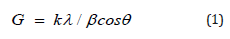Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Article(ISSN: 2638-6070) 
Complementary and Alternative Medicine: Impact of Consciousness Energy Healing Treatment on the Cholecalciferol (Vitamin D3)
Volume 1 - Issue 5Gopal Nayak1*, Mahendra Kumar Trivedi1, Alice Branton1, Dahryn Trivedi1 and Snehasis Jana2
- 1 Trivedi Global, Inc., Henderson, USA
- 2 Trivedi Science Research Laboratory Pvt. Ltd., Bhopal, India
Received: January 29, 2019; Published: February 20, 2019
*Corresponding author:Gopal Nayak, Trivedi Global, Inc., Henderson, USA
DOI: 10.32474/SJFN.2019.01.000121
Abstract
Cholecalciferol is a steroid hormone (7-dehydroxycholesterol), which helps in the absorption of dietary minerals like zinc, calcium, magnesium, iron, and phosphate and also responsible for other biological activity. In this research study, the influence of the Trivedi Effect®-Consciousness Energy Healing Treatment on the physicochemical and thermal properties of cholecalciferol was evaluated using the modern analytical technique. The cholecalciferol test sample was divided into two parts. One of the test samples was considered as a control sample, which did not receive the Biofield Energy Treatment; whereas, the other part was treated with the Consciousness Energy Healing Treatment remotely by a well-known Biofield Energy Healer, Gopal Nayak and termed as a treated sample. The particle size values in the treated cholecalciferol powder sample were significantly decreased by 78.94% (d10), 26.21% (d50), 22.01% (d90), and 29.04% {D (4,3)}; thus, the specific surface area of the treated cholecalciferol was significantly increased by 174.12% compared to the control sample. The XRD peak intensities and crystallite sizes of the treated cholecalciferol powder sample were significantly altered ranging from -44.37% to 370.49% and -74.48% to 91.52%, respectively; therefore, the average crystallite size of the treated cholecalciferol was significantly decreased by 36.21% compared to the control cholecalciferol. The latent heat of fusion of the treated cholecalciferol was significantly increased by 25.89% compared to the control cholecalciferol. The weight loss was decreased by 1.48%; whereas, the residue amount was significantly increased by 107.02% in the treated sample compared with the control sample. Thus, the Trivedi Effect®-Consciousness Energy Healing Treatment generated a new polymorphic form of cholecalciferol which might offer better solubility, absorption, bioavailability, and be thermally more stable compared with the control sample. Henceforth, the Consciousness Energy Healing Treated cholecalciferol would be more beneficial to maintain the overall quality of life and it would be more useful in designing novel nutraceutical/pharmaceutical formulations for the better therapeutic responses against deficiency of vitamin D, osteoporosis, rickets, cardiovascular diseases, diabetes mellitus, cancer, mental disorders, multiple sclerosis, etc.
Keywords:Complementary and Alternative Medicine; Vitamin D3; The Trivedi Effect®; Consciousness Energy Healing Treatment; Particle size; Surface area; PXRD; DSC; TGA/DTG
Introduction
Vitamin D3 (cholecalciferol or 7-dehydroxycholesterol) is a steroid hormone produced in the skin once exposed to ultraviolet light and also obtained from dietary sources. It is a fat-soluble vitamin helps in the absorption of the dietary minerals like zinc, calcium, magnesium, iron, and phosphate and responsible for other biological activity, i.e., maintain healthy immune, skeletal, cardiovascular, and reproductive systems [1-3]. The major dietary sources of vitamin D are the cod liver oil, fatty fish like salmon and tuna, milk, nutraceutical and pharmaceutical supplements [2]. It is used for the prevention and treatment of several diseases like hypovitaminosis D, rickets, osteoporosis, diabetes mellitus, cardiovascular diseases, mental disorders, infections, cancer, multiple sclerosis, etc. [4-6]. Cholecalciferol nutritional deficiency mostly under-diagnosed and under-treated is pandemic all over the world [7]. The 1,25-Dihydroxycholecalciferol is the biologically active form of cholecalciferol known as calcitriol [5- 7]. The overdosing of cholecalciferol can be the cause of vomiting, constipation, hypercalcemia, polyuria, polydipsia, insomnia, confusion, kidney stone, weakness, and mental retardation [1].
The bioavailability of cholecalciferol is very poor. Similarly, some of the other factors that directly affect the bioavailability of cholecalciferol are dietary fiber, genetic factors, and cholecalciferol status [8,9]. The stability, solubility, and bioavailability of cholecalciferol are the major concern for the storage and nutraceutical/pharmaceutical formulations point of views, as it is insoluble to water and sensitive to the light and air [10,11]. The pharmaceutical and nutraceutical scientists are working hard for the improvement of physicochemical properties of the compounds for better dissolution, absorption, and bioavailability in the body [12]. In this concern, the Consciousness Energy Healing Treatment (the Trivedi Effect®) has been proved experimentally that, it has a substantial impact on various physicochemical properties and also bioavailability of nutraceutical and pharmaceutical entities [13-16]. The Trivedi Effect® is natural and only proven scientific phenomenon in which a specialist can harness this intelligent energy from the “Universe” and transfer it anywhere on the planet through the possible mediation of neutrinos [17]. Around the body of every living organism an infinite and para-dimensional unique electromagnetic field exists created from the continuous moment of the charged particles like ions, cells, blood, etc. is called a “Biofield”. The Biofield Energy Therapies have been reported with significantly beneficial outcomes against various disease [18]. The National Center for Complementary and Alternative Medicine (NCCAM) and the National Institutes of Health (NIH) have recommend and included the Energy therapy under the Complementary and Alternative Medicine (CAM) along with other therapies, i.e., homeopathy, acupuncture, Ayurvedic medicine, naturopathy, acupressure, Tai Chi, Qi Gong, Reiki, hypnotherapy, etc. The CAM has been accepted by most of the USA population [19,20]. On the other hand, the Consciousness Energy Healing Treatment (the Trivedi Effect®) has gained popularity all over the world and reported with the substantial impact on the physicochemical and behavioural properties of metals, ceramics, polymer, organic compounds, microorganisms, cancer cells, crops, etc. [21-31]. In this study the impact of the Trivedi Effect®-Consciousness Energy Healing Treatment on the physicochemical and thermal properties of cholecalciferol was evaluated using particle size analysis (PSA), powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC), and differential thermogravimetric analysis (DTG)/ thermogravimetric analysis (TGA).
Materials and Methods
Chemicals and Reagents
The cholecalciferol powder sample (> 98%) was purchased from Sigma-Aldrich, India and reaming chemicals were of analytical grade purchased in India. Consciousness Energy Healing Treatment Strategies The test sample vitamin D3 was equally divided into two parts. One part of the vitamin D3 sample was received the Consciousness Energy Healing Treatment (the Trivedi Effect®) remotely provided standard laboratory conditions for 3 minutes by a well-known Biofield Energy Healer, Gopal Nayak, India, known as a Biofield Energy Treated sample. Though, the second part of vitamin D3 sample did not receive the Consciousness Energy Healing Treatment but treated with a “sham” healer called as a control sample. The “sham” healer doesn’t know anything about the Biofield Energy Treatment. After the treatment, both the samples were kept in sealed conditions and characterized using modern analytical techniques.
Characterization
The PSA of vitamin D3 was performed using Malvern Mastersizer 2000 (the UK) using the wet method [32,33]. The PXRD analysis of vitamin D3 powder sample was performed with the help of Rigaku Mini Flex-II Desktop X-ray diffractometer (Japan) [34,35]. The crystallites size was calculated from XRD data using the Scherrer’s formula (1)
Where k is the equipment constant, G is the crystallite size in nm, λ is the radiation wavelength, β is the full-width at half maximum, and θ is the Bragg angle [36,37].
Similarly, the DSC analysis of vitamin D3 was performed with the help of DSC Q200, TA instruments. The TGA/DTG thermograms of vitamin D3 were obtained with the help of TGA Q50 TA instruments [32,33]. The % change in particle size, specific surface area, peak intensity, crystallite size, latent heat, melting point, weight loss and the maximum thermal temperature of the Biofield Energy Treated vitamin D3 was calculated compared with the control vitamin D3 using the following equation 2:
Results and Discussion
Particle Size Analysis (PSA)
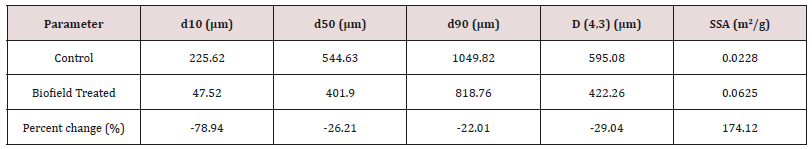
The particle sizes of the control and Biofield Energy Treated cholecalciferol were analyzed, and the data are presented in Table 1. The particle size values in the treated cholecalciferol were significantly decreased by 78.94%, 26.21%, 22.01%, and 29.04% at d10, d50, d90, and D (4,3), respectively compared to the control cholecalciferol. Therefore, the specific surface area of the Biofield Energy Treated cholecalciferol (0.0625 m2/g) was significantly increased by 174.12% compared to the control sample (0.0228 m2/g). The results indicated that the Trivedi Effect®-Consciousness Energy Healing Treatment supposed to act as an external force for breaking the larger particles of cholecalciferol to smaller one; hence increased the surface area. The particle properties (size, shape, and surface area) of a drug molecule have a significant impact on the solubility, absorption, bioavailability, and the therapeutic efficacy [12, 38]. As cholecalciferol is a lipophilic compound and the solubility of it is very poor in water accountable for the poor bioavailability in the body [8,9]. The Consciousness Energy Healing Treated cholecalciferol may show better solubility, absorption, and therapeutic efficacy in the body.
Powder X-Ray Diffraction (PXRD) Analysis
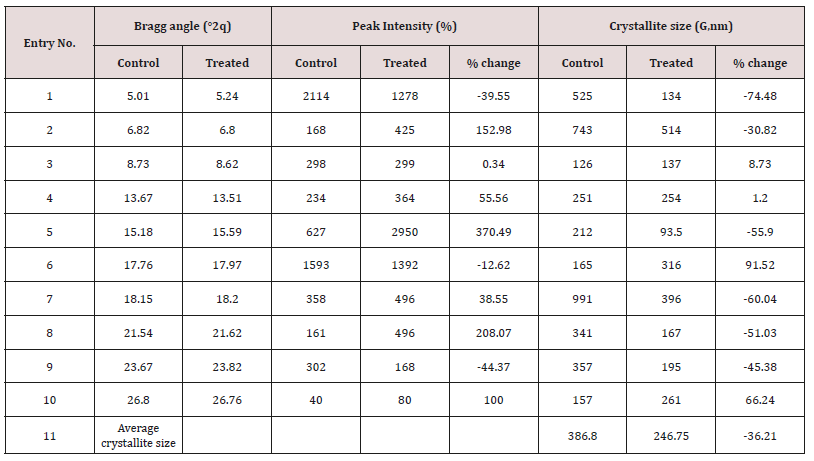
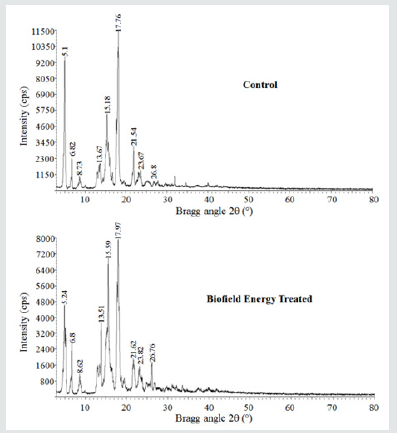
The PXRD diffractograms of both the samples showed sharp and intense peaks (Figure 1), indicated that both the samples were crystalline. The control and Biofield Energy Treated cholecalciferol samples showed the highest peak intensity at 2θ equal to 17.76° and 17.97° in the powder XRD diffractograms (Table 2). The peak intensities of the treated cholecalciferol were significantly altered ranging from -44.37% to 370.49% compared to the control sample. Similarly, the crystallite sizes of the treated cholecalciferol sample were significantly altered ranging from -74.48% to 91.52% compared to the control sample. Largely, the average crystallite size of the Biofield Energy Treated cholecalciferol (246.75 nm) was significantly decreased by 36.21% compared with the control sample (386.8 nm). The change in the peak intensity of the crystalline compound indicated the alterations in the crystal morphology [38]. The alterations in the powder XRD pattern provide the proof of polymorphic transitions [39,40]. The Trivedi Effect®-Consciousness Energy Healing Treatment assumed to be responsible for the new polymorphic form of cholecalciferol probably through the Consciousness Energy via neutrino oscillations [17]. The drug performance, i.e., bioavailability, therapeutic efficacy, and toxicity of the pharmaceutical compound would be different from the original one due to different polymorphic forms [41-43]. Hence, the Consciousness Energy Healing Treated sample would be more efficacious novel pharmaceutical/nutraceutical formulations for the treatment of cholecalciferol deficiency disease.
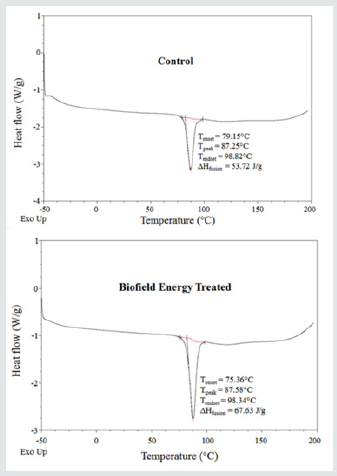
Differential Scanning Calorimetry (DSC) Analysis
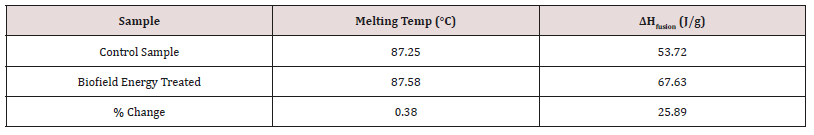
The cholecalciferol control and treated samples showed the sharp endothermic peak in the thermograms (Figure 2). The literature data closely match the experimental results [42]. The melting point and latent heat of fusion (ΔH fusion) of the Biofield Energy Treated cholecalciferol were increased by 0.38% and 25.89%, respectively compared with the control sample (Table 3). The change in the latent heat of fusion can be directly related to the disrupted intrinsic molecular chain and the crystal structure [43]. As per the result the thermal stability of the Consciousness Energy Healing Treated cholecalciferol was improved compared to the thermal stability of the control sample.
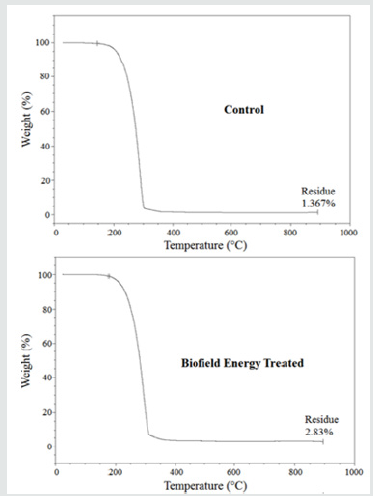
Thermal Gravimetric Analysis (TGA) / Differential Thermogravimetric Analysis (DTG)
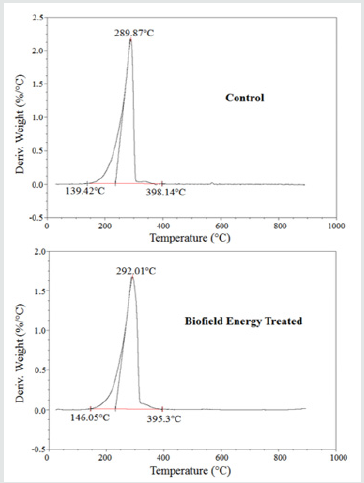
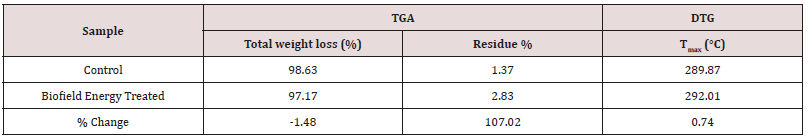
The control and Biofield Energy Treated samples showed one step of thermal degradation (Figure 3). The weight loss of the Biofield Energy Treated cholecalciferol was decreased by 1.48%; whereas, the residue amount was significantly increased by 107.02% compared to the control sample (Table 4). Similarly, the control and Biofield Energy Treated cholecalciferol showed one peak of maximum thermal degradation temperature (Tmax) in the thermograms (Figure 4). The Tmax of the treated sample (292.01°C) was increased by 0.74% compared to the control sample (289.87°C). Overall, thermal analysis data of cholecalciferol samples indicated that the thermal stability of the Biofield Energy Treated sample was increased compared with the control sample.
Conclusions
The Consciousness Energy Healing Treatment (the Trivedi Effect®) has shown the significant effects on the particle and crystallite size, surface area, peak intensity, and thermal behavior of cholecalciferol. The particle size values in the Biofield Energy Treated cholecalciferol powder sample were significantly decreased by 78.94% (d10), 26.21% (d50), 22.01% (d90), and 29.04% {D (4,3)}; thus, the specific surface area of the Biofield Energy Treated cholecalciferol was significantly increased by 174.12% compared to the control cholecalciferol. The powder XRD peak intensities and crystallite sizes of the Biofield Energy Treated cholecalciferol powder sample were significantly altered ranging from -44.37% to 370.49% and -74.48% to 91.52%, respectively; therefore, the average crystallite size of the Biofield Energy Treated sample was significantly decreased by 36.21% compared with the control sample. The ΔH fusion of the Biofield Energy Treated cholecalciferol was significantly increased by 25.89% compared to the control cholecalciferol. The total weight loss was decreased by 1.48%; whereas, the residue amount was significantly increased by 107.02% in the Biofield Energy Treated sample compared with the control sample. Thus, the Trivedi Effect®-Consciousness Energy Healing Treatment generated a new polymorphic form of cholecalciferol which might offer better solubility, absorption, bioavailability, and be thermally more stable compared with the control sample. Henceforth, the Consciousness Energy Healing Treated cholecalciferol would be more beneficial to maintain the overall quality of life and it would be more useful in designing novel nutraceutical/pharmaceutical formulations for the better therapeutic responses against deficiency of vitamin D, osteoporosis, rickets, cardiovascular diseases, diabetes mellitus, cancer, mental disorders, multiple sclerosis, etc.
Acknowledgements
The authors are grateful to Central Leather Research Institute, SIPRA Lab. Ltd., Trivedi Science, Trivedi Global, Inc., Trivedi Testimonials, and Trivedi Master Wellness for their assistance and support during this work.
References
- Kulie T, Groff A, Redmer J, Hounshell J, Schrager S (2009) Vitamin D: An evidence-based review. J Am Board Fam Med 22: 698-706.
- Zhang R, Naughton DP (2010) Vitamin D in health and disease: Current perspectives. Nutr J 9: 65.
- Gouni Berthold I, Krone W, Berthold HK (2009) Vitamin D and cardiovascular disease. Curr Vasc Pharmacol 7(3): 414-422.
- Simana E, Simian R, Portnoy S, Jaffe A, Dekel BZ (2015) Feasibility Study -Vitamin D loading determination by FTIR-ATR. Information & Control Systems 76: 107-111.
- Ritu G, Gupta A (2014) Vitamin D Deficiency in India: Prevalence, Causalities and Interventions. Nutrients 6(2): 729-775.
- Lawson DE, Wilson PW, Kodicek E (1969) Metabolism of vitamin D. A new cholecalciferol metabolite, involving loss of hydrogen at C-1, in chick intestinal nuclei. Biochem J 115(2): 269-277.
- Mattila P, Lehikoinen K, Kiiskinen T, Piironen V (1999) Cholecalciferol and 25-hydroxycholecalciferol content of chicken egg yolk as affected by the cholecalciferol content of feed. J Agric Food Chem 47(10): 4089- 4092.
- Lehmann U, Hirche F, Stangl GI, Hinz K, Westphal S, et al. (2013) Bioavailability of vitamin D (2) and D (3) in healthy volunteers, a randomized placebo-controlled trial. J Clin Endocrinol Metab 98(11): 4339-4345.
- Borel P, Caillaud D, Cano NJ (2015) Vitamin D bioavailability: State of the art. Crit Rev Food Sci Nutr 55(9): 1193-1205.
- Koshy KT, Beyer WF (1984) Vitamin D3 (Cholecalciferol) in Analytical Profiles of Drug Substances. Florey K (Edn.), Academic Press, Inc, Orlando, USA 13: 656-707.
- Collins ED, Norman AW (2001) Vitamin D in Handbook of Vitamins. (3rd edn.) Rucker RB, Suttie JW, McCormick DB, Machlin LJ, Marcel Dekker, Inc, New York, USA, pp. 51-114.
- Chereson R (2009) Bioavailability, bioequivalence, and drug selection. In: Makoid CM, Vuchetich PJ, Banakar UV (Eds) Basic pharmacokinetics (1st edn) Pharmaceutical Press, London, UK.
- Nayak G, Trivedi MK, Branton A, Trivedi D, Jana S (2018) Evaluation of the Effect of Consciousness Energy Healing Treatment on the Physicochemical and Thermal Properties of Selenium. Journal of New Developments in Chemistry 2(1): 13-23.
- Branton A, Trivedi MK, Trivedi D, Nayak G (2018) Evaluation of the physicochemical and thermal properties of the biofield energy healing treated ofloxacin. J Pharm Pharmaceutics 5: 80- 87.
- Branton A, Jana S (2017) The use of novel and unique biofield energy healing treatment for the improvement of poorly bioavailable compound, berberine in male Sprague Dawley rats. American Journal of Clinical and Experimental Medicine 5: 138-144.
- Branton A, Jana S (2017) Effect of the biofield energy healing treatment on the pharmacokinetics of 25-hydroxyvitamin D3 [25(OH)D3] in rats after a single oral dose of vitamin D3. American Journal of Pharmacology and Phyto therapy 2: 11-18.
- Trivedi MK, Mohan TRR (2016) Biofield energy signals, energy transmission and neutrinos. American Journal of Modern Physics 5: 172-176.
- Rubik B, Muehsam D, Hammerschlag R, Jain S (2015) Biofield science and healing: History, terminology, and concepts. Glob Adv Health Med 4: 8-14.
- Barnes PM, Bloom B, Nahin RL (2008) Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 12: 1-23.
- Koithan M (2009) Introducing complementary and alternative therapies. J Nurse Pract 5: 18-20.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O, et al. (2015) Characterization of physical and structural properties of brass powder after biofield treatment. J Powder Metall Min 4: 134.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O (2015) Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after bio field treatment. Ind Eng Manage 4: 161.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Mishra R (2015) Influence of biofield treatment on physicochemical properties of hydroxyethyl cellulose and hydroxypropyl cellulose. J Mol Pharm Org Process Res 3: 126.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Sethi KK, et al. (2016) Isotopic abundance ratio analysis of biofield energy treated indole using gas chromatography-mass spectrometry. Science Journal of Chemistry 4: 41-48.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Panda P, et al. (2016) Gas chromatography-mass spectrometric analysis of isotopic abundance of 13C, 2H, and 18O in biofield energy treated p-tertiary butylphenol (PTBP). American Journal of Chemical Engineering 4: 78-86.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Shettigar H, et al. (2015) Antibiogram of multidrug-resistant isolates of Pseudomonas aeruginosa after biofield treatment. J Infect Dis Ther 3: 244.
- Trivedi MK, Branton A, Trivedi D, Shettigar H, Nayak G, et al. (2015) Assessment of antibiogram of multidrug-resistant isolates of Enterobacter aerogenes after biofield energy treatment. J Pharma Care Health Sys 2: 145.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. J Integr Oncol 4: 141.
- Trivedi MK, Patil S, Shettigar H, Gangwar M, Jana S (2015) In vitro evaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. J Cancer Sci Ther 7: 253-257.
- Nayak G, Altekar N (2015) Effect of a biofield treatment on plant growth and adaptation. J Environ Health Sci 1: 1-9.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Bairwa K, et al. (2015) Physical, thermal, and spectroscopic characterization of biofield energy treated murashige and skoog plant cell culture media. Cell Biology 3: 50- 57.
- Trivedi MK, Sethi KK, Panda P, Jana S (2017) A comprehensive physicochemical, thermal, and spectroscopic characterization of zinc (II) chloride using X‑ray diffraction, particle size distribution, differential scanning calorimetry, thermogravimetric analysis/ differential thermogravimetric analysis, ultraviolet-visible, and Fourier transform‑infrared spectroscopy. International Journal of Pharmaceutical Investigation 7: 33-40.
- Trivedi MK, Sethi KK, Panda P, Jana S (2017) Physicochemical, thermal and spectroscopic characterization of sodium selenate using XRD, PSD, DSC, TGA/DTG, UV-vis, and FT-IR. Marmara Pharmaceutical Journal 21(2): 311-318.
- (1997) Desktop X-ray Diffractometer “MiniFlex+”. The Rigaku Journal 14: 29-36.
- Zhang T, Paluch K, Scalabrino G, Frankish N, Healy AM, et al. (2015) Molecular structure studies of (1S,2S)-2-benzyl-2,3-dihydro-2- (1Hinden-2-yl)-1H-inden-1-ol. J Mol Struct 1083: 286-299.
- Langford JI, Wilson AJC (1978) Scherrer after sixty years: A survey and some new results in the determination of crystallite size. J Appl Cryst 11: 102-113.
- Khadka P, Ro J, Kim H, Kim I, Kim JT, et al. (2014) Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm Sci 9(6): 304-316.
- Raza K, Kumar P, Ratan S, Malik R, Arora S (2014) Polymorphism: The phenomenon affecting the performance of drugs. SOJ Pharm Pharm Sci 1: 10.
- Brittain HG (2009) Polymorphism in pharmaceutical solids in Drugs and Pharmaceutical Sciences, Vol 192, (2nd edn), Informa Healthcare, Inc, New York, USA.
- Censi R, Martino PD (2015) Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 20: 18759-18776.
- Blagden N, de Matas M, Gavan PT, York P (2007) Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv Drug Deliv Rev 59(7): 617-630.
- Vora L, Sita V G, Vavia P (2017) Zero order-controlled release delivery of cholecalciferol from injectable biodegradable microsphere: In-vitro characterization and in-vivo pharmacokinetic studies. European Journal of Pharmaceutical Sciences 107: 78-86.
- Zhao Z, Xie M, Li Y, Chen A, Li G, et al. (2015) Formation of curcumin nanoparticles via solution-enhanced dispersion by supercritical CO2. Int J Nanomedicine 10: 3171-3181.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...