). As objectives of this research, it was established to compare in organic farming conditions and in wild conditions: 1) the nutritional characteristics of two species; 2) the bioactive components present. Since wild species are well adapted to local environmental conditions, they can ensure constant production under adverse environmental conditions and could be considered as vegetables for the local productive structure, as well as a beneficial source for health.
Materials and Methods
Plant material and sample preparation
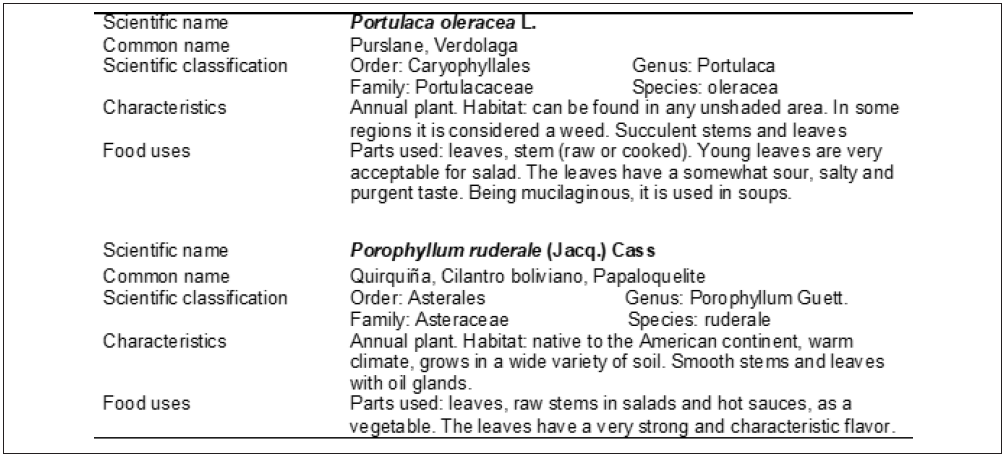
The harvest was carried out in the spring-summer season of 2020 under two growing conditions: 1) organic farming conditions; 2) wild conditions in the same area of the Valencian coast within latitudes N 39º45’13’’ and longitudes W 0º12’21’’. The wild plants were collected in the areas close to the cultivation area and their brief description is summarized in Table 1. P. oleracea is a widespread edible and medicinal plant with expanding interest as some previous research indicates that its shoots are a rich source of bioprotective nutrients [12-16].
P. ruderale has also been used in traditional medicine [17], although the review work by Marques et al. [18] highlights the lack of scientific work on this plant, except for some research on the chemical characterization of its essential oil [19,20]. The phytochemical constituents of the studied plants have some bioactive properties that are summarized in Figure 1. Approximately 1 kg of each species was collected. The leaves and tender stems were separated and used for the extraction and quantification of bioactive compounds: total antioxidants, total polyphenols, chlorophylls and other chemicals (pH, nitrates, total acidity). The rest of the plant material was dehydrated in an oven at 70 °C (J.P. Selecta) and subsequently ground (Retsch KG-5657 Haan Remscheid Germany) for proximal analysis.
Reagents and Standards
The 80% (v/v) methanol and 80% (v/v) acetone solutions were prepared from the analytical grade purity reagents. Sodium carbonate; citric acid, boric acid, sulfuric acid, hydrochloric acid and phosphoric acid; lanthanum (III) chloride, sodium hydroxide from Scharlau. Trolox (6-hydroxy-2,5,7,8-tetramethyl-chroman- 2-carboxylic acid); 2,2´-azobis-2-methyl-propanimidamine; 1,1-diphenyl-2-picrylhydrazyl (DPPH); Folin-Ciocalteu reagent, gallic acid from Sigma-Aldrich Co.
Proximate analysis and minerals determination
The analysis was carry out by the official methods: moisture (AOAC 984.25), proteins (AOAC 984.13), fat (AOAC 983.23), fiber (AOAC 991.43) and ashes (AOAC 923.03). Digestion was performed according to AOAC method 985.35. Carbohydrate content was calculated by difference. The minerals were analyzed by atomic absorption spectroscopy. (Thermo Elemental AA series), except for phosphorus, which was analyzed by colorimetry [21].
Bioactive components evaluation
All analytical methods applied were optimized and validated for specific vegetal samples analyzes. The total antioxidant content was determined by the DPPH method following the modified methodology of Brand-Willams [22]: the extract was obtained by mixing 0.8 g of the sample with 5 mL of 80% methanol for 1 hour at room temperature in orbital shaker SO1 (Stuart Scientific); then an aliquot of the extract (100 μL) was allowed to react for 45 minutes in the dark with the reagent (0.025 g/L of DPPH methanolic solution). The absorbance was measured at 515 nm (Schott UVline9400). The calibration curve was obtained with Trolox as standard. The results were expressed in μmoles of Trolox equivalent in 100 g of fresh weight (μmol TE 100 g-1 fw). The total phenolic content was performed by mixing an aqueous extract aliquot (0.5 mL) with the Folin-Ciocalteu reagent and sodium carbonate. The absorbance was measured at 750 nm in the UV/V spectrophotometer (JENWAY 6715/UV-Vis) after 1 hour of incubation. Gallic acid was used for the calibration curve. The results were expressed as mg of gallic acid equivalents in 100 g of fresh weight (mg GAE 100 g-1 fw). The chlorophylls content was determined using the adapted Hansmann method (1973): the extract was obtained with 80% acetone.
Once filtered, the absorbance was measured at 645nm, 653nm and 663nm (Schott UVline9400). The results were expressed as milligrams of chlorophyll in 100 g of fresh weight (mg·100 g-1 fw).
Other chemicals: nitrates, pH and total acidity
Aqueous extracts of aerial parts were prepared in 1:2 w/v ratio (plant / 80% acetone). Nitrates and pH were measured directly with the respective electrodes by pH and ION-Meter GLP 22+ equipment (CRISON). The total acidity was determined potentiometrically with a 0.05 N NaOH solution. The results were expressed as a percentage of citric acid.
Statistical analysis
Each sample was analyzed in triplicate. The analysis of variance and the significant difference between means were tested by multivariate ANOVA at a significant level of p<0.05. Differences between groups were determined by comparisons of means (Tukey contrast). All analysis was processed using Statgraphics Plus version 5.1 (Manugistics Inc., Rockville, MD, USA)
Result and Discussion
Proximate analysis
Table 2 shows the percentage contents of the nutrients, as well as the macro and microminerals values. It is observed that the wild species (P. ruderale with 84.70% and P. oleracea with 88.39%) have a higher humidity level than their cultivated counterparts with a significant difference (p=0.0000). On the contrary, the ash content is higher in P. oleracea than in P. ruderale and in both species it is lower in wild conditions vs. cultivated conditions. Crude protein had a higher value in P. ruderale under wild conditions, and in P. oleracea no significant differences were observed for this nutrient according to the growth system. The fat content in P. ruderale was higher in wild conditions (5.50%) vs. cultivated conditions (3.57%), while in P. oleracea the same parameter was higher in cultivated conditions (0.99%). For both species, the differences between cultivation systems were significant with p=0.0156 (P. ruderale) and p=0.0000 (P. oleracea). Crude fiber did not present significant differences between the growing systems in any of the species, showing the highest level in P. ruderale (5.50%). Carbohydrates indicated high levels in cultivated conditions vs. wild conditions for both species. The characterization of the nutrients in vegetables is an important contribution to the daily requirements of a balanced diet. Moisture content determines freshness of vegetables and color characteristics appreciable by consumers [23]. On the other hand, the stability and quality of food are also affected by the moisture content. Its higher levels in the wild conditions for both species are probably related to their ability to retain water when exposed to higher stress agroclimatic conditions than their cultivated counterparts. Moisture values similar to those found in this study are reported for conventional vegetables such as parsley (88%) [24], and higher values have been found in P. oleracea (97.3%) from Sudan [25].
Table 2: Nutritional and mineral components of the aerial parts of each species: mean ± standard error and probability (p-value) for the significance differences between growth conditions.

Ash is the inorganic part of the plant that is closely related to the mineral content. The ashes presented a variation between 0.44- 0.67% in P. ruderale, values lower than those reported by Carillo [26] for the same Mexican species (2.04%). The variation between 2.62-3.39% in P. oleracea is much higher than that reported by USDA [27] for the same American species (1.36%). Crude protein showed a significant difference between the growth conditions in P. ruderale (p=0.0002) and no difference in P. oleracea. These results that both species are not a protein source of alternative nutrition, unlike other wild edible species such as C. album (26.42%) or the genus Amaranthus (21.38%) [28]. In general, the amount of protein in the species studied is close to that of common lettuce (1.13%) or escarole (1.6%) [24]. The fat content was significantly influenced by the growth conditions in P. ruderale and P. oleracea, where the value found tripled that of the organic growth conditions. The fat content is very similar to that reported for the wild species P. oleracea Portuguese (0.39%) by Pinela et al. [29]. In general, all the values were below 1.0%, confirming that the plants studied are a low-fat source, which could be considered to design healthy diets [30, 31]. The crude fiber varied between 2.39-5.50%, without presenting differences between cultivation systems for any of the species. The fiber content in this study can be considered high since it exceeds the range reported by Kim et al. [32] for iceberg, romaine, green and purple leaf lettuce from 0.9 to 2.1 g 100 g-1 in fresh weight or reported by Tardío et al. [33] for P. oleracea Mediterranean (1.20%). These results indicate that the aerial parts of both species are a good source of crude fiber whose daily intake is beneficial for health. Carbohydrate concentration varied in the range 4.72-8.41% (P. oleracea) and 6.80-17.55% (P. ruderale). The carbohydrate content in P. oleracea exceeded that reported by Tardío et al. [33] for the same species with 1.98%. The estimated caloric values of the plants studied in two growth conditions fluctuated between 28.0-78.65%, where P. ruderale had the highest caloric value in cultivated conditions (Table 1). However, both species are oriented as low-calorie foods and 100g of their consumption contributes only about 4% considering the referenced energy value (2000 kcal/ day for adults) [34]. In this way, the studied species are appropriate food for low-calorie diets.
Minerals
Human nutritional requirements require at least 23 elements that are differentiated into macrominerals (they require higher daily amounts) and microminerals (their required daily amounts are very small) [35]. The mineral composition of the two undervalued species is presented in Table 1. The calcium (Ca) range is between 110.59 mg·100 g-1 (P. oleracea) and 687.49 mg·100 g-1 (P. ruderale), both under cultivated conditions. Species P. ruderale stood out as a source of calcium since 100 g fresh edible portion represents a contribution of more than 55% of daily requirements for adults of this element when estimating the reference dietary intake of Ca in 1200 mg per day [36]. Vegetables rich in calcium are kale, broccoli, and watercress, which provide between 100 and 150 mg per 100 g [37], although the impact that food has on total calcium intake depends on food consumption patterns of the population. Magnesium (Mg) was between 91.68 mg 100 g-1 (P. oleracea) and 185.54 mg 100 g-1 (P. ruderale), both cultivated, and between 131.15 mg 100 g-1 and 165.33 mg 100 g-1, respectively, in their wild counterparts. The amount of magnesium depends more on the growth conditions in P. oleracea than in P. ruderale with a significant difference (p=0.0000). In general, magnesium is a critical element in many cellular functions, being a cofactor in more than 300 enzymes in the body and has functions that affect nerve conduction [38]. Studies indicate that Mg intake below 237 mg/day is associated with poor bone health [39]. The mean value of potassium (K) ranged between 515.28 and 776.67 mg·100 g-1 in the wild species. The functions of this element in plants are related to osmotic regulation and electroneutrality of cells (Renna et al., 2015). The superiority of K under wild conditions is possibly related to its availability in more rustic areas. The phosphorus concentration ranged from 33.67 to 84.57 mg·100 g-1 with significant differences between the culture systems in both species, surpassing the cultivated ones vs. the wild ones. In the case of wild conditions its low content may be related to deficient levels of this element in the soil, and, in the case of cultivation conditions, it may be influenced by agricultural practices and the contributions made in the form of organic matter.
In relation to sodium, the significant difference (p=0.0000) was only observed in P. oleracea, doubling its content in wild vs. cultivated conditions. In general, for humans, sodium plays a vital role in regulating fluid balance and blood pressure (Renna et al., 2015). The WHO [40] recommends its intake below 2 g per day to prevent cardiovascular diseases attributed to high blood pressure. The result of the present study showed that all the species analyzed can be considered as contributors of low daily sodium intake. Among the microminerals, the one with the highest concentration was iron (Fe) for both species under wild conditions, followed by zinc (Zn). In the human body, Fe acts as an oxygen carrier and its deficiency is the most common nutritional disorder. Considering that the requirement of its daily intake is 8 and 10 mg [36], the species in this study could provide around 18% of the requirement. Zn is also an essential component for humans since it participates in the synthesis and degradation of carbohydrates, lipids, proteins, and nucleic acids. Its central role in the immune system is supported by several studies [36]. If the recommended daily amount of Zn (10 mg/day, Odhav et al., [41]) is considered, the study plants can provide between 4.5-10% of what is recommended. The microelement with the lowest concentration was copper (Cu) in wild conditions for both plants. On the contrary, a high content of Cu was found in both species collected under cultivated conditions, exceeding by more than 2.5 times the content of this element in their wild counterparts. This element is vital for certain enzymes and proteins (Renna et al., 2015), as well as it is required in the synthesis of collagen and mobilization of iron [42]. The edible parts of the plants studied are shown to be a moderate source of this trace element, which represents 15.6% (wild species) and 41.1% (cultivated species) of 100 g of their fresh consumption, considering that the recommended daily consumption is 0.9 mg/ day [29].
Bioactive components
Figure 2: Bioactive compounds: mean values: AOT=total antioxidants (μmol TE·100 g-1pf); PFT= total polyphenols (mg GAE·100 g-1); total Chl=total chlorophyll ( as chlorophyll a + b) (mg·100 g-1).
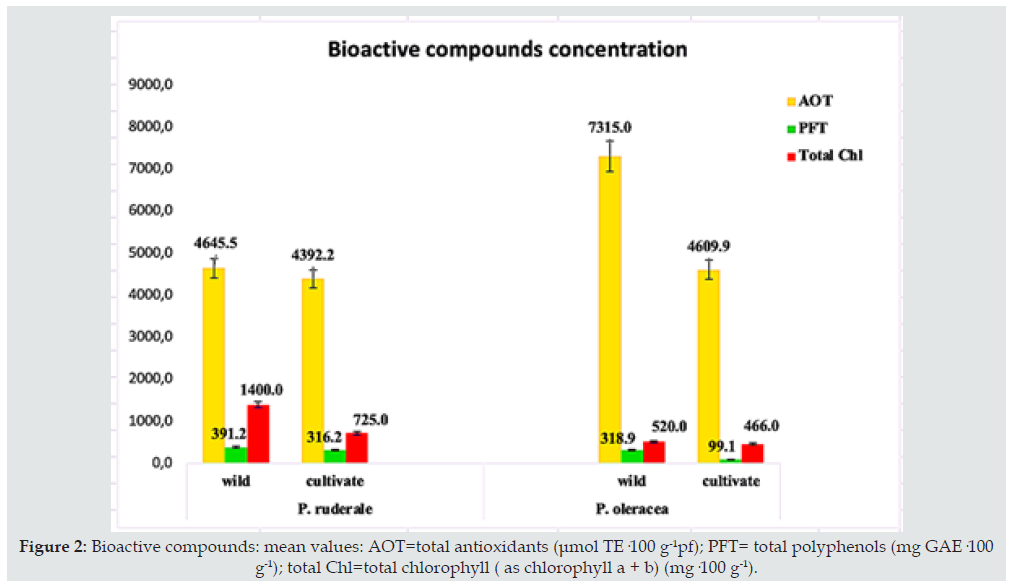
The bioactive components of this study that are antioxidants, phenolic compounds, and chlorophylls, in plants are involved in growth, reproduction and defense against pathogens and known as secondary metabolites. For humans, these compounds exert beneficial effects due to their biological activity that promotes health [43]. Therefore, these bioactive molecules affect the nutritional quality of foods since they provide functionality to the food. The content of total antioxidants (TAO), total phenolic compounds (PFT) and total chlorophyll (Chl) of the undervalued wild plants are presented in Figure 2. The amount of total antioxidants in the fresh samples of both plants ranged from 4645.53 to 7135.0 (μmol TE·100 g-1) for the wild species compared to their cultivated counterparts (4392.2-4609.9 μmol TE·100 g-1) with significant differences between both growth systems. On the contrary, the content of total phenolic compounds did not present significant differences between the growth systems for P. ruderale species. However, in P. oleracea a significant difference was observed between cultivated (99.1 mg GAE·100 g-1) and wild (318.9 mg GAE·100 g-1). The amount of chlorophylls in the fresh samples was higher in the wild species of both plants, although there were only significant differences in P. oleracea. In general, both species exhibit high levels of bioactive compounds, especially total antioxidants, which are higher in wild species. In some plants, antioxidant activity is correlated with phenolic compounds. [17]. In our study the positive correlation was only observed in the case of the species P. oleracea. The total content of phenolic compounds is much higher than that reported by Kim et al. [32] for the green leaves of the “Simpson Elite” variety lettuce (65-67 mg 100 g-1) and the red leaves of the “Red Cross” variety (250-260 mg 100 g-1). In contrast, the wild leaves of Tunisian P. oleracea studied by Dabbou et al. [44] contain a higher amount of phenols than that found in this study for the same species. The leaves of Mediterranean P. oleracea reported by Tardío et al. [33] showed results in phenolic compounds (270 mg 100 g-1) lower than that of this study, for the same species in wild conditions and higher than in its cultivated counterpart.
Chlorophylls were analyzed in this study as part of the bioactive compounds and are the pigments responsible for the green color of leaves. In all the samples analyzed, the concentration of chlorophyll a was higher than that of chlorophyll b, and the sum of both corresponds to the amount of total chlorophyll. Its quantity prevailed in the wild species of both plants and its range was between 520 (P. oleracea) and 1400 mg·100 g-1 pf (P. ruderale). The study on total chlorophyll in the leaves of Tunisian P. oleracea (742 mg in 100 g) carried out by Dabbou et al. [44] reported an amount of total chlorophyll greater than that found in this study. The higher amount of bioactive compounds under wild conditions is probably due to the fact that these secondary metabolites are responsible for the plant defense system. Being subjected to conditions of greater stress, the total content of phenols in the leaves can be increased. Also, the stage of harvest has a significant effect on the total phenolic content, as well as the age of the plant [45]. Chlorophyll content is one of the most significant parameters to evaluate the physiological state of plants that can be used as an index of the nutritional and stress state of the plant [46]. As bioactive compounds, chlorophylls have antioxidant and antimutagenic activity, as well as cancer prevention [47]. Its use is better when the plants are consumed fresh, since these natural pigments are thermolabile and are destroyed by heat.
Other chemicals
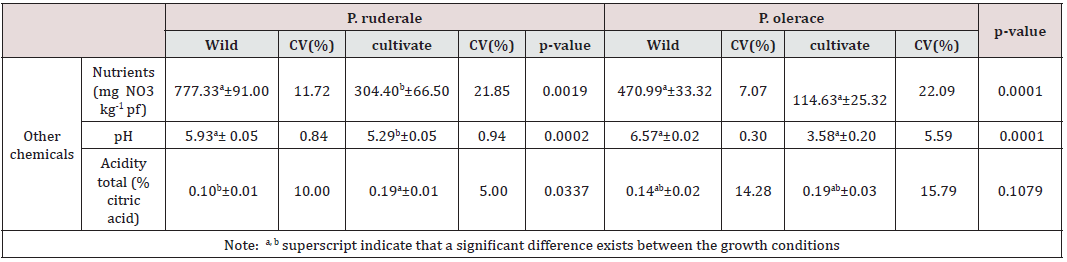
Determined in this study are nitrates, pH and total acidity and their content is shown in Table 3. Nitrates and pH showed significant differences (p<0.05) between growth systems, both in the species P. ruderale and in the species P. oleracea. On the contrary, total acidity did not present significant differences between plants growth systems. As there is a natural nitrogen cycle in plants, it can be modified by applied agronomic practices and climatic conditions, as well as by storage conditions during post-harvest. The toxicity of nitrates increases when reduced to nitrites. However, the WHO/ FAO indicates that the acceptable daily intake of nitrates expressed as ions is 3.7 mg NO3-·kg-1 of body weight. Hence the importance of determining the amount of nitrates in edible plants. All these chemicals interfere with the organoleptic qualities of the species and are considered the internal quality parameters of vegetables in the agri-food industry [48-53].
Table 3: Other chemicals of the aerial parts of each species: mean values ± standard error, coefficient of variability (CV) and p- value for the significance of differences between growth conditions.
Conclusions and Recommendations
The influence of growth conditions on the nutritional composition and bioactive components of two undervalued wild plants P. ruderale and P. oleracea was evaluated. The proximal analysis of the aerial parts revealed a high content of crude fiber and carbohydrates. In the mineral content, the concentration of calcium and potassium stood out with its higher level in the cultivated conditions for P. ruderale and in the wild conditions for P. oleracea. The trace element iron was relevant in both plants and in both growth conditions. Likewise, significant levels of bioactive compounds were found in the two species studied that can provide functionality when consuming these vegetables. These results suggest that undervalued wild plants can be part of diets as an alternative to commonly used vegetables, reinforcing regional practices of consumption of wild species. Simultaneously, they can be postulated as new crop sources.
References
- Organización de Naciones Unidas (ONU, 2019) Informe de los Objetivos de Desarrollo Sostenible.
- González Turmo I, Medina, FX (2012) Retos y responsabilidades tras la declaración de la dieta mediterránea como patrimonio cultural inmaterial de la humanidad (UNESCO).
- Mosconi L, Murray J, Tsui WH, Li Y, Davies M, et al. (2014) Mediterranean diet and magnetic resonance imaging-assessed brain atrophy in cognitively normal individuals at risk for Alzheimer's disease. J prev Alzheimer's Dis 1(1): 23.
- Safouris A, Tsivgoulis G, Sergentanis T, Psaltopoulou T (2015) Mediterranean diet and risk of dementia. Curr Alzheimer Res 12(8): 736-744.
- Gardener H, Caunca MR (2018) Mediterranean Diet in Preventing Neurodegenerative Diseases. Curr Nutr Rep 7: 10-20.
- Gubert C, Kong G, Renoir T, Hannan AJ (2020) Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol dis 134: 104621.
- Torrija Isasa M, Matallana González M (2016) A Historical Perspective of Wild Plant Foods in Mediterranean Area. In: Mediterranean wild edible plants: ethnobotany and food composition tables. Sánchez-Mata, M Tardío J (Eds.), Springer, New York, HY, USA.
- Tardío J, Pardo de Santayana M, Morales R (2006) Ethnobotanical review of wild edible plants in Spain. Botanical Journal of the Linnean Society 152(1): 27-71.
- van Breda SG, de Kok TM (2018) Smart combinations of bioactive compounds in fruits and vegetables may guide new strategies for personalized prevention of chronic diseases. Molecular Nutrition & Food Research 62(1): 1700597.
- Demasi S, Caser M, Donno D, Enri SR, Lonati M, et al. (2021) Exploring wild edible flowers as a source of bioactive compounds: New perspectives in horticulture. Folia Horticulturae 33(1): 27-48.
- Medina FX (2017) Reflexiones sobre el patrimonio y la alimentación desde las perspectivas cultural y turística. In: Anales de antropología 51(2): 106-113.
- Spina M, Cuccioloni M, Sparapani L, Acciarri S, Eleuteri AM, et al. (2008) Comparative evaluation of flavonoid content in assessing quality of wild and cultivated vegetables for human consumption. Journal of the Science of Food and Agriculture 88(2): 294-304.
- Yang Z, Liu C, Xiang L, Zheng Y (2009) Phenolic alkaloids as a new class of antioxidants in Portulaca oleracea. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 23(7): 1032-1035.0020.
- Franco JA, Cros V, Vicente MJ, Martínez Sánchez JJ (2011) Effects of salinity on the germination, growth, and nitrate contents of purslane (Portulaca oleracea L.) cultivated under different climatic conditions. The Journal of Horticultural Science and Biotechnology 86(1): 1-6.
- Iranshahy M, Javadi B, Iranshahi M, Jahanbakhsh SP, Mahyari S, et al. (2017) A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. J ethnopharmacol 205: 158-172.
- Li YH, Lai CY, Su MC, Cheng JC, Chang YS (2019) Antiviral activity of Portulaca oleracea L. against influenza A viruses. J Ethnopharmacol 241: 112013.
- Conde Hernández LA, Guerrero Beltrán JÁ (2014) Total phenolics and antioxidant activity of Piper auritum and Porophyllum ruderale. Food chemistry 142: 455-460.
- Marques ÉA, de Oliveira JA, Coelho AD, Salimena JP, Gavilanes ML (2020) Porophyllum ruderale (Jacq.) Cass. uma revisão dos últimos 39 anos. Research Society and Development 9(7): e944975215.
- Fonsceca MC, Barbosa LC, Nascimento EA, Casali VW (2006) Essential oil from leaves and flowers of Porophyllum ruderale (Jacq.) Cassini (Asteraceae). Journal of Essential Oil Research 18(3): 345-347.
- Santos V, Sussa FV, Gonçalez E, Silva PS, Felicio JD (2016) Comparative study of the essential oil effects on the Aspergillus flavus growth. In: PETERS, MIRANDA (ed.). Essential Oils: Historical Significance, Chemical Composition, and Medicinal Uses and Benefits. Hauppauge, New York, USA: Nova Science Publishers, 2016. cap. 8. p. 139-152.
- AOAC (2005) Official Methods of Analysis of Offial Analytical Chemist, 18th; Association of Official Analytical Chemist (AOAC International): Washington, DC, USA.
- Brand Williams W, Cuvelier ME, Berset C (1995) Use of free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28: 25-30.
- Kibar H, Sönmez F, Temel S (2021) Effect of storage conditions on nutritional quality and color characteristics of quinoa varieties. Journal of Stored Products Research 91: 101761.
- BEDCA (2022) Base de datos Española de Composición de Alimentos.
- Obied WA, Mohamoud EN, Mohamed OSA (2003) Portulaca oleracea (purslane): nutritive composition and clinic-pathological effects on Nubian goats. Small Ruminant Research 48(1): 31-36.
- Carillo SR (2014) Estudio del papaloquelite (Porophyllum ruderale) como alimento funcional.
- USDA (2019). https://fdc.nal.usda.gov/fdc-app.html#/food-details/169274/nutrients
- Ozbucak TB, Akçin ÖE, Yalçın S (2007) Nutrition contents of some wild edible plants in Central Black Sea Region of Turkey. International Journal of Natural and Engineering Sciences 1: 11-13.
- Pinela J, Carvalho AM, Ferreira IC (2017) Wild edible plants: Nutritional and toxicological characteristics, retrieval strategies and importance for today's society. Food and Chemical Toxicology 110: 165-188.
- Kaur N, Chugh V, Gupta AK (2014) Essential fatty acids as functional components of foods- a review. J Food Sci Technol 51(10): 2289-2303.
- Marrelli M, Statti G, Conforti F (2020) A review of biologically active natural products from Mediterranean wild edible plants: benefits in the treatment of obesity and its related disorders. Molecules 25(3): 649.
- Kim MJ, Moon Y, Tou JC, Mou B, Waterland NL (2016) Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). Journal of Food Composition and Analysis 49: 19-34.
- Tardío J, Sánchez Mata MDC, Morales R, Molina M, García Herrera P, et al. (2016) Ethnobotanical and food composition monographs of selected Mediterranean wild edible plants. In Mediterranean wild edible plants pp. 273-470.
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council. of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission regulation (EC) No 608/2004. Off J Eur Union 54: 18-63.
- Quintaes KD, Díez García RW (2015) The importance of minerals in the human diet. Handbook of mineral elements in food. John Wiley & Sons, Ltd, Chichester UK pp. 1-21.
- WHO: World Health Organization (2004) Vitamin and mineral requirements in human nutrition, 2nd ed.; Geneva, Switzerland.
- Cormick G, Belizán JM (2019) Calcium Intake and Health. Nutrients 11(7): 1606.
- Nielsen F (2018) Magnesium deficiency and increased inflammation: current perspectives. J inflamm res 11: 25-34.
- Nielsen F, Lukaski H, Johnson L, Roughead Z (2011) Reported zinc, but not copper, intakes influence whole-body bone density, mineral content and T score responses to zinc and copper supplementation in healthy postmenopausal women. Br J Nutr 106(12): 1872-1879.
- WHO World Health Organization (2006) Reducing salt intake in populations–Report of a WHO forum and technical meeting.
- Odhav B, Beekrum S, Akula US, Baijnath H (2007) Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. Journal of Food Composition and Analysis 20(5): 430-435.
- Kibar B, Temel S (2015) Evaluation of mineral composition of some wild edible plants growing in the eastern Anatolia region grasslands of turkey and consumed as vegetable. J of Food Processing and Preservation, 40(1): 56-66.
- Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2(5): 270-278.
- Dabbou S, Lahbib K, Pandino G, Dabbou S, Lombardo S (2020) Evaluation of pigments, phenolic and volatile compounds, and antioxidant activity of a spontaneous population of Portulaca oleracea L. grown in Tunisia. Agriculture 10(8): 353.
- Petropoulos SA, Fernandes Â, Tzortzakis N, Sokovic M, Ciric A, et al. (2019) Bioactive compounds content and antimicrobial activities of wild edible Asteraceae species of the Mediterranean flora under commercial cultivation conditions. Food Research International 119: 859-868.
- Silla F, González Gil A, González Molina ME, Mediavilla S, Escudero A (2010) Estimation of chlorophyll in Quercus leaves using a portable chlorophyll meter: effects of species and leaf age. Annals of Forest Science 67(1): 108.
- Kang YR, Park J, Jung SK, Chang YH (2018) Synthesis, characterization, and functional properties of chlorophylls, pheophytins, and Zn-pheophytins. Food chemistry 245: 943-950.
- Cajamar GC (2014) Parámetros de Calidad Interna de Hortalizas y Frutas en la Industria Agroalimentaria.
- Nemzer B, Al Taher F, Abshiru N (2020) Phytochemical composition and nutritional value of different plant parts in two cultivated and wild purslane (Portulaca oleracea L.) genotypes. Food Chem 320: 126621.
- Pawłowska KA, Baracz T, Skowrońska W, Piwowarski JP, Majdan M, et al. (2022) The contribution of phenolics to the anti-inflammatory potential of the extract from Bolivian coriander (Porophyllum ruderale ruderale). Food Chem 371: 131116.
- Takahashi HT, Novello CR, Ueda Nakamura T, Filho BPD, Palazzo de Mello JC, et al. (2011) Thiophene derivatives with antileishmanial activity isolated from aerial parts of Porophyllum ruderale (Jacq.) Cass. Molecules 16(5): 3469-3478.
- Vázquez Atanacio MJ, Bautista Ávila M, Velázquez González C, Castañeda Ovando A, González Cortazar M, et al. (2021). Porophyllum genus compounds and pharmacological activities: A review. Scientia Pharmaceutica 89(1): 7.
- Zhou YX, Xin HL, Rahman K, Wang SJ, Peng C, et al. (2015) Portulaca oleracea L.: a review of phytochemistry and pharmacological effects. BioMed Res Int article ID 925631.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...