Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Article(ISSN: 2638-6070) 
Development and Application of HPLC/Post-Column Method for Analysis of Low Molecular Weight Organic Acids in Beers, Wines and Fruit Juices
Volume 1 - Issue 4Joon-Kwan Moon1, Kyu-Won Hwang1, Hoon Choi2, Woo Suk Bang3 and Takayuki Shibamoto4*
- 1Department of Plant Life and Environmental Sciences, Hankyong National University, Ansung, Gyoung-gi, Republic of Korea, 17579
- 2Division of Food and Environmental Sciences, Wonkwang University, Iksan, Republic of Korea, 54538
- 3Department of Food and Nutrition, Yeungnam University, Gyeongsan, Gyeongbuk, Republic of Korea, 38541
- 4Department of Environmental Toxicology, University of California at Davis, One Shields Avenue, USA, CA95616
Received: October 05, 2018; Published: December 13, 2018
*Corresponding author: T Shibamoto, Department of Environmental Toxicology, University of California at Davis, One Shields Avenue, USA, CA95616
DOI: 10.32474/SJFN.2018.01.000119
Abstract
A post-column reaction method, which involves use of the pH indicator BTB, was developed and applied to the analysis of low molecular weight organic acids in commercial beers, wines, and fruit juices. Organic acids identified in these beverages were formic, acetic, citric, pyruvic, tartaric, malic, succinic, lactic, and pyroglutamic. Amounts of total acids in samples ranged from 980.4 mg/L to 513.5 mg/L in beers, from 7,502.3 mg/L to 5,573.3 mg/L in wines, and from 11,162.8 mg/L (orange) to 2,995.8 mg/L (mango) in fruit juices. Citric acid was found in the greatest amount-ranging from 286.7 ± 4.5 mg mg/L to 139.0 ± 5.3 mg mg/L in beers. The greatest level of malic acid was in a wine with 4,248.4 mg/L. The method developed is applicable to determine amounts of organic acids, formic and acetic acids, in beverages quickly and accurately.
Keywords: Organic acids analysis; HPLC/post-column; Beers; Fruit juices; Wines
Introduction
Low molecular weight organic acids (LMWOAs), including formic, acetic, malic, citric, tartaric, lactic, succinic, and oxalic acid, are found in various beverages, such as beers, wines and fruit juices (citrus, apple, grape, and melon), as well as in some foods [1,2]. These acids contribute characteristic tastes to beverages. For example, formic acid possesses pungent odor and sour taste in proper dilution. It is particularly adaptable to the pineapple flavor. Acetic acid also has a pungent and stinging sour odor, but it gives a clean-sour and acid taste at dilute concentrations (lower than 1%) in water and been used in flavor compositions, such as butter, chocolate, grape strawberry and wine. Malic acid is present in plum, peach, apricot and related fruits and used for imitation fruit flavors, such as maple [3]. Citric acid is the major acid component of citrus species and possesses a clean acid taste in an aqueous solution [3,4]. In addition to the role of organic acids in taste and flavor of beverages, they also play an important role in quality of beverages, including beers [5], wines [6] and fruit juices [7]. A capillary electrophoresis (CE) with spectrophotometric method achieved successfully analysis of a limited number of LMWOAs [8].
This method has been widely used for LMWOAs analysis in foods and beverages [9]. However, the resolution and detection of LMWOAs formic and acetic, achieved better by chromatographic methods than by the spectrophotometric method [10]. In the case of the most commonly used gas chromatography (GC), a tedious derivatization of organic acids is required to prepare samples for analysis because they are highly soluble in water [11]. On the other hand, HPLC, which can take aqueous samples directly, has been widely used to analyze LMWOAs in water samples; such as wine, beer [12], vinegar and fruit juices [13], coffee [14] and alcoholic beverage meads [15]. A HPLC interfaced to enzyme reactor analyzed oxalic acid in fruit and vegetable juices [16]. After the electronspray ionization system was advanced to interface between HPLC and mass spectrometers (LC/MS), LC/MS application for the analysis of water-soluble chemicals, including LMWOAs, developed significantly [17]. However, separation of LMWOAs by HPLC, formic acid and acetic acid, remains relatively difficult.
In the present study, a previously reported simple and accurate post-column reaction method for LMWOAs [18] was improved and successfully applied to analyze LMWOAs, including formic and acetic acids, in beers, wines, and fruit juices.
Materials and Methods
Beverage Samples
All commercial beverages-10 kinds of beers, 6 types of wines (5 red and 1 white), and 25 kinds of fruit juices-were bought from a local market in Seoul, Korea.
Chemicals and Reagents
Bromothymol blue (BTB, 95%), sodium phosphate dibasic anhydrous (98.5%), sodium hydroxide (98%), perchloric acid (67– 72%), oxalic acid (99.5–100.2%), phosphoric acid (85%), citric acid (99.5%), tartaric acid (99.5%), malic acid (95–100%), quinin acid (100%), succinic acid (99%), fumaric acid (99%), lactic acid (85%), formic acid (95%), acetic acid (99.7%), pyroglutamic acid (99%) were purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA) or TCI Chemical Co. Ltd. (Tokyo, Japan). The purified water was prepared with a Zener Power II (Human Co., Korea). A stock solution of standard organic acids (10g/L) was prepared in purified water for preparation of standard solutions and spike analysis.
Preparation of Beer and Wine Samples
Beer and wine samples (2 mL each) were filtered with an Arcadis Syringe Filter with 0.45 mm PVDF membrane (Waters Co, Milford, MA).
Preparation of Fruit Juice Samples
Well-mixed juice samples were centrifuged at 10,000 rpm for 5min at 4 ºC to collect the supernatant. The supernatant was filtered with an Arcadis Syringe Filter with 0.45mm PVDF membrane (Waters Co, Milford, MA) and were analyzed with HPLC.
Analysis of Organic Acids in Beverages
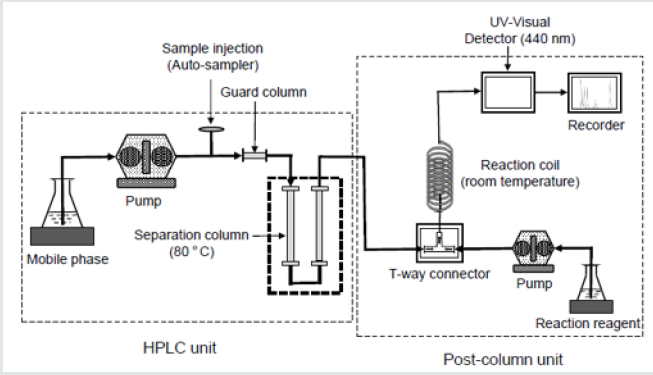
Figure 1 shows the systematic diagram of the post-column method used in the present study. A prepared beverage sample was injected (10mL) into an Agilent 1100 model HPLC equipped with a 5cm x 8.0 mm i.d. Shorex RS pack KC-LG guard column, which was connected to two 30 cm x 8.0 mm i.d. KC-811 separation columns in series. The mobile phase was a 3mmol perchloric acid solution with a 0.7 mL/min flow rate. Temperature of the separation columns was 80ºC. The eluate from HPLC was mixed with a reaction solution containing 0.2mmol bromothymol blue (BTB), 15 mmol Na2HPO4, and 2 mmol NaOH. The reaction solution was purged at a 0.7mL/ min flow rate in a T-way connector as shown in Figure 1. Adducts formed from the acids and BTB in a 50 cm x 0.25 mm i.d. stainless reaction coil was monitored with a UV detector at l = 440 nm.
Results and Discussion
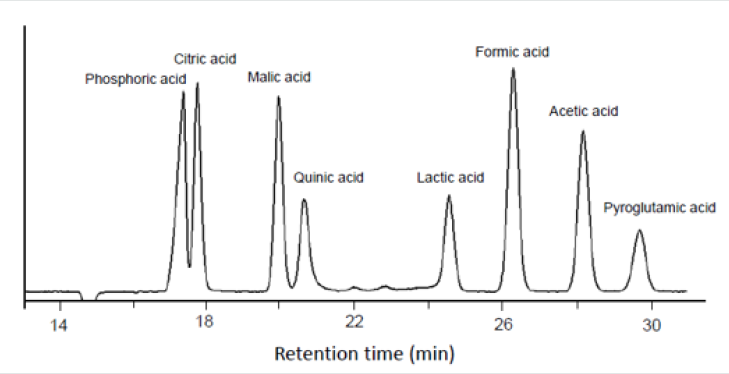
As mentioned above, analysis of LMWOAs is a difficult process. There have been advances in HPLC/MS methods recently but some problems, such as insufficient resolution of formic acid and acetic acid, remain. In order to resolve these problems, we developed a post-column reaction method, which involved use of pH indicator BTB. This method and related theory were originally advanced nearly three decades ago [18]. Figure 2 shows an HPLC postcolumn chromatogram of standard acids. This chromatogram shows satisfactory resolution of low molecular weight acids for quantitation. Phosphoric acid peak in this chromatogram may come from a phosphate in a reaction solution. However, phosphoric acid is not organic acid, which is out of scope of this study. The limits of detection (LOD) of acids were 25.0mg/L for citric, malic and quinin, lactic; 12.5mg/L for formic and acetic; and 50mg/L for pyroglutamic. The limits of quantitation (LOQ) of each acid were 75.0mg/L for citric, malic, quinin, and lactic; 30.0 mg/L for formic; 50.0 mg/L for acetic; and 150 mg/L for pyroglutamic.
Organic Acids Found in Beer Samples
Table 1: Organic Acid Contents in Beer Samples (mg/L). Values are Mean ± Standard deviation (SD, n = 3).

Table 1 shows the results of LMWOAs analysis in beer samples. There are three monoprotic acids (lactic, acetic and pyroglutamic), two diprotic acids (malic and succinic), and one triprotic acid (citric acid). Amounts of total acids in beer samples varied considerably, ranging from 1,801.3 mg/L (brand E) to 776.0 mg/L (brand D). Among the acids identified in the beer samples, citric acid was generally found in the greatest amount-ranging from 286.7 ± 4.5 mg/L (brand E) to 139.0 ± 5.3 mg/L (brand G), followed by lacticranging from 274.3 ± 5.6 mg/L (brand E) to 19.3 ± 2.2 mg/L (brand G), and pyroglutamic acid-ranging from 264.9 ± 1.4 mg/L (brand E) to 105.5 ± 2.4 mg/L (brand D. ) Previously, the electrophoresis method was successfully used to analyze LMWOAs in beers. Klampft [19] found citric acid with level of 193 ± 2.4 mg/L in Chinese rice beer, 171 ± 1.5 mg/L in white beer and 178 ± 1.1 mg/L in Lager beer. Cordeiro-Ramirez et al. [20] reported citric acid (ND – 59 ± 2 mg/L), fumaric acid (ND – 5.9 ± 0.7 mg/L), malic acid (106 ± 12 mg/L), pyroglutamic acid (92 ± 6 mg/L) in 6 different beers. Citric acid exhibited synergistic effects with lactic acid-producing bacteria toward inhibition of pathogenic bacteria grow [21]. The amounts found in these acids in the present study were like these reports. For example, the present study found citric acid with amounts ranging from 146.2 ± 3.8 mg/L brand D to 286.7 ± 4.5 mg/L in brand E. In addition, the present study found pyroglutamic acid with levels ranging from 105.5 ± 2.4 mg/L in brand D to 264.9 ± 1.7 mg/L in brand E. The level of acetic acid ranged from 98.2 ± 3.0 mg/L (brand C) to not detected (brand H) in the present study, whereas acetic acid was not reported from the studies conducted with the electrophoresis method.
Organic Acids Found in Wine Samples
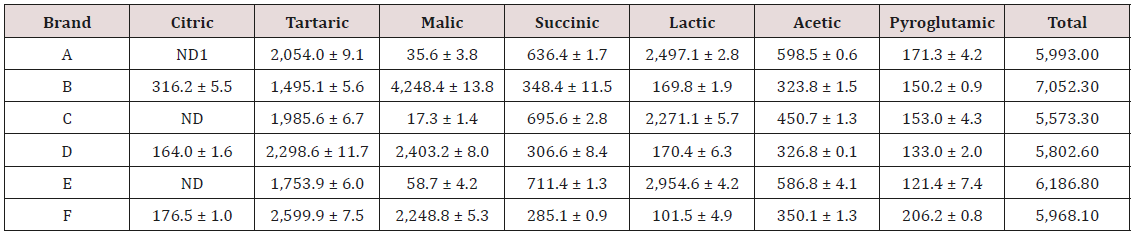
Table 2 shows the results of LMWOAs analysis in wine samples. The total amounts of acids were similar in all wine samples, ranging from 7,052.3mg/L (brand B) to 5,573.3mg/L (brand C). Tartaric acid was generally found in the highest concentration, ranging from 2,599.9 ± 7.5mg/L (brand F) to 1,495.1 ± 5.6mg/L (brand B). Amounts of citric acid, malic acid and lactic acid varied significantly among brands. Lactic acid was found at high levels in brands A (2,497.1 ± 2.8mg/L), C (2,271.1 ± 5.7 mg/L), and E (2,954.6 ± 4.2mg/L), but at relatively low levels in brands B (169.8 ± 1.9mg/L), D (170.4 ± 6.3 mg/L), and F (101.5 ± 4.9mg/L). On the other hand, high levels of malic acid were found in brands B (4,248.4 ± 13.8 mg/L), D (2,403.2 ± 8.0mg/L), and F (2,248.8 ± 5.3mg/L), whereas its concentrations in A was only 35.6 ± 3.8 mg/L in, C was 17.3 ± 1.4 mg/L and in E was 58.7 ± 4.2mg/L. A similar trend was observed in the case of citric acid. A previous study found lactic and tartaric acids in Ribeiro Sacra wines as the predominant organic acid components [9]. Lactic acid and tartaric acid levels ranged from 3,784mg/L to 452mg/L and 1978mg/L 866mg/L. These results are consistent to the ones from the present study. As mentioned above, the quality of wines depends on, in part, acidity associated with the composition of acids [22]. The results in the present study demonstrate that the composition of acids in different brands of wines varies. Therefore, the analytical method developed can be useful in evaluating wine quality as well as a guide to improvement in the winemaking process.
Organic Acids Found in Fruit Juice Samples
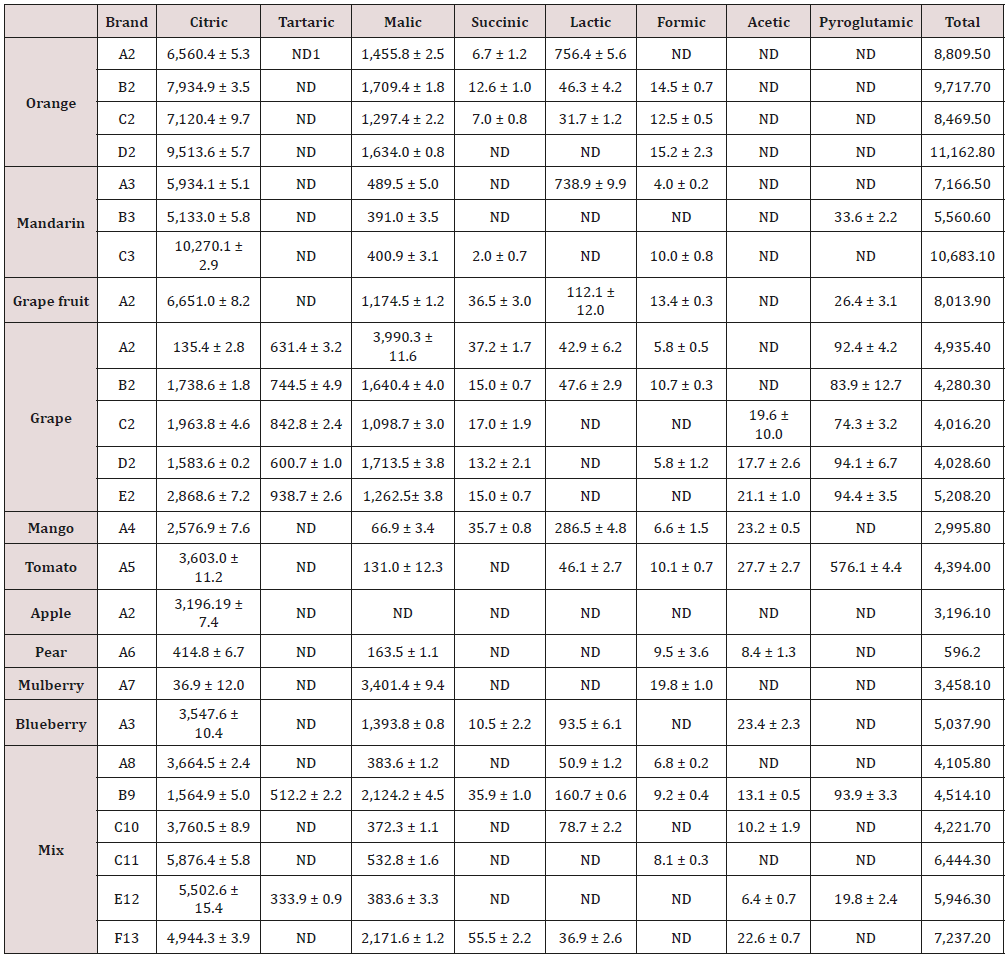
Table 3 shows the results of LMWOAs analysis in fruit juice samples. Formic acid and acetic acid, which are the lowest and 2nd lowest molecular weight acids among LMWOAs and difficult to determine by a CE method [10], were satisfactorily analyzed in the present study. The levels of formic and acetic acids found ranged from 19.8 ± 1.0mg/L (mulberry) to 4.0 ± 0.2mg/L (mandarin) and from 27.7 ± 2.7mg/L (tomato) to 17.7 ± 2.6mg/L (grape), respectively. Orange juice samples had the greatest total concentration of acids, ranging from 11,162.8 mg/L (brand D) to 8,469.5 mg/L (brand C), followed by mandarin juice samples, ranging from 10,683.1mg/L (brand C) to 5,560.6 mg/L (brand B) and grape juice samples, ranging from 5,208.2 mg/L (brand E) to 4,016.2 mg/L (brand C). Citric acid and malic acid were generally detected in higher levels than other acids, ranging from 10,270.1 ± 2.9mg/L (mandarin) to 36.9 ± 12.0mg/L (mulberry) and from 3,990.3 ± 11.6mg/L (Grape) to 66.9 ± mg/L (mango), respectively. The pear juice sample contained the lowest level of total acid with 596.2 mg/L. The total amounts of acids in mixed juices ranged from 7,237.2mg/L (brand F) to 4,105.8 mg/L (brand A), which are comparable to those of grape juices.
One previous report demonstrated satisfactory analysis of organic acids in fruit juices (apple, peach, pear and apricot) using HPLC with an ion- exclusion column [23]. Another previous study reported the amounts of citric, malic, quinin and tartaric acids in apple, orange, cranberry, white/red grapes, and pomegrante juices analyzed using LC/MS/MS [24]. Effect of malic acid for the inactivation of common food pathogens on fresh-cut lettuce was reported, suggesting that malic acid possesses some biological activity [25]. The presence of formic and acetic acids was, however, not reported in these studies. On the other hand, the present study found 9.5 ± 3.6 mg/L formic acid in pear juice, 4.0 ± 0.2 – 10.0 ± 0.8 mg/L in mandarin juice, 12.5 ± 0.5mg/L – 15.2 ± 2.4mg/L in orange juice, 13.4 ± 0.3 mg/L in grape fruit, 5.8 ± 0.5 – 10.7 ± 0.3 mg/L in grape juice, 6.6 ± 1.5mg/L in mango juice, 10.1 ± 0.7 mg/L in tomato juice, and 19.8 ± 1.0 mg/L in mulberry juice. In addition, acetic acid was determined in pear juice (8.4 ± 1.3 mg/L), grape juice (17.7 ± 2.6 mg/L – 21.1 ± 1.0 mg/L), mango (23.2 ± 0.5 mg/L), and tomato (27.7 ± 2.7 mg/L). However, it is difficult to compare the results from the present study to those from the previous study because the compositions of the juices vary in the different brands.
Conclusion
The present study demonstrates that the method developed was useful to determine LMWOAs, formic acid and acetic acid, present in samples with complex matrices of beverages. Acids composition are important to evaluate quality of beverages. Therefore, the method developed in the present study would useful to analyze LMWOAs levels for evaluating quality of beverages. The CE method is also well-established method for LMWOAs analysis. Therefore, it is recommended to use both methods, the present method for formic acid and acetic acid.
References
- Quitmann H, Fan R, Czermak P (2014) Acidic organic compounds in beverage, food and feed production. Advances in Biochemical Engineering/Biotechnology 143: 91-141.
- Xiang J, Zhu W, Han J, Li Z, Ge H, et al. (2012) Analysis of organic acids in Chinese raisin tree (Hovenia dulcis) peduncle and their changes in liquid fermentation process. Food Science and Biotechnology 21: 1119-1127.
- Arctander S (1969) Perfume and Flavor Chemicals. Published by the author, Montclair, CA.
- Penniston K, Nakada S, Holmes RP, Assimos DG (2009) Quantitative assessment of citric acid in lemon juice, lime juice, and commerciallyavailable fruit juice products. Journal of Endourology 22(3): 567-570.
- Guido LF, Curto AF, Boivin P, Benismail N, Cristina R Gonçalves, et al. (2007) Correlation of malt quality parameters and beer flavor stability. Multivariate Analysis 55(3): 728-733.
- Prenesti E, Berto S, Toso S, Caniele PG (2012) Acid-base chemistry of white wine: Analytical characterization and chemical modelling. The Scientific World Journal 2012: 249041, p. 7.
- Etienne A, Génard M, Lobit P, Mbeguié-A-Mbeguié D, Bugaud C (2013) What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. Journal of Experimental Botany 64(6): 1451- 1469.
- Kowalski P, Plenis A (2007) Comparison of HPLC and CE methods for the determination of cetirizine dihydrochloride in human plasma samples. Biomedical Chromatography 21(9): 903-911.
- Castiñeira A, Peña RM, Herrero C, Garcia Martin S (2002) Analysis of organic acids in wine by capillary electrophoresis with direct UV detection. Journal of Food Composition and Analysis 15(3): 319-331.
- Klampfl CW (2007) Determination of organic acids by CE and CEC methods. Electrophoresis 28: 3362-3378.
- Ferreira AM, Laespada ME, Pavón JL, Cordero BM (2013) In situ aqueous derivatization as sample preparation technique for gas chromatographic determination. Journal of Chromatography A 1296: 70-83.
- Park JM, Shin JA, Lee JH, Lee KT (2017) Development of quantitative method for organic acid in wine and beer using high performance liquid chromatography. Food Science and Biotechnology 26(2): 349-355.
- Zhang A, Fang Y, Meng JF, Wang H, Chen S, et al. (2011) Analysis of low molecular weight organic acids in several complex liquid biological systems via HPLC with switching detection wavelength. Journal of Food Composition and Analysis 24(3): 449-455.
- Rodrigues CI, Marta L, Maia R, Miranda M, Ribeirinho M, et al. (2007) Application of solid-phase extraction to brewed coffee caffeine and organic acid dtermination by UV/HPLC. Journal of Food Composition and Analysis 20(5): 440-448.
- Švecová B, Bordovská M, Kalvachová D, Hájek T (2015) Analysis of Czech meads: Sugar content, organic acid content and selected phenolic compounds content. Journal of Food Composition and Analysis 38: 80- 88.
- Siener R, Seidler A, Voss S, Hesse A (2016) The oxalate content of fruit and vegetable juices, nectars and drinks. Journal of Food Composition and Analysis 45: 108-112.
- Friedman M, Kozukue N, Kim HJ, Choi SH, Mizuno M ( 2017) Glycoalkaloid, phnolic, and flavonoid content and antioxidant activities of conventional nonorganic and organic potato peel powders from dommercial gold, red, and Russet potatoes. Journal of Food Composition and Analysis 62: 69- 75.
- Wada A, Bonoshita M, Tanaka Y, Hibi KA (1984) Study of a reaction system for organic acid analysis using a pH indicator as post-column reagent. Journal of Chromatography 291: 111-118.
- Klampfl CW (1999) Analysis of organic acids and inorganic anions in different types of beer using capillary zone electrophoresis. Journal of Agricultural and Food Chemistry 47(3): 987-990.
- Cortacero-Ramirez S, Carretero-Segura A, Hernainz-Bermudez de Castro M, Fernandez-Gutierrez A (2005) Determination of low-molecular-mass organic acids in any type of beer samples by coelectroosmotic capillary electrophoresis. Journal of Chromatography A 1064(1): 115-119.
- Seo S, Jung D, Wang X, Seo DJ, Lee MH, Choi C, et al. (2013) Combined effect of lactic acid bacteria and citric acid on Escherichia coli O157:H7 and Salmonella Typhimurium. Food Science and Biotechnology 22(4): 1171-1174.
- Volschenk H, van Vuuren HJJ, Viljoen-Bloom M (2006) Malic acid in wine: Origen, function and metabolism during vinification. South African Journal of Enology and Viticulture 27(2): 123-136.
- Chinici F, Spinabelli U, Riponi C, Amati A (2005) Optimization of the determination of organic acids and and sugars in fruit juices by ionexclusion liquid chromatography. Journal of Food Composition and Analysis 18(2-3): 121–130.
- Ehling S, Cole S (2011) Analysis of organic acids in fruit juices by liquid chromatography-mass spectrometry: An enhanced tool for authenticity testing. Journal of Agricultural and Food Chemistry 59(6): 2220-2234.
- Kim JH, Kwon KH, Oh SW (2016) Effects of malic acid or/and grapefruit seed extract for the inactivation of common food pathogens on fresh-cut lettuce. Food Science and Biotechnology 25(6): 1801-1804.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...