Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Article(ISSN: 2638-6070) 
Effect of different freezing methods on the quality changes of precooked freshwater crayfish(Procambarus clarkii) during frozen storage
Volume 4 - Issue 2Yanfei Sang1, Jingjing Zheng1, Li Yang2, Shaotong Jiang1,3, Lin Lin1,3,4,*, Jianfeng Lu1,3,4*
- 1School of Food and Biological Engineering, Hefei University of Technology, Hefei, China
- 2Anhui Fuhuang Sungem Food Group Co. Ltd., Chaohu, China
- 3Key Laboratory for Agricultural Products Processing of Anhui Province, Hefei, China
- 4Engineering Research Centre of Bio-process, Ministry of Education of China, Hefei, China
Received: March 24, 2022 Published: March 31, 2022
*Corresponding author:Lin Lin and Jianfeng Lu, School of Food and Biological Engineering, Hefei University of Technology, No. 420 Feicui Road, Hefei 230601, Anhui, China
DOI: 10.32474/SJFN.2022.04.000185
Abstract
The effects of three freezing methods, including refrigerator, immersion solution, and liquid nitrogen freezing, on quality changes of crayfish during frozen storage were investigated. The pH value, total volatile basic nitrogen (TVB-N), water holding capacity (WHC), lipid oxidation, texture and water distribution of crayfish meat were determined. The results showed that the pH value of crayfish decreased first and then increased during storage. The TVB-N and malonadehyde (MDA) of crayfish frozen by all three methods increased in 12 weeks of frozen storage. The WHC and the texture properties of crayfish got worse during frozen storage. The water relaxation time T20 and T21 increased slowly, while T22 decreased significantly during storage. Moreover, the crayfish frozen with liquid nitrogen had a higher free water proportion than by refrigerator and immersion solution at the end of storage. In general, the freezing method had significant effects on the properties of crayfish during frozen storage. Lower freezing temperatures (-90 °C and -35 °C) can form an intracellular crystal, as well as larger electrostatic repulsion, which helps maintain the protein tertiary structure, preventing protein aggregation.
Keywords:Lin Lin and Jianfeng Lu, School of Food and Biological Engineering, Hefei University of Technology, No. 420 Feicui Road, Hefei 230601, Anhui, China
Introduction
Crayfish (Procambarus clarkii), also called red swamp or freshwater crayfish, is one of the most popular and important commercial freshwater species in China and is highly appreciated by consumers on account of the high nutritive value and great flavor [1]. According to China Fishery Statistical Yearbook [2], the crayfish yield in China was 2,089,604 tons in 2019 which has increased by 27.52% compared to 1,638,662 tons in 2018. In 2019, 509,938-ton crayfish have been processed into products, and it was 124.67% as much as the output in 2018. From April to October, crayfish is at a high yield and low price, therefore the freezing treatment and frozen storage are widely used in crayfish processing to balance the yield and price. Common freezing methods used in food processing include air blast freezing, contact plate freezing, immersion freezing, and liquid nitrogen freezing. In the process of common freezing, the freezing rate and temperature are the main factors that affect the size and quantity of ice crystals, as well as their dis tribution in the cell and intercellular space. Immersion freezing (IF) is a kind of freezing technology with a high freezing rate and low energy consumption resulting in instantaneous and uniform nucleation throughout foods [3]. It can greatly improve the quality of frozen products and storage stability. Liquid nitrogen freezing has an extremely fast freezing rate due to its low heat resistance and has been widely used in shrimp, fish, mushrooms, and fruit freezing [4]. When aquatic products are exposed to temperature changes during frozen storage, biochemical reactions will occur, such as modification of amino acid side chain residues, oxidation of sulfhydryl groups, an increasing concentration of cytosol etc. [5]. These will lead to the oxidation or denaturation of lipids and proteins of the products [6]. Recent research on the freezing of fresh crayfish showed that the temperature difference between freezing and storage and freezing rate both influence the final quality of crayfish products. The recommended shelf-life of crayfish should be 1 month, which should be freezing at -30 °C and stored at -18 °C [7]. Improved protective atmospheres can also delay the quality changes of crayfish tails [8]. In practical applications the crayfish was normally cooked before freezing by the factories to keep a better quality. In the present study, the cooked crayfish were frozen by three methods (refrigerator, immersion solution and liquid nitrogen freezing), and the quality changes of crayfish during frozen storage were observed and compared.
Materials and Methods
Pretreatment of crayfish
The alive crayfish (Procambarus clarkii) (about 18.0±2.0 g each) was purchased from the local aquatic market and transported to the laboratory with ice for 1 h in a foam box. The crayfish was cleaned with tap water and cooked in boiling water for 10 minutes. After cooling, the head and claws of the crayfish was removed, and the tail was kept with the shell. Then the crayfish tails were wiped dry and packed in polyethylene bags. Each unit weighed about 30 g.
Freezing of crayfish
Refrigerator Freezing (RF)
The crayfish samples were frozen directly in the refrigerator, set at -20 ℃, until the meat core temperature reached below -18 ℃, measured with a thermometer.
Immersion Freezing (IF)
The packaged crayfish was immersed in the freezing solution (Rens Agricultural Science and Technology Co., Ltd. Jiangsu, China) channel (-35 ℃) for 20 - 30 min until the central temperature of the crayfish was lower than -18 ℃. Then, the residual liquid on the surface of the package was removed and dried.
Liquid nitrogen freezing (LNF)
The type liquid nitrogen freezing equipment (Cryogenic Science & Technology Co. Ltd., Beijing, China) was precooled for 30 min and reached -90 °C. The packaged crayfish passed through the liquid nitrogen quick freezing tunnel for about 20-30 min until the central temperature of the crayfish was below -18 °C.
Frozen storage of crayfish
The frozen crayfish was stored at -18 °C for 12 weeks, and the changes in physicochemical properties were measured every two weeks. The samples were randomly selected and thawed at 4 °C for 12 h before the determination of physicochemical properties.
Determination of physicochemical properties of crayfish pH
The pH value of crayfish was determined according to the method of Chinese National standard GB 5009.237-2016 (2016). Five grams of crayfish meat were chopped and added to 45 mL of distilled water and then crushed with the homogenizer (T18, IKA Co., Ltd, Germany) for 30 seconds. Allow the solution to rest for 30 minutes. Determine the pH value of its filtrate with a pH meter (PHS-3C, Shanghai Hongyi Instrumentation Co., Ltd., Shanghai, China).
Total Volatile Base Nitrogen (TVB-N)
TVB-N was determined by the method of Chinese national standard GB 2009.228-2016 [9]. Five grams of crayfish meat with 0.6 mol/L of perchloric acid solution (45 mL) were homogenized for 2 min, then filtered and kept the filtrate at 2-6 ℃ for analysis. The filtrate was distilled through the Kjeldahl distillation unit (K9840, Jinan Hanon Instruments Co., Ltd., Jinan, Shandong, China). The distillate was absorbed by 40 mL of boric acid (30 g/L) containing a mixed indicator produced from 0.2 g of methyl red and 0.1 g of methylene blue to 100 mL ethanol. Afterward, the boric acid solution was titrated with a 0.01 mol/L hydrochloric acid (HCl) standard solution. The TVB-N (mg/ 100 g) was calculated as following equation (1):

Where V1 is the volume of hydrochloric acid standard solution consumed by sample (mL), V2 is the volume of hydrochloric acid standard solution consumed by blank control (mL), and m is the mass of sample (g).
Water Holding Capacity (WHC)
WHC was determined by the centrifugal method described by Li, Liu, Su, Cai and Li [10]. Minced crayfish meat (10 g) wrapped with absorbent cotton was put in a 50 mL centrifuge tube and centrifuged for 10 min at 9000 rpm/min and 4 ℃ in a refrigerated centrifuge (CT15RT, Techcomp Shanghai Instrument Ltd., Shanghai, China). After centrifuge, the absorbent cotton wrapped the crayfish meat was peeled off, and the crayfish meat was weighed again. The WHC (%) was calculated as equation (2).

Where m1 is the weight of the sample (g) before centrifugation, and m2 is the weight of the sample (g) after centrifugation.
Thiobarbituric Acid Reactive Substances (TBARS)
TBARS were determined according to the method of Song et al. [11] and the secondary lipid oxidation products malonaldehyde (MDA) were measured. Five grams of crayfish meat were dispersed in 50 mL of 7.5% of trichloroacetic acid (TCA) solution (containing 0.1% of EDTA). The mixture was sealed and heated in a water bath for 10 min at 50 ℃. Then the mixture was cooled and centrifuged at 3600 g for 2 min at room temperature. The supernatant (5 mL) reacted with 5 mL thiobarbituric acid solution (0.02 mol/L) for 30 min at 90 ℃. Afterwards, the absorbance of the mixture was measured at 532 nm using a spectrophotometer (TU-1901, Beijing PERSEE General Instrument Co., Ltd., Beijing, China). The standard curve was prepared using malondialdehyde (MDA) and TBARS was expressed as mg MDA/kg sample.
Texture
Texture profile analysis (TPA) of crayfish meat was performed using a TA-XT plus texture analyzer (Stable Micro systems Ltd., Surry, UK). The second and the third segment of the crayfish tail was cut into 8 mm × 8 mm × 6 mm uniform square samples and was compressed perpendicularly in a two-cycle compression test using a P/36R stainless steel cylindrical probe. The sample was measured with a 5 g trigger force at a constant test speed of 1 mm/s and press down distance of 5 mm. TPA parameters, including hardness (N), elasticity, resilience and chewiness were recorded. Each sample was tested repeatedly 10 times.
Low field nuclear magnetic resonance (LF-NMR) analysis
NMR relaxation measurement was performed using NMR imaging and analyzing system (NMI20, Shanghai Numag Electronic Technology Co., Ltd., Shanghai, China). Take the whole shrimp meat, which is between 2 and 2.5cm in diameter. The sample (about 2 g) was wrapped with a bag and put in a glass tube of 25 mm diameter. The experimental temperature is set at 32 °C. The T2 signal was collected using Carr-Prucell-Meiboom-Gill (CPMG) pulse sequence with a τ value of 120 μs. Data were acquired from 7000 echoes, and three measurements were carried out on each sample. Three relaxation times (T20, T21 and T22) and their corresponding water populations (P20, P21 and P22) were obtained as outputs.
Statistical analysis
All data were presented as means ± standard deviation and were analyzed of variance by one-way analysis of variance (ANOVA) using SPSS for Windows 19.0 (IBM Corp., Armonk, NY, USA). A difference in means was determined using the Duncan’s multiple range test at P<0.05.
Results and Discussion
pH
pH is one of the vital symbols to judge the freshment of aquatic products. Generally, due to the decomposition of protein and other nitrogen components in muscle by endogenous enzymes and microorganisms, which would generate ammonia, trimethylamine, histamine and other alkaline substances, the pH of aquatic products progressively increased during storage [12]. As shown in Figure 1, with the increasing time of frozen storage, the pH originally went down and then up. After 12 weeks of storage at - 18 ℃, the pH of RF group, IF group and LNF group were 8.45, 8.36 and 8.35 respectively. It showed a little difference after 12 weeks of storage, however, it could be told from the overall trend that the lower freezing temperature could maintain the lower pH during post frozen storage. The rise in pH reduced the electrostatic repulsion that causes protein aggregation. The closer to the initial pH value, the less damage was to the quality of crayfish [13].
Figure 1: Effects of different freezing methods on pH value of crayfish meat. RF: Refrigerator freezing; IF: Immersion freezing; LNF: Liquid nitrogen freezing.

TVB-N
The decay degree of crayfish muscle treated with three different frozen methods was determined by measuring TVB-N. When frozen, the protein of crayfish muscle is decomposed by spoilage microorganisms and endogenous enzymes to produce ammonia and other alkaline nitrogenous volatile compounds [14]. However, during frozen storage, the spoilage microorganisms are usually inhibited. So, the endogenous enzymes should be mainly responsible for the increase of the TVB-N content of crayfish during frozen storage. The limit of TVB-N content in shrimp as acceptable for human consumption is usually lower than 30 mg/100g [15]. The content of TVB-N of fresh crayfish was 7.09 mg/100g. The TVB-N value all showed an upward trend followed with time, however it went up slowly in the later stage (Figure 2). That may be due to the deamination of adenine nucleotides at the initial stage of storage, and the formation of organic acids, aldehydes, ketones and other odor metabolites by microorganisms and other spoilage bacteria in the later stage. During the frozen period, the TVB-N value of the RF group was significantly higher than the other two groups (P<0.05). The freezing speed of the IF and LNF group was faster than the RF group, and smaller ice crystals have been formed. It suggested that IF and LNF groups have less damage to the tissue cells of crayfish meat. On the other hand, the lower freezing temperature could effectively inhibit the activity of enzymes, so as to reduce the decomposition rate of protein in crayfish meat, which is more conducive to maintaining the quality of frozen crayfish.
Figure 2: Effects of different freezing methods on pH value of crayfish meat. RF: Refrigerator freezing; IF: Immersion freezing; LNF: Liquid nitrogen freezing.

TBARS
Lipid oxidation in crayfish meat treated with different freezing methods was determined by measuring the content of MDA content (Figure 3). Though freezing can inhibit bacteria growth effectively, long-term freezing storage does involve lipid and protein oxidation for seafood products. Proteins, particularly myofibrillar proteins (MPs), are susceptible to oxidation in the muscular system through interactions between free radicals and amino acid residues. Furthermore, SHs of proteins are highly susceptible to being oxidized into S—S and other sulfur-containing oxides in the presence of hydrogen peroxide [16]. Crude fat content in crayfish is about 1 g/100g wet weight. The MDA content of crayfish increased during the storage period, with potential cytotoxicity and fishy odor [16]. The MDA content of fresh crayfish was 0.131 mg/kg. After 8 weeks of frozen storage, the MDA content of RF, IF and LNF groups were 0.534 mg/kg, 0.510 mg/kg and 0.461 mg/kg respectively, which indicated that the lipid of crayfish had been oxidized [12]. At the end of frozen storage, the MDA content of crayfish of all three groups was within the acceptable range of less than 1 mg/kg [17]. During frozen storage, the MDA content of crayfish meat in LNF group was the lowest, indicating that liquid nitrogen freezing could effectively slow down the lipid oxidation, which may be due to the lower freezing temperature can slow the process of lipid oxidation.
Figure 3: Effects of different freezing methods on MDA content of crayfish meat. RF: Refrigerator freezing; IF: Immersion freezing; LNF: Liquid nitrogen freezing

WHC
The changes in water holding capacity (WHC) of crayfish subjected to different freezing methods were shown in Figure 4. During frozen storage, the WHC of three groups decreased at various degrees. Among them, the WHC of the RF group decreased from 84.1% to 64.51% after 12 weeks, while the WHC of IF and LNF groups decreased to 65.62% and 68.48%, respectively. With the prolongation of storage time, the myofibril gets broken down under the action of protein denaturation, resulting in the decrease of WHC. The WHC of LNF group was always higher than the RF and IF group during storage. In the process of cryopreservation, the degree of damage to cell structure is related to the size of ice crystals. The freezing speed of liquid nitrogen group was fast, and the ice crystals were small and evenly distributed, which could prevent protein denaturation and delay the water loss during storage [4]. Balan et al. [18] also confirmed that rapid freezing could effectively maintain the WHC of mutton, and also maintained the flavor and nutrition better. Gao et al. [4] believed that the lower the quick-freezing temperature was, the faster the frozen sample could pass through the zone of maximum ice crystal formation, and the smaller the ice crystals were formed, It would lead to a less damage to the cell structures,and maintain a better WHC (Figure 4).
Water contribution and mobility analysis
The moisture distribution of the crayfish meat was determined by low field nuclear magnetic resonance (LF-NMR). Table 1 shows the variation in moisture distribution of crayfish meat under different freezing methods. The relaxation time shows that there are three forms of water in crayfish meat. These include bound water (0.1< T20 < 1, T21< 10 ms) and immobilized water (10 < T22 <100 ms) [10]. In general, both T20 and T21 refer to water that is tightly bound to protein macromolecules, and T22 represents water that is not easily flowable being trapped in a complex network of myo- fibrillar proteins, accounting for more than 90% of the total signal, which is mainly located between actin and myosin filaments [19]. The variation of T2 value is influenced by the mobility of water in crayfish meat and its degree of binding to environmental substances. According to Table 1, the storage time and freezing method had a significant effect on the distribution of T2. In the control group (day 0), T20, T21 and T22 were 0.15, 2.03 and 40.56 ms, respectively, while after freezing they, fluctuated in the ranges of 0.23-0.30, 2.58-3.43 and 34.49-37.40 ms, respectively. Both T20 and T21 increased slowly, while T22 decreased significantly (P<0.05). There was a decrease in P20 and an increase in P21, but it was not significant (P>0.05). It indicates that freezing promotes water release, with the possibility of sequential transformation in the order of bound water, less mobile water to free water [7]. And it may be due to the disruption of the three-dimensional network structure by ice crystals. In contrast to the view of zhao [20], our study found that the decrease of T22 indicates that freezing caused the protein to collapse from the three-dimensional structure into spherical aggregates, resulting in the loss of water. In addition, the reduction of P22 may be the result of thawing loss (Table 2).
Figure 4: Effects of different freezing methods on water holding capacity (WHC) of crayfish meat. RF: Refrigerator freezing; IF: Immersion freezing; LNF: Liquid nitrogen freezing

Table 2: Changes in corresponding relative populations P2 of crayfish meat frozen by different methods during storage.

Figure 5 shows the moisture distribution of the three groups of crayfish meat at the end of storage. The T22 of crayfish meat in the LNF group was 37.40 ms, which was higher than that in the RF and IF groups (34.49 ms). The P22 of the LNF group was higher than that of the other two groups, and the P22 of the RF and IF groups were similar. These results are consistent with the changes in WHC, suggesting that liquid nitrogen freezing can reduce the damage to protein structure and water loss during frozen storage. This would be due to the effect of internal stress during freezing, resulting in low-temperature fracture or changes in protein structure and then polypeptide chains were separated. This physical change has enlarged the space of the net structure of the protein, leading to the muscle expansion and increasing water fluidity.
Figure 5: Water relaxation time (T2) of fresh crayfish and frozen samples after 12 weeks of storage. RF: Refrigerator freezing; IF: Immersion freezing; LNF: Liquid nitrogen freezing. Bars of same relaxation time with different letter are significantly different (p<0.05).

Texture
Generally, the changes in elasticity, hardness, chewiness and resilience of crayfish treated with three freezing methods had a similar trend during frozen storage. The hardness of crayfish meat frozen with different ways remained stable during early storage (0 to 6 weeks) but decreased significantly from 6 to 12 weeks (P<0.05) (Figure 6A). Moreover, the hardness of LNF group was significantly higher than the other two groups(P<0.05). The elasticity of crayfish decreased followed by the storage time (Figure 6B). The crayfish meat of LNF group had higher elasticity as well as a smaller decline rate, than the other two groups, and there was no significant difference in elasticity between the RF and IF groups. The resilience (Figure 6C) and chewiness (Figure 6D) of crayfish meat of LNF, IF and RF have decreased by 16%,14%, 29% and 30%, 34%, 42%, respectively.
Figure 6: Effects of different freezing methods on (A) hardness, (B) elasticity, (C) resilience, and (D) chewiness of crayfish meat. RF: Refrigerator freezing; IF: Immersion freezing; LNF: Liquid nitrogen freezing

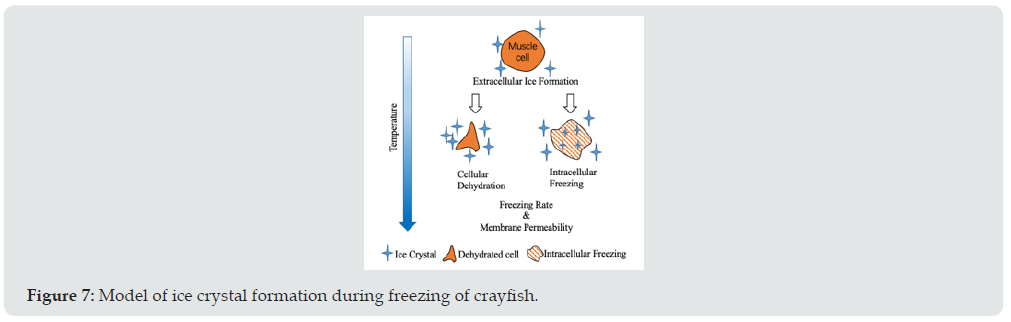
Effects of freezing temperature on ice formation during frozen storage
Upon freezing, the extracellular solution first crystallizes as it has a higher freezing point due to a lower content of solutes. Once ice crystals form in the extracellular space, solutes get concentrated and therefore there will be osmotic pressure across the sarcolemma (i.e., cell membrane). To achieve a balanced state again, cells can respond in two ways: cellular dehydration or intracellular ice formation (Figure 7). These subsequent events during cooling depend mainly on the cooling rate and membrane permeability to water [21]. The cooling rate can be assessed by the characteristic freezing time (tc), and tc is defined as time needed for temperature to decrease from −1 °C (beginning of freezing) to −7 °C (freezing of 80% of the water present). Intracellular ice formation may happen in LNF and IF treatment, because these treatments enabled less time spent, to transverse freeze to the fibers. As long as the protein is not highly denatured, the intracellular ice can be absorbed, and the muscle cell can be largely recovered [22, 23]. It indicated that LNF and IF could slow down the decline of the structure of crayfish meat. The present results indicated that freezing with LNF and IF was effective to retard the change of texture parameters in crayfish meat during frozen storage due to a high rate rate of freezing and small ice crystals formation [24-26].
Conclusion
The crayfish was stored at -18 ℃ for 12 weeks after freezing by refrigerator (RF), immersion solution (IF) and liquid nitrogen (LNF). Their freezing temperatures were -20 °C, -35 °C and -90 °C, respectively. With the increase in frozen storage duration, the pH, TVB-N, and TBARS of crayfish meat significantly increased (P<0.05) and WHC, texture, and T2 relaxation time decreased (P<0.05). LNF , while IF have better effects on retarding the process of protein deterioration as well as lipid oxidation. It also suggested that the liquid nitrogen group has less alteration of the three-dimensional network of the proteins. The lower the freezing temperature, like IF and LNF, could help reduce the damage to the quality of crayfish meat, which was more conducive to the quality maintenance of crayfish in frozen storage. In our study, liquid nitrogen freezing had the best effect on the quality of crayfish during frozen storage. However, the effects of different freezing methods on the properties and structure of crayfish protein should be further explored.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Funding
This work was supported by China Agriculture Research System of MOF and MARA, Anhui Provincial Modern Agro-industry Technology Research System (AHCYJSTX-08) and the Fundamental Research Funds for the Central Universities of China (Grant No. PA2021GDSK0102).
References
- Shao Y, Xiong G, Ling J, Hu Y, Shi L, et al. (2018) Effect of ultra-high-pressure treatment on shucking and meat properties of red swamp crayfish (Procambarus clarkia). LWT - Food Sci Technol 87: 234-240.
- Bureau of Fisheries of Ministry of Agriculture of China. 2020. China Fishery Statistical Yearbook. Beijing: Chinese Agriculture Press. (in Chinese).
- Liang D, Lin F, Yang G, Yue X, Zhang Q, et al. (2015) Advantages of immersion freezing for quality preservation of litchi fruit during frozen storage. LWT - Food Sci Technol 60(5): 948-956.
- Gao W, Huang Y, Zeng X-a, Brennan MA (2019) Effect of soluble soybean polysaccharides on freeze-denaturation and structure of myofibrillar protein of bighead carp surimi with liquid nitrogen freezing. Int J Biol Macromol 135: 839-844.
- Tian J, Walayat N, Ding Y, Liu J (2021) The role of trifunctional cryoprotectants in the frozen storage of aquatic foods: Recent developments and future recommendations. Compr Rev Food Sci F 21(1): 321-339.
- Yi SK, Li YH, Shi LL, Zhang L, Li QB, et al. (2018) Characterization of Population Genetic Structure of red swamp crayfish, Procambarus clarkii, in China. Sci Rep 8: 5586.
- Tan M, Lin Z, Zu Y, Zhu B, Cheng S (2018) Effect of multiple freeze-thaw cycles on the quality of instant sea cucumber: Emphatically on water status of by LF-NMR and MRI. Food Res Int 109: 65-71.
- Cremades O, Álvarez Ossorio C, Gutierrez Gil JF, Parrado J, Bautista J (2011) Quality changes of cooked crayfish (Procambarus clarkii) tails without additives during storage under protective atmospheres. J Food Process Pres 35: 898-906.
- Chinese National Standard GB 5009.228-2016. 2016. National food safety standard: Determination of total volatile base nitrogen in food. Beijing: Standards Press of China. (in Chinese)
- Li XX, Liu S, Su W, Cai L, Li J (2017) Physical quality changes of precooked Chinese shrimp Fenneropenaeus chinensis and correlation to water distribution and mobility by low-field NMR during frozen storage. J Food Process Pres 41(6): e13220.
- Song Y, Liu L, Shen H, You J, Luo Y (2011) Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control 22: 608-615.
- Anacleto P, Teixeira B, Marques P, Pedro S, Nunes ML, et al. (2011) Shelf-life of cooked edible crab (Cancer pagurus) stored under refrigerated conditions. LWT – Food Sci Technol 44(6): 1376–1382.
- Yasemi M (2017) Prevention of denaturation of freshwater crayfish muscle subjected to different freeze-thaw cycles by gelatin hydrolysate. Food Chem 234: 199-204.
- Yuan G, Lv H, Tang W, Zhang X, Sun H (2016) Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control 59: 818-823.
- Shi J, Lei Y, Shen H, Hong H, Yu X, et al. (2019) Effect of glazing and rosemary (Rosmarinus officinalis) extract on preservation of mud shrimp (Solenocera melantho) during frozen storage. Food Chem 272: 604-612.
- Guan W, Nong W, Wei X, Zhu M, Mao L (2021) Impacts of a novel live shrimp (Litopenaeus vannamei) water-free transportation strategy on flesh quality: Insights through stress response and oxidation in lipids and proteins. Aquaculture 533: 736168.
- McKenna DR, Mies PD, Baird BE, Pfeiffer KD, Ellenbracht JW, et al. (2005) Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci 70(4): 665-682.
- Balan P, Kim YHB, Stuart AD, Kemp R, Staincliffe M, et al. (2019) Effect of fast freezing then thaw-aging on meat quality attributes of lamb M. longissimus lumborum. Anim Sci J 90(8): 1060-1069.
- Qian S, Li X, Wang H, Sun Z, Zhang C, et al. (2018) Effect of sub-freezing storage (-6, -9 and -12 °C) on quality and shelf life of beef. Int J Food Sci Technol 53: 2129-2140.
- Zhao X, Chen L, Wongmaneepratip W, He Y, Zhao L, et al. (2021) Effect of vacuum impregnated fish gelatin and grape seed extract on moisture state, microbiota composition, and quality of chilled seabass fillets. Food Chem 354: 129581.
- Elliott G, Wang S, Fuller BJ (2017) Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 76: 74-91.
- Bao Y, Ertbjerg P, Estévez M, Yuan L, Gao R (2021) Freezing of meat and aquatic food: Underlying mechanisms and implications on protein oxidation. Compr. Rev Food Sci F 20(6): 5548-5569.
- Mendes R, Huidobro A, Caballero E (2002) Indole levels in deepwater pink shrimp (Parapenaeus longirostris) from the Portuguese coast. Effects of temperature abuse. Eur Food Res Technol 214: 125-130.
- Tsironi T, Dermesonlouoglou E, Giannakourou M, Taoukis P (2009) Shelf-life modeling of frozen shrimp at variable temperature conditions. LWT - Food Sci Technol 42(2): 664-671.
- Yamagata M, Low LK (1995) Banana shrimp, Penaeus merguiensis, quality changes during iced and frozen storage. J Food Sci 60(4): 721-726.
- Chinese National Standard GB 5009.237-2016. 2016. National food safety standard: Determination of pH value of food. Beijing: Standards Press of China. (in Chinese).

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...