Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Article(ISSN: 2638-6070) 
Effect of Different Processing Methods on Nutritional Composition of Lemna Paucicostata as Inclusion in Fish Feed
Volume 4 - Issue 3Abdullahi AI1*, Auta J2, Abdullahi SA2, Bolorunduro P3 and Onimisi HU3
- 1Department of Fisheries and Aquaculture, University of Maiduguri, Borno State, Nigeria
- 2Department of Biology, Faculty of Life Sciences, Ahmadu Bello University, Zaria-Nigeria
- 3National Agricultural Extension and Research Liaison Services (NAERLS), Ahmadu Bello University, Zaria-Nigeria
Received: Aprial 24, 2022 Published: May 09, 2022
*Corresponding author:Abdullahi AI, Department of Fisheries and Aquaculture, University of Maiduguri, Borno State, Nigeria
DOI: 10.32474/SJFN.2022.04.000186
Abstract
The effects of different processing methods on the nutritional composition and anti-nutritional factors of Lemna paucicostata were evaluated. Lemna paucicostat was subjected to four processing methods (blanching, fermentation, sun-drying and soaked in potash) to determine the effect of these treatments on proximate composition and anti-nutritional factors. Five samples of L. paucicostata were analyzed, raw, blanched, fermented, sun-dried and soaked in potash. The tests were carried out in triplicate for each treatment. Blanched L. paucicostata led to a significant (P≤0.05) increase in crude protein (37.13%) while the sample soaked in potash had the least crude protein (34.12%) when compared with other treatments. Highest percentage of ash content (22.12%) was obtained in the potash treated L. paucicostata while the raw and sun-dried L. paucicostata had the highest percentage nitrogen free extract with a value 22.60 and 22.15, respectively. Analysis of anti-nutritional factors indicated that all the components determined were greatly reduced in all the processed L. paucicostata when compared with the raw L. paucicostata. Therefore, all the processing methods used in this study reduced anti-nutritional factors of L. paucicostata while blanching method is the best in terms of improving the nutrient composition of L. paucicostata.
Keywords:Nutritional Composition; Anti-Nutritional Factors; Lemna paucicostata; Blanching; Fermentation; Sun Drying
Introduction
The use of conventional plant protein sources in fish diet may not be sustainable because they are good sources of protein for humans. There is therefore an urgent need to identify other protein rich plant resources that could substitute conventional protein feed ingredients. Although, limitations do exist to the use of plant sources in fish diets because of certain problems which includes low amount of protein, presence of anti-nutrients which will no doubt affect palatability and digestibility of feed by fish [1]. However, over the years, measures like drying soaking, fermentation, toasting etc. have been taken to either reduce to a bare minimum or to remove the anti-nutrients in these unconventional feed stuffs [2]. Lemna paucicostata is an aquatic plant which is commonly known as “Duckweed”. It is a monocotyledonous, floating plant, and is the world’s smallest and simplest flowering plant. L. paucicostata consists of little more than two, poorly differentiated fronds, a combination of leaf and stem. The tissue is composed principally of chlorenchymatous cells, separated by large intercellular spaces that provide buoyancy. The upper epidermis is cutinized and sheds water. Generally, duckweed is suitable for animal consumption and is rich in invaluable nutrients [3]. There are about 40 species of duckweed plant species worldwide and the major ones are of the four genera: Lemna, Spirodela, Wolfilla and Wolffiella. Generally, duckweed contains 6.8 to 45% crude protein (CP), 1.8 to 9.2% crude lipid (CL), 5.7 to 16.2% crude fiber, 12 to 27.6% ash, and the carbohydrate content are in the range of 14.1-43.6% on a dry matter basis [4]. The nutrient composition in each duckweed species varies depend- ing on the condition of the water environment. Fresh duckweed has been successfully used as feedstuffs for common carp, Thai sharputi, raj puti, silver carp and tilapia [1]. Despite the potentials of Lemna paucicostata as an excellent plant protein source in fish diets, there is no research work on the effects of different processing methods on its composition. Therefore, this study aims to evaluate the effect of different processing methods on nutritional composition of Lemna paucicostata as plant protein in fish diet.
Materials and Methods
Collection of Duckweed (Lemna paucicostata)
Duckweed (Lemna paucicostata) was harvested from a still water near ABU Press Limited, Zaria with the aid of a hand net and was transported in a nylon bags.
Preparation of duckweed meal
The freshly collected Lemna paucicostata was washed properly to get rid of any foreign materials before treatment using blanching, fermentation, sun drying and treated with potash.
Blanching of Lemna paucicostata
Duckweed (Lemna paucicostata) was boiled in water for 5 minutes at 100 °C. This treatment is referred to as blanching following the methods described by Sogbesan and Ugwumba [5].
Fermentation
The freshly collected was put inside an air-tied container for 72 hours before it was taken for analysis.
Sun-drying of Lemna paucicostata
This treatment was formed by sun drying of fresh duckweed (Lemna paucicostata) under hygienic conditions for three (3) days.
Treatment with potash solution
Five (5g) of maize-cob ash (Potassium hydroxide) was dissolved into a solution at 5 g/l and used to soak duckweed for 24 hours following the method of Vadivel and Pugalenthi [6].
Proximate Analysis
The proximate composition of raw and processed Lemna paucicostata were determined using the standard methods of the Association of Official Analytical Chemists [7].
Moisture Content
A clean crucible was dried to a constant weight in an air oven at 110 oC, cooled in a desiccator and weighed (W1). 2g of finely pulverized sample was weighed in the crucible and then re-weighed (W2). The crucible and its content were dried in an oven to a constant weight (W3). The percentage moisture was calculated thus.
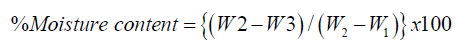
Ash content
The porcelain crucible was dried in an oven at 100 °C for 10 minutes, cooled in a desiccator and weighed (W1). 2g of finely pulverized sample was weighed (W2) into the previously weighed clean crucible which was ignited in the muffle furnace at 550oC for 1 hour and cooled in a desiccator. The crucible and its content were transferred into the muffle furnace and the temperature was gradually increased until it reached 550 °C, the sample was then left in the furnace for 8 hours to ensure proper ashing. The crucible containing the ash was allowed to cool to 200 oC, the crucible was removed and cooled in a desiccator until constant weight was obtained (W3).
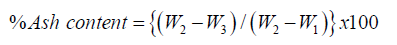
Crude Protein
Two grams of the sample was weighed into 100 cm3 Kjeldahl digestion flask and about lg of catalyst mixture (K2S04 and CuS04) was added to speed up the reaction. 25ml of concentrated sulphuric acid was added into the flask. The content in the Kjeldahl digestion flask was heated slowly at first in Kjeldahl heating unit frotting subsides and then more vigorously with occasional rotation of the flask to ensure even digestion and avoid over heating of the content. The heating continued until a clear solution is obtained. After cooling, the solution was transferred into 100cm3 volumetric flask and diluted to mark with distilled water. 10ml aliquot of the diluted solution or digest was pipette into Markham semi macro nitrogen steel and 10cm3 of 40% sodium hydroxide solution was added. The liberated ammonia was trapped in a 100 cm3 conical flask containing l0 cm3 of 40% boric acid and 2 drops of methyl red indicator. Distillation was allowed to continue until pink colour of the indicator turn green. The content of the conical flask was titrated with 0.1M HC1, with end point indicated by a change from green to pink colour. The volume of the acid used for the distillate as well as the blank was noted.
% Nitrogen = {(0.014 x M x (V1-V0)}/ {weight of test sample} x 100
Where M = actual molarity of acid,
V1 = volume of HC1 required for 10ml sample solution,
V0 = volume of HC1 required for the blank
Atomic weight of nitrogen = 0.014
% Crude = % Nitrogen (N2) × 6.25
Crude Lipid Content
Four grams (4g) of sample was weighed (W1) into a clean, dried 500ml round bottom flask containing few anti-bumping granules and was weighed (W2) and 300ml of petroleum ether (40-60 °C) for the extraction was poured into the flask fitted with soxhlet extraction unit. The round bottom flask and a condenser will be con nected to the soxhlet extractor, and cold water circulation was put on. The heating mantle was switched on and the heating rate adjusted until the solvent was refluxing at a steady rate. Extraction was carried out for 6 hours. The solvent was recovered, and the oil was dried in the oven at 70oC for 1 hour. The round bottom flask and oil were cooled and then weighed (W3).
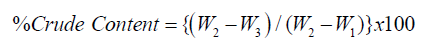
Crude Fibre
Two grams of finely pulverized sample was weighed into an extraction apparatus, fat was extracted with liquid petroleum spirit (40-60 °C) the extracted was removed and dried at 105 °C for 30 minutes. Two grams of the defatted sample was weighed into a dry 600cm round bottom flask. 100cm3 of (0.023M) sulphuric acid was added and the mixture boiled under reflux for 30 minutes. The hot solution was quickly filtered under suction. The insoluble matter was washed several times with hot water until it was acid free. It was quantitatively transferred into the flask and 100cm3 of hot (0.312) sodium hydroxide solution was added and the mixture boiled under reflux for 30 minutes and quickly filtered under suction. The insoluble residue was washed with boiling water until it was base free. It was then dried to constant weight in the oven set at 100 °C, cooled in a dessicator and weighed (C2). The weighed residue was incinerated in a muffle furnace at 550 °C for 2 hours, cooled in a dessicator and reweighed (C3).
The loss in weight on ashing (incineration) = C2 - C3
Weight of original sample = W
% Crude Fibre = {C2-C3)/W} x 100
Nitrogen Free Extract
The total carbohydrate content was determined by difference method. The sum of the percentage moisture, % ash, %crude lipid, % crude protein and % crude fibre was subtracted from 100.
NFE = 100 - (ash+ crude lipid + crude protein + crude fibre)
Determination of Anti-nutritional Factors
Triplicate samples of the raw and treated Lemna paucicostata was analysed according to methods described by AOAC (2006).
Alkaloid
The alkaloid content was determined gravimetrically where 5 g of sample was weighed and dispersed in 10 % acetic acid solution in ethanol to form a ratio of 1:10. The mixture was allowed to stand for 4 hours at 28. It was later filtered via Whatman No. 42 grade paper, the filtrate was concentrated to one quarter its original volume by evaporation and treated with drop wise addition of concentrated aqueous NH2OH until the alkaloid was precipitated. The alkaloid precipitate was received in a weighed filter paper, washed with 1% ammonia solution and dried in the oven at 80. Alkaloid content was calculated and expressed as a percentage of the weight of sample analysed:
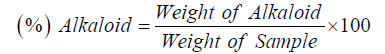
Oxalate
Two grams (2g) of aliquot of the ground sample was weighed to a 250ml flask, 190ml distilled water and 10ml of 6m hydrochloric acid was added. The mixture was digested for 1 hour on boiling water bath, then cooled, transferred in to a 250ml volumetric flask, diluted to volume and filtered. Four drops of methyl indicator were added followed by concentrated ammonia until the solution turn to faint yellow. It was then heated to 100 oC and allowed to cool and filtered. The filtrate was boiled and 10ml of 5% calcium chloride was also added with constant stirring and was allowed to stand overnight. The mixture was filtered through whatman No. 40 filtered paper. The precipitate was rinsed several times with distilled water, transferred to a beaker and 5ml of 25% sulphoric acid was added to dissolve the precipitate. The resultant solution was maintained at 80 oC then cooled and titrated against 0.5% potassium permanganate until the pink colour persisted for approximately one minute. Blank test was also run for the test sample. From the amount potassium permanganate, the oxalate was calculated. Thus,
1ml of potassium permanganate = 2.24mg oxalate.
Phytate
A known weight of each ground sample was soaked into 100ml of 2% HCl in a conical flask, 50cm3 of 0.3% potassium thiocynate solution was added. The mixture was titrated in a standard solution of FeCl3 until a brownish-yellow colour persisted for 5 min. The concentration of the FeCl3 will be 1.04% w/v
Mole ratio of Fe to Phytate = 1:1
Conc. of phytatephosporus
Saponin
A gravimetric method employing the use of soxhlet extractor, and two different organic solvents was used. The first solvent extracts lipids and interfering pigments while the second solvent extracts the saponin proper. Ten grams (10g) of the dried ground sample was weighed and fitted unto the soxhlet apparatus (bearing the sample containing thimble) and methanol poured into the flask. The methanol was enough to cause a reflex. The saponin was then exhaustively extracted for 3 hours. The flask was re-weighed. The difference in weight represents the weight of saponin extracted.
Tannin
Two grams (2g) of ground sample was defatted for 2 hours using Soxhlet extraction apparatus. The residue was placed in an oven for 24 hours, retrieved and boiled at 100˚C with 300ml of distilled water, diluted to 500ml in a standard volumetric and filtered through non-absorbent cotton wool. A volume of 25ml of the infusion was measured into 2litre porcelain dish and titrated with 0.1N oxalic acid until blue solution changed to green, then few drops of 0.1 potassium permanganates was added. The difference between the two titration was multiplied by 0.006235 to obtain the amount of tannin in sample, since 0.1N oxalic acid = 0.006235g tannin.
Data Analysis
Data obtained were subjected t-test and one-way Analysis of Variance (ANOVA) to test for significant differences between the various treatment means. Least significant difference (LSD) test was used to rank and separate means where ANOVA shows significant differences. SPSS Version 16 was adopted (statistical package) to show mean deviation and standard errors, at 0.05 significant level (P≤0.05).
Results
The mean proximate composition of raw and differently processed Lemna paucicostata is presented in Table 1. Highest percentage of crude protein content of 37.13±0.51 was obtained in blanched L. paucicostata and the least value 34.12±0.81 was recorded in the sample treated with potash solution. There was no significant different (P>0.05) in the lipid content of the raw and blanched L. paucicostata but they differ significantly (P≤0.05) among the other treatments. Highest crude fibre content with a values 4.72±0.67, 4.23±0.62 and 4.63±0.32 were obtained in the raw, fermented and potash treated samples, respectively. The blanched L. paucicostata obtained the least value 3.34±0.88 of crude fibre among the treatments. The percentage nitrogen free extract or carbohydrate were recorded as 22.60±0.70, 20.04±0.63, 20.04±0.63, 21.05±0.76 and 22.15±0.61 for raw, blanched, fermented, potash treated and sundried samples, respectively. Analysis of anti-nutritional factors as presented in Table 2 indicates that all the components determined in the all differently processed L. paucicostata were greatly reduced when compared with raw sample. Sun-dried L. paucicostata obtained the least concentration of alkaloid of 0.40±0.06 than all the other treatments. There was not significant different (P>0.05) in the concentrations of oxalate and phytate in the raw and all the processed L. paucicostata. Fermented L. paucicostata had the least concentration of saponin with the value of 0.80±0.06 while the potash treated sample obtained the least concentration of tannin with a value of 0.90±0.06.
Key: RLP= Raw Lemna paucicostata, BLP= Blanched L. paucicostata, FLP= Fermented L. paucicostata, PLP= Potash treted L. paucicostata, SLP= Sun-dried L. paucicostata, CP= Crude Protein, CF= Crude Fiber, NFE= Nitrogen Free Extract
Table 2: Different Processing Methods on Anti-Nutritional Compositions (g/100g) of Raw and Processed Lemna paucicostata.
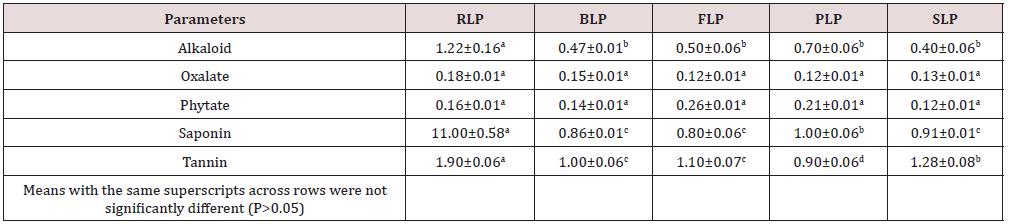
Key: RLP= Raw Lemna paucicostata, BLP= Blanched L. paucicostata, FLP= Fermented L. paucicostata, PLP= Potash treted L. paucicostata, SLP= Sun-dried L. paucicostata
Discussion
The proximate composition of all differently processed L. paucicostata indicates a significant increase in the crude protein composition from 36.25% in the fermented L. paucicostata to 37.13% in the blanched L. paucicostata except the potash treated L. paucicostata which was reduced to 34.12%. The crude protein composition obtained in this study was within the range of 6.8 to 45% reported by Christine et al. [4] for general duckweed plants. The highest percentage of crude protein 37.13% obtained in this study was higher than 27.4%, 29.1% and 29.28% reported by Hlophe and Moyo [8], Heuze and Tran [9] and Sogbesan et al. [10], respectively while it was lower than 38.0%37.7% and 40.2 reported by Tavares et al. [11], Du et al. [12] and Khanum et al. [13] respectively. The percentage ash content of the raw and differently processed L. paucicostata obtained was higher than 14.6%, 12.3%, 3.8%, 14.0%,15.34% and 15.9% reported by Tavares et al. [11], Hlophe and Moyo [8], Du et al. [12], Khanum et al. [13], Sogbesan et al. [10] and Heuze and Tran [9], respectively. The percentage lipid content, crude fiber and nitrogen free extract obtained were all within range reported by Christine et al. [4]. The differences observed in this study may be as a result of differences in geographical location, harvesting time and the variety the L. paucicostata. The results of the anti-nutritional factors of the raw and the differently processed L. paucicostata showed significant reduction of these factors following the drying of the processing methods. This significant reduction of the anti-nutritional factors could be as a result of efficacy of all the processing methods in removing anti-nutrients in L. paucicostata.
Conclusion
All the processing methods observed in this study improved the nutritional composition and reduced the anti-nutrients of L. paucicostata to the acceptable limit. This indicates that L. paucicostata could serves as an excellent source of plant protein in fish diet. Therefore, fish farmers are advice to incorporate L. paucicostata as plant protein source in their feed formulation in order to reduce the cost of feed and make their venture profitable.
References
- Dorothy MS, Sudhanshu R, Vipin N, Khushvir S, Yogananda T, et al. (2018) Use of Potential Plant Leaves as Ingredient in Fish Feed - A Review. International Journal of Current Microbiological Application Sciences 7 (7): 112-125.
- Olasunkanmi JB, Julius OT, Babalola TO, Jimoh JO, Ariyomo TO (2021) Alternative feed resources in Aquaculture: The role of underutilized plants–A Review. IOP Conference Series: Earth and Environmental Science 655(1): 012008.
- Mwale M, Gwaze FR (2013) Characteristics of duckweed (Lemna paucicostata) and its potential as feed source for chickens reared for meat production: A review. Scientific Research and Essays 8(18): 689-697.
- Christine A, Annita SKY, Ching FF (2018) Supplementation of duckweed diet and citric acid on growth performance, feed utilization, digestibility and phosphorus utilization of TGGG hybrid grouper (Epinephelus fuscoguttatus x Epinephelus lanceolatus) juvenile. Journal of Science and Technology 40(5): 1009-1016.
- Sogbesan OA, Ugwumba AAA (2008) Nutritional values of some non-conventional animal protein feedstuffs used as fishmeal supplement in aquaculture practices in Nigeria. Turkey Journal of Fish Aquatic Sciences 8: 159-164.
- Vadivel V, Pugalanthi M (2008) Removal of Anti-nutritional/toxic substances and improvement in protein digestibility of velvet bean seeds during various processing methods. Journal of Food Science Technology 45(3): 242-246.
- AOAC (2006) Official method of analysis K Helrich (ed). 17th edition, vol 1, AOAC, Arlington, VA, pp. 684.
- Hlophe SN, Moyo NAG (2011) The effect of different plant diets on the growth performance, gastric evacuation rate and carcass composition of Tilapia rendalli. Asian Journal of Animal Vetenarian Advances 6(10): 1001-1009.
- Heuze V, Tran G (2015) Duckweed: Feedipedia, a programme by inra, cirad, afz and fao.
- Sogbesan OA, Onoja CF, Adedeji HA, Idowu TA (2015) Utilization of treated duckweed meal (Lemna pausicostata) as plant protein supplement in African mud catfish (Clarias gariepinus) juvenile diets. Fish and Aquaculture Journal 6: 141.
- Tavares FA, Lapolli FR, Roubach R, Jungles MK, Fracalossi DM, et al. (2010) Use of domestic effluent through duckweeds and red tilapia farming in integrated system. Pan-American Journal of Aquatic Sciences 5 (1): 1-10.
- Du TH, Linh NQ, Everts H, Beynen AC (2012) Ideal and total tract digestibility in growing pigs fed cassava root meal and rice bran with inclusion of cassava leaves, sweet potato vine, duckweed and stylosanthes foliage. Livestock Resources and Rural Development pp. 21-12.
- Khanum J, Chwalibog A, Huque KS (2012) Study on digestibility and feeding systems of duckweed (Lemna paucicostata) in growing ducks. Livestock Research and Rural Development pp. 17-50.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...