
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Article(ISSN: 2638-6070) 
Chlorophyll Extraction from Wood Plants at Elevated Temperatures
Volume 4 - Issue 1Yao Tsung Lee1, Donyau Chiang2, Mao Kuo Wei3 and Sanboh Lee1*
- 1Department of Materials Science and Engineering, National Tsing Hua University, Taiwan
- 2Taiwan Instrument Research Institute, National Applied Research Laboratories, Taiwan
- 3Department of Materials Science and Engineering, National Dong Hwa University, Taiwan
Received:June 10, 2021; Published:June 22, 2021
*Corresponding author:Sanboh Lee, Department of Materials Science and Engineering, National Tsing Hua University, Hsinchu 30013, Taiwan
DOI: 10.32474/SJFN.2021.04.000178
Abstract
Chlorophyll is a significant component in tea leaves and plays a vital role in health and industrial applications, but its extraction kinetics using solvent did not report in the literature. The chlorophyll extract from Shy Jih Chuen, Ttes No 12, and Chin Shin Oolong were conducted at different temperatures using acetone, ethanol, and methanol. Shy Jih Chuen, which harvests all year round, has the most remarkable chlorophyll extraction rate; Ttes No 12, offspring of Chin Shin Oolong, has a higher extraction rate than Chin Shin Oolong. The amounts of chlorophyll increased with the increase of temperature and time. The chlorophyll extraction from the fresh leaves is faster than that from the dried leaves due to thermal aging. The solvent to decrease the chlorophyll extraction rate follows the sequence acetone > ethanol > methanol, implying that the lower polarity solvent achieves the higher extraction rate because chlorophyll is hydrophobic. A diffusion model coupled with surface wetting is used to explain the extraction kinetics. Surface structure affects the epidermis wetting. The diffusivity, wetting time, and relaxation time satisfy the Arrhenius equation. The activation energies of chlorophyll diffusion and surface wetting are greater for wood plants than for herbaceous plants, implying that the chlorophyll extraction rate of the former is lower than that of the latter.
Keywords:Tea Leaf; Chlorophyll Extraction; Diffusion; Activation Energy
Introduction
Tea is one of the popular beverages consuming more than 3.5 billion cups per day [1-3] due to its antioxidant, anti-inflammatory, cholesterol-lowering, antimicrobial, neuroprotective and anticarcinogenic properties [4]. Many works of literature reported that tea affected significantly on many chronic diseases, such as cancer [5, 6], obesity [7], diabetics [8], and cardiovascular [9-11]. In addition to beverages, tea leaf contains a significant amount of chlorophyll, a valuable bioactive compound. Chlorophyll has antioxidant and antimutagenic properties. Chlorophyll and its derivatives are used as a natural color agent and applied in different manufacturers, including candy, soft drinks, cosmetics, mouthwash, ink, toothpaste, and soap [12,13]. The demand for chlorophyll has dramatically increased, which led to many attempts to extract the chlorophyll from the green plant leaves [14,15].
Chlorophyll in plants confines in chloroplasts where it complexes with phospholipids, polypeptides, and tocopherols and protected by a hydrophobic membrane [16] that water is not a good candidate for chlorophyll extraction. There are two chlorophyll extraction methods of organic solvent extraction and supercritical fluid extraction. This paper focused on organic solvent extraction, which is simple, but its disadvantage is environmental contamination. The organic solvent extraction involves the organic solvent penetrating the cell membrane and dissolving the lipids and the lipoproteins of the chlorophyll membrane, and chlorophyll diffuses out. Simon and Helliwell [17] studies organic solvent extraction of microalgal chlorophyll and found methanol has more extraction amount of chlorophyll than acetone. On the contrary, Hung et al. [18] explored the isothermal chlorophyll extraction from herbaceous plants and illustrated that acetone extracted chlorophyll faster than methanol. The solvent extraction depends on the capability of solvent penetration into the cell membrane for dissolving phospholipids, polypeptides, and tocopherols. Shy Jih Chuen, Ttes No 12, and Chin Shin Oolong, grown popularly in Taiwan, belong to the wood plant. Shy Jih Chuen harvests all year round, and Chin Shin Oolong is one of the most popular drinks in Taiwan. Ttes No 12 is the offspring of Ttes No 8 (mother) and Hard Branch Heart (father, Chin Shin Oolong); that harvest has 20%~50% more than Chin Shin Oolong. We have reported the chlorophyll extraction from the herbaceous plants [18], which prompted us to study chlorophyll extraction from woody plants. The aim is to use a diffusion model coupled with surface wetting based on a thermodynamics approach to analyze the chlorophyll extraction and compared chlorophyll extraction from herbaceous plants and woody plants.
Experimental Procedure
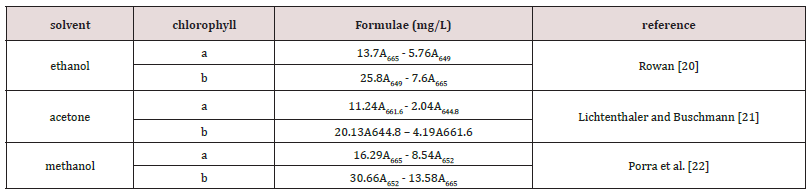
The fresh leaves and dried leaves obtained from the same tea farm, where the Shy Jih Chuen and Ttes No 12 took from Tontas Tea House (Mingjian town, Nantou County, Taiwan) and Chin Shin Oolong was from Chasyu Tea House (Sanzhi Dist. New Taipei City, Taiwan), respectively. As-received leaves were clean with distilled water before the chlorophyll extraction. Methanol, ethanol, and acetone of analytic grade purchased from Echo Chemical Co. (Miaoli, Taiwan). Some researchers reported the detailed procedures of chlorophyll extraction [1,4], and we described it briefly here. The ratios of fresh and dried leaf weights to the solvent volume were 0.25 g to 40 ml and 0.05 g to 40 ml, respectively. Several glass bottles contained solvent placed in a thermostatic water bath maintained at a given temperature. A tea leaf is immersed in the solvent bottle. Then, the 3 mL solution had the extracted chlorophyll poured in a cuvette for the chlorophyll measurement using a spectrophotometer (U-3010, Hitachi High-Technologies Corp, Tokyo, Japan). After eliminating the turbidity line and constraining the spectra from 710 nm to 750 nm to be zero, we obtained the absorbance with spectra wavelengths of 400 nm to 750 nm. The concentrations of chlorophylls a and b dissolved in the different solvents calculated based on the equations listed in Table 1 [2,3,19]. After finishing the absorbance measurement, the 3 mL solution was immediately poured back into the bottle to maintain the original solvent volume. The solvent is added into the bottle to compensate for the solvent evaporation, if necessary. The process of absorbance measurement continued until the extracted chlorophyll concentration reached a plateau.
Figure 2: The plots of amount, Mt, of (a) chlorophyll a and (b) chlorophyll b for Ttes No 12 fresh leaves, (c) chlorophyll a and (d) chlorophyll b for Shy-Jih-Chuen fresh leaves, and (e) chlorophyll a and (f) chlorophyll b for Chin-Shin Oolong fresh leaves extracted by ethanol with time at different temperatures. M0 is the extracted amount at time infinity
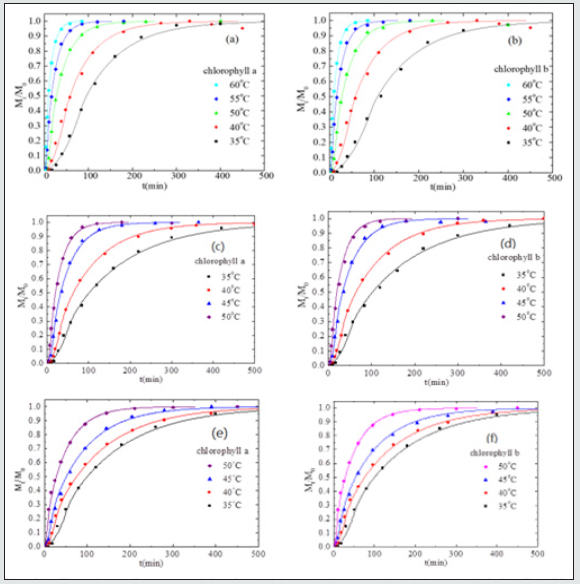
Figure 3: The amount, Mt, of chlorophyll varies with time for the dried leaves of (a) Ttes No 12, (b) Shy-Jih-Chuen, and (c) Chin- Shin Oolong extracted by ethanol at different temperatures
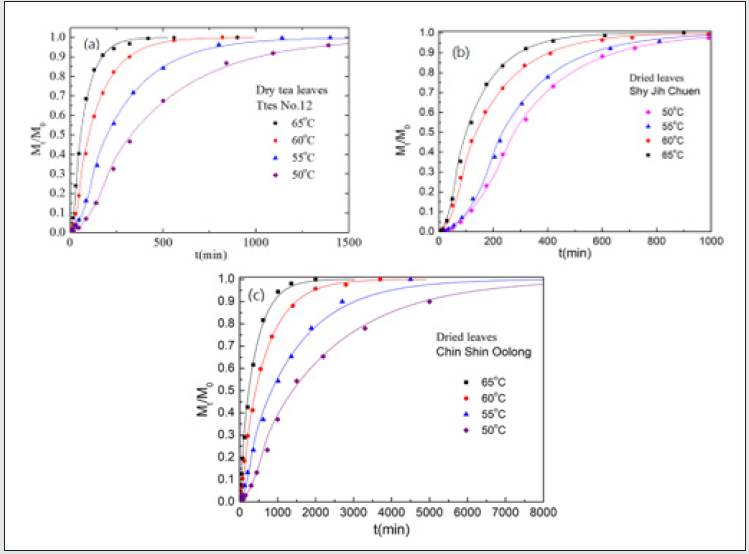
Figure 6: The total chlorophyll amount, Mt extracted by (a) acetone and (b) methanol with time for Shy-Jih-Chuen at different temperatures where M0 is the chlorophyll amount at time infinity.
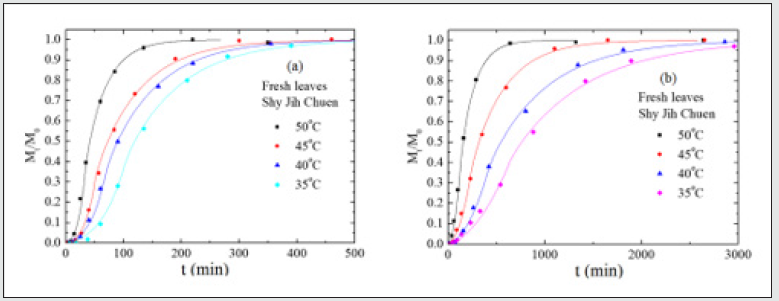
Figure 7: The plots of the total amount, Mt, of chlorophyll extracted from tea leaves of Ttes No12 by (a) acetone, (b) methanol versus extraction time at different temperatures where M0 is the amount of caffeine at time infinity
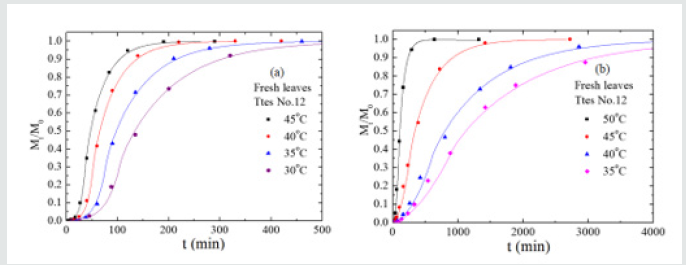
Table 1: The formulae are used to calculate chlorophyll a and b from UV-VIS absorbance at various wavelengths in different solvents. A665 and A649 are the specific absorbance coefficients at 665 nm 649 nm wavelengths, respectively.
Results and Discussion
Figure 1(a), (b), and (c) exhibit the amounts of chlorophyll, Mt, extracted from fresh leaves of Ttes No 12, Shy Jih Chuen, and Chin Shin Oolong by ethanol at different temperatures, respectively, where M0 is the total extracted amount of chlorophyll at time infinity. For a given time, the amount of extracted chlorophyll increases with the increase of temperature in the range from 35 oC to 60 oC. The higher the extracted temperature, the earlier the complete extraction process reaches. For a given temperature, Mt increases gradually initially, climbs rapidly, and finally approaches a plateau. The shape of Mt versus time looks like a character S, and the S-shape becomes more apparent at the lower temperatures. The data of Mt of the extracted chlorophylls a and b versus time for the fresh leaves of tea Ttes No 12, Shy Jih Chuen, and Chin Shin Oolong at different temperatures exhibit in Figs. 2(a), 2(b), 2(c), 2(d), 2(e), and 2(f) in Supplementary Information, respectively. The amount of chlorophyll extracted from fresh leaves of Ttes No 12 shown in Figure 1(a) is the sum of the extracted chlorophylls a and b illustrated separately in Figure 2(a) and 2(b). Similarly, the amount of chlorophyll extracted from fresh leaves of Shy Jih Chuen shown in Figure 1(b) and Chin Shin Oolong shown in Figure 1(c) are the sum of chlorophylls a and b illustrated in Figure S1(c) and S1(d), Figure 2(e) and 2(f), respectively. Again, Mt increases gradually in the beginning, then moves quickly, and finally approaches a plateau with the increase of time. In the following sections, the amount of chlorophyll indicates a sum of chlorophylls a and b except otherwise states.
Instead of fresh leaves, Figure. 3(a), 3(b), and 3(c), respectively, show plots of the amount of chlorophyll versus time at different temperatures for the dried leaves of Ttes No 12, Shy Jih Chuen, and Chin Shin Oolong. To compare the effect of fresh leaves and dried leaves of three tea types on the chlorophyll extraction, we plot the amount of chlorophyll versus time for fresh and dried leaves of three kinds of tea at the temperature of 50 oC shown in Figure 4. The fresh leaves take less than 500 min to complete the chlorophyll extraction process at the temperature of 35 oC, but the dried tea leaves spend 1500 min at 50 oC. Even more significant difference in Chin Shin Oolong, the complete chlorophyll extraction process is longer for dried leaves than for fresh leaves by a factor of 16 times at the lower extraction temperatures (See Fig. 3(c) and Figure 4). The Ttes No 12 has a higher extraction rate than its parent, Chin Shin Oolong, for the same sample preparation.
The experimental data indicated by symbols shown in Figures Figs. 2(a)~(f), Figs. 3(a)~(c), and Figure 4 for the chlorophyll extracted from the fresh and dried leaves of three types of tea by using ethanol can fit by the following equations obtained by Hung et al. [18], who solved the diffusion equation coupling with the surface wetting theory, as
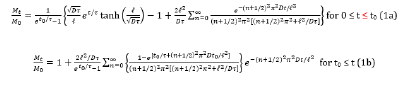
where Mt and M0 are the total extracted amounts of chlorophyll at time t and infinity, respectively. Equations (1a) and (1b) satisfy the initial condition and boundary condition of Eqs. (2) and (3),
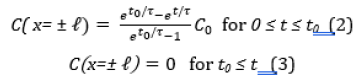
where τ and t0 are structure relaxation time and surface wetting time. C and Co are the concentration at time t and constant at the initial time, respectively. ℓ is the half-thickness of the sample. One can see that the experimental data shown by symbols are in good agreement with the theoretical model indicated by the solid lines. Tables 2, 3, and 4 list the physical dimension of the leaves, leaf weight, the diffusion coefficient, surface wetting time, relaxation time, and the ratio of chlorophyll a to b for Ttes No 12, Shy Jih Chuen, and Chin Shin Oolong, respectively. One can see from Tables 2, 3, and 4 that the extracted amount of the chlorophyll a was higher about 2.6~3.1 times than that of removed chlorophyll b for the fresh leaves, and the ratio of chlorophyll a to chlorophyll b was 4.7~8.3 times for the dried leaves. For example, the total amount of extracted chlorophyll a from the fresh leaves of Ttes No12 at 40 oC was 9.88 mg/L, and that of chlorophyll b at the same condition was 3.65 mg/L. The ratio of chlorophyll a to chlorophyll b is 2.706, which lists a row of a/b of Table 2. The ratio of chlorophyll a to b is in good agreement with the previous reports of approximately 3 to 1 in fresh plant leaves [5,6]. Comparing dried leaves with fresh leaves, the ratios of thickness shrinkage to weight loss are at 64% and 84% for tea Ttes No12, 49% and 82% for Shy Jih Chuen, and 79% and 80% Chin Shin Oolong, respectively. The significant components of weight loss are moisture and volatile chemicals [5]. In summary, chlorophyll a is dominant for all tea plants regardless of the tea process.
Table 2: The chlorophyll diffusion coefficient, D, surface wetting time, t0, relaxation time, τ, leaf thickness, 2ℓ, leaf weight, Mleaf, and chlorophyll ratio a/b of fresh and dried leaves tea Ttes No12 using 99.5% ethanol.
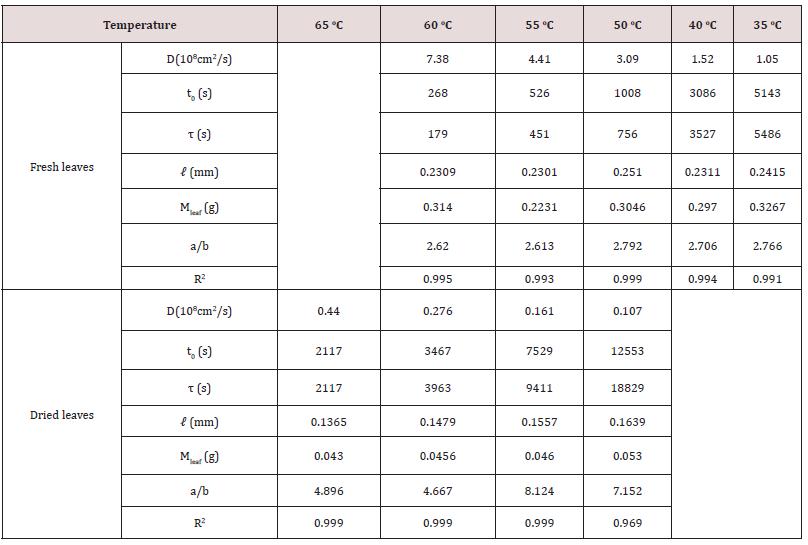
Table 3: The chlorophyll diffusion coefficient, D, surface wetting time, t0, relaxation time, τ, leaf thickness, 2ℓ, leaf weight, Mleaf, and chlorophyll ratio, a/b, of fresh and dried leaves of tea Shy Jih Chuen using 99.5% ethanol.
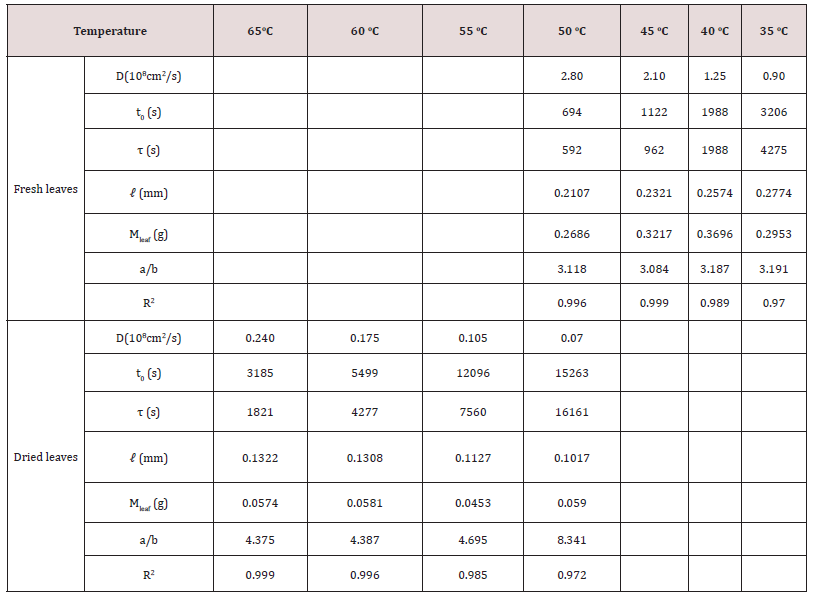
Table 4: The chlorophyll diffusion coefficient, D, surface wetting time, t0, relaxation time, τ, leaf thickness, 2ℓ, leaf weight, Mleaf, and chlorophyll ratio, a/b, of fresh and dried leaves of tea Chin Shin Oolong using 99.5% ethanol.
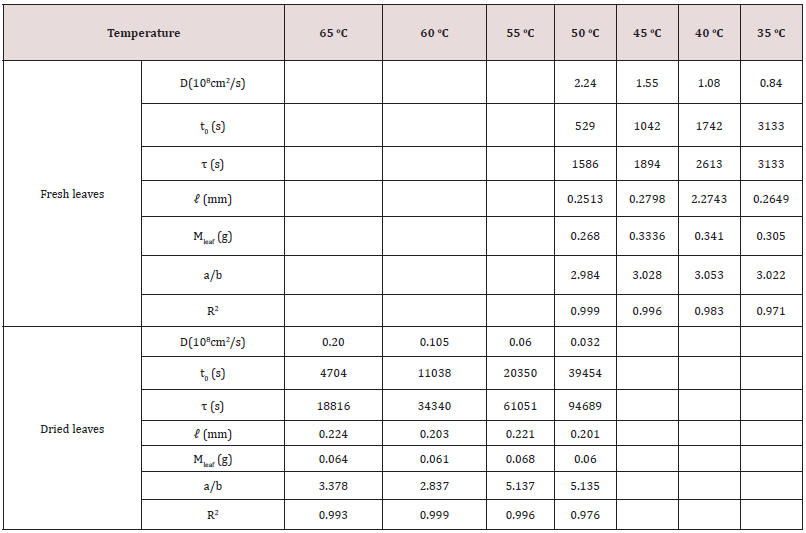
Table 5: The diffusion coefficient, D, surface wetting time, t0, relaxation time, τ, leaf thickness, 2ℓ, of fresh leaves of tea Shy Jih Chuen using three solvents to extract the chlorophyll and their activation energies.
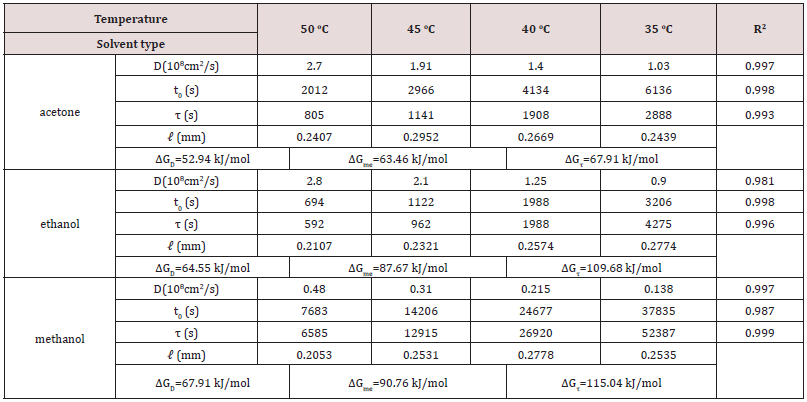
Table 6: Diffusion coefficient D, surface wetting time t0, relaxation time τ, leaf thickness 2ℓ of fresh leaves of tea Ttes No 12 using different solvents to extract the chlorophyll and activation energies.
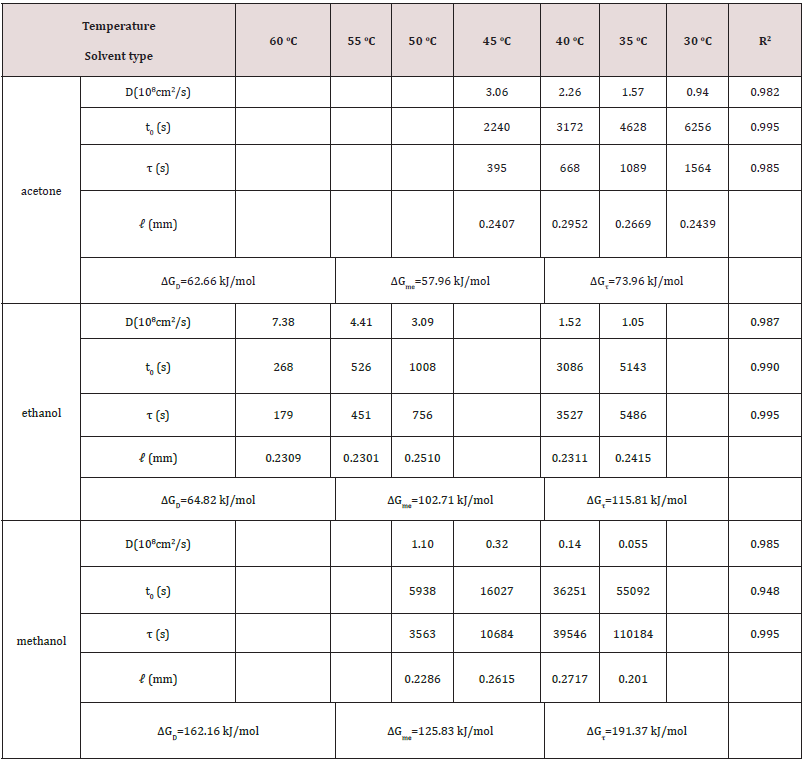
Figure 1: The plots of the amount of chlorophyll, Mt, extracted from fresh leaves of (a) Ttes No 12, (b) Shy Jih Chuen, and (c) Chin Shin Oolong conducted at different temperatures using ethanol versus time where M0 is the extracted amount of chlorophyll at time infinity
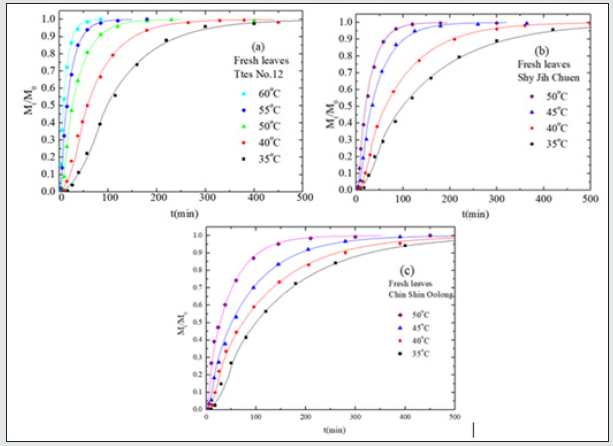
Figure 4: The amount of total chlorophyll, Mt, as a function of time for fresh leaves and dried leaves of three teas conducted at 50 oC using ethanol where M0 is the total chlorophyll at time infinity.
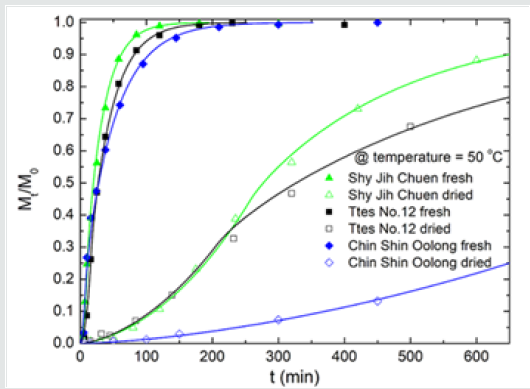
Figure 5: The plots of (a) log(D), (b) log(T/t0), and (c) log(1/) versus 1/T for the total chlorophyll extracted from fresh and dried leaves of three types of tea using ethanol.
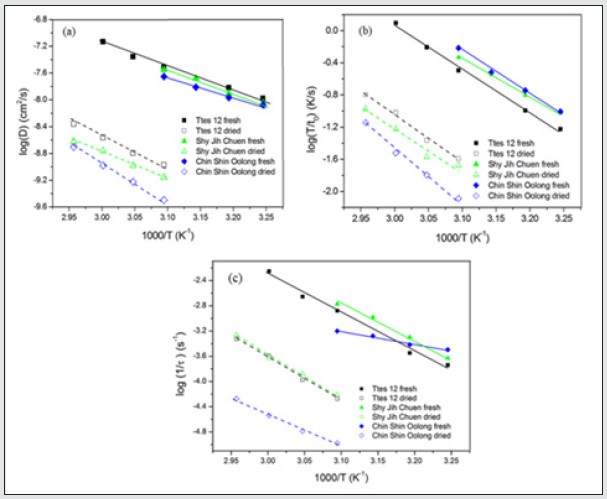
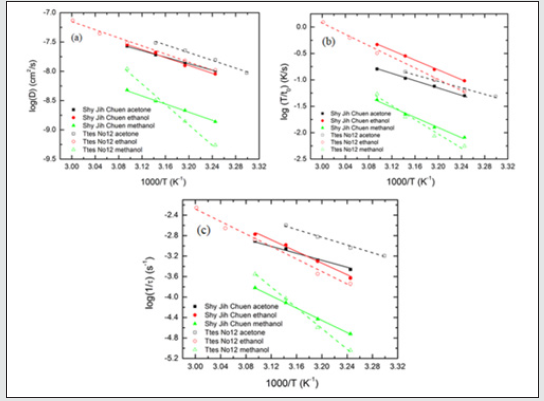
Figure 8: The plots of log(D), (b) log(T/t0), and (c) log(1/) versus 1/T for the chlorophyll extraction from fresh leaves of Shy Jih Chuen and Ttes No 12 using different solvents.
Figures 5(a), 5(b), and 5(c) show the plots of diffusion coefficient, surface wetting time, and structure relaxation time for both fresh and dried leaves of three types of tea using ethanol, respectively. All lines satisfy the Arrhenius relation. Diffusion, surface wetting, and structure relaxation are thermal-activated processes with activation energies (or energy barriers). Because of the significant differences in diffusion coefficient, surface wetting time, and structure relaxation time between the fresh leaves and dried leaves, one observed the two regimes in Figure 5. For a given temperature and the same tea species, the diffusion coefficient of fresh leaves is ca. 30 times higher than that of dried leaves. The Ttes12 has a more significant diffusion coefficient for a given temperature than its parent, Chin Shin Oolong, for fresh and dried leaves. However, their slopes of the two regimes are similar except that the slope of the relaxation time of fresh leaves of Chin Shin Oolong possesses the smallest value. When the extracted temperature increases from 35 oC to 50 oC for fresh leaves of Chin Shin Oolong, the relaxation time increases approximately twice.
Nevertheless, a difference of seven times in relaxation time of the fresh leaves occurs for the other two tea types. The slight change of relaxation time in Chin Shin Oolong does not significantly affect the chlorophyll extraction efficiency compared to the rest two tea types. From the slope, we obtain the activation energies of chlorophyll diffusion obtained for fresh leaves of Ttes No 12, Shy Jih Chuen, and Chin Shin Oolong being 64.82, 64.55, and 54.58 kJ/ mol, respectively, and those for dried leaves of Ttes No 12, Shy Jih Chuen, and Chin Shin Oolong being 86.75, 73.86, and 110.93 kJ/ mol. One expects that for all types of tea trees, the activation energy of chlorophyll extracted from the fresh leaf by ethanol is smaller than that extracted from the dried leaf. The energy barriers of surface wetting for fresh leaves of Ttes No 12, Shy Jih Chuen, and Chin Shin Oolong are 102.71, 87.67, and 99.37 kJ/mol, and those energy barriers for dried leaves of Ttes No 12, Shy Jih Chuen, and Chin Shin Oolong are 113.74, 97.01, and 137.65 kJ/mol, respectively. The energy barrier of surface wetting is smaller for the fresh leaf than for the dried leaf, regardless of the type of tea. The activation energies of relaxation time for fresh leaves of Ttes No 12, Shy Jih Chuen, and Chin Shin Oolong are 115.81, 109.68, and 28.51 kJ/mol, and those energies of relaxation time for dried leaves of Ttes No 12, Shy Jih Chuen and Chin Shin Oolong are 134.73, 130.10, and 108.46 kJ/mol, respectively. For ethanol extraction, the fresh leaf has smaller activation energies of structure relaxation than the dried leaf for all types of tea. Because of the larger diffusivity and the smaller surface wetting time and structure relaxation time for fresh leaves, the chlorophyll extraction rate is more significant for the fresh leaves of three types of tea than for the dried leaves for all three species. Note that fresh leaves have more chlorophyll than dried leaves due to thermal aging. Figs. 6(a) and 6(b) show the chlorophyll extracted from the fresh leaves of the Shy Jih Chuen using acetone and methanol at different temperatures, respectively. Similarly, Figures 7(a) and 7(b) exhibit chlorophyll extraction from the fresh leaves of Ttes No 12 using acetone and methanol, respectively. One obtains the solid lines in Figures 6 and 7 using Eq. (1), and tabulates the diffusion coefficient, D, surface wetting time, t0, relaxation time, τ, and leaf thickness 2ℓ in Tables 5 and 6 for fresh leaves Shy Jih Chuen and Ttes No 12, respectively. In addition to acetone and methanol, Tables 5 and 6 also include the parameters of D, t0, τ, and 2ℓ for the fresh leaves of both tea types using ethanol. The times to approach the extraction plateau are roughly 500 min for the fresh leaves of Shy Jih Chuen and Ttes No 12 extracted by the ethanol and acetone, respectively. However, it takes over six times longer to approach a plateau using methanol than using the other two solvents.
From the data of Tables 5 and 6, we plot the curves of the logarithm of D, T/t0, and 1/τ to 1/T for fresh leaves of Shy Jih Chuen and Ttes No 12 extracted by three solvents in Figs. 8(a), 8(b) and 8(c), respectively. All lines satisfy the Arrhenius plot, and one calculates the corresponding activation energies listed in Tables 5 and 6. One can see from Fig. 8 that the lines corresponding to extracted acetone and ethanol are higher than that corresponding to methanol. Compared to ethanol and acetone, the chlorophyll extraction from fresh tea leaves using methanol has a smaller D and larger t0 and τ than ethanol and acetone. That is, methanol has the lowest efficiency of chlorophyll extraction among the above three solvents. The activation energies of chlorophyll diffusion (ΔGD), surface wetting (ΔGme), and structure relaxation (ΔGτ) processes for Shy Jih Chuen are 52.94, 63.46, and 67.91 kJ/mol using acetone, 64.55, 87.67, and 109.68 kJ/mol using ethanol, and 67.91, 90.76, and 115.04 kJ/mol using methanol, respectively. The activation energies of chlorophyll diffusion, surface wetting, and structure relaxation processes for Ttes No12 are 62.66, 57.96, and 73.96 kJ/ mol using acetone, 64.82, 102.71, and 115.81 kJ/mol using ethanol, and 162.16, 125.83, and 191.37 kJ/mol using methanol, respectively. The relative polarities of methanol, ethanol, and acetone are 0.767, 0.654, and 0.355, respectively [23]. The polarity strength of the solvent arranged in ascending order is methanol > ethanol > acetone. Note that the relative polarity of water is unity. The higher the polarity of the solvent used to extract the chlorophyll from the fresh leaves of the teas is, the larger the activation energies of the extraction process are. One infers that it is more difficult to extract the chlorophyll from the tea leaves and complete the extraction using a solvent with greater polarity. It is because chlorophyll is hydrophobic. Note that water was not found to extract chlorophyll in plants. Hung et al. [18] studied chlorophyll extracted from herbaceous plants by ethanol and acetone. The activation energies of chlorophyll diffusion in creep oxalis, nodalflower synedrella, and broomjute are 14.75±0.65, 5.89±0.09, and 8.17±0.23 kJ/mol using 80% acetone, whereas that in creep oxalis is 22.25±075 kJ/ mol using ethanol. For the same solvent, the activation energy of diffusion in herbaceous plants is lower than that in woody plants. The diffusion coefficient of chlorophyll in herbaceous plants is greater than that in wood plants by three orders of magnitude. On the other hand, the activation energies of surface wetting in creep oxalis, nodalflower synedrella, and broomjute are 47.8, 21.75±0.15, and 30.2 kJ/mol using 80% acetone, and 40.0±0.4 kJ/mol in creep oxalis using ethanol. Again for the same solvent, the energy barriers of chlorophyll to cross surface in herbaceous plants is lower than those in woody plants. In summary, herbaceous plants have more efficiency in chlorophyll extraction than woody plants using a solvent.
Summary and Conclusions
Chlorophyll is a significant component in tea leaves, which is essential to human health and industrial applications. Chlorophyll extraction from the fresh leaves and dried leaves of Shy Jih Chuen, Ttes No 12, and Chin Shin Oolong using methanol, ethanol, and acetone are studied where Ttes No 12 is an offspring of Chin Sin Oolong, and Shy Jih Chuen harvests all year round. The Shy Jih Chuen has the most remarkable chlorophyll extraction rate among three tea species for a given temperature. In the comparison of the dried leaves to the fresh leaves on extraction efficiency, the former has higher efficiency of chlorophyll extraction than the latter due to thermal aging. The dried leaves have a higher chlorophyll extraction rate for Ttes No 12 than for Chin Shin Oolong at the same temperature and solvent. The increasing efficiency of chlorophyll extraction follows the sequence methanol, ethanol, and acetone opposite to relative polarity because chlorophyll is hydrophobic. We infer that water is a poor solvent using chlorophyll extraction. We propose a diffusion model coupled with surface wetting to explain the chlorophyll extraction. The activation energies of diffusion and surface wetting for Shy Jih Chuen, Ttes No 12, and Chin Shin Oolong for a given solvent such as acetone, ethanol, and methanol are greater than those for creep oxalis, nodalflower synedrella, and broomjute, respectively. We conclude that herbaceous plants have more efficiency in chlorophyll extraction than woody plants using a solvent.
Acknowledgment
We would like to thank the Ministry of Science and Technology, Taiwan, for financial support. We declare that we have no interests in conflict.
References
- Franks M, Lawrence P, Abbaspourrad A, Dando R (2019) The influence of water composition on flavor and nutrient extraction in green and black tea. Nutrients 11(1): 80.
- Roshanak S, Rahimmalek M, Goli SAH (2016) Evaluation of seven different drying treatments in respect to total flavonoid, phenolic, vitamin C content, chlorophyll, antioxidant activity and color of green tea (Camellia sinensis or C. assamica) leaves. J Food Sci Technol 53(1): 721-729.
- Vuong QV (2014) Epidemiological evidence linking tea consumption to human health: a review. Crit Rev Food Sci Nutr 54(4): 523-536.
- Sanlier N, Gokcen BB, Altuğ M (2018) Tea consumption and disease correlations. Trends in Food Science & Technology 78: 95-106.
- Lee AH, Su D, Pasalich M and Binns CW (2013) Tea consumption reduces ovarian cancer risk. Cancer Epidemiol 37(1): 54-59.
- Mao JT, Nie WX, Tsu IH, Jin YS, Rao JY, et al. (2010) White tea extract induces apoptosis in non–small cell lung cancer cells: the role of peroxisome proliferator-activated receptor-γ and 15-lipoxygenases. Cancer Prev Res 3(9): 1132-1140.
- Cheng M, Zhang X, Miao Y, Cao J, Wu Z, et al. (2017) The modulatory effect of (-)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3 ″Me) on intestinal microbiota of high fat diet-induced obesity mice model. Food Res Int 92: 9-16.
- Fu QY, Li QS, Lin XM, Qiao RY, Yang R, et al. (2017) Antidiabetic effects of tea. Molecules 22(5): 849.
- Pang J, Zhang Z, Zheng Tz, Bassig BA, Mao C, et al. (2016) Green tea consumption and risk of cardiovascular and ischemic related diseases: A meta-analysis. Int J Cardiol 202: 967-974.
- Zhong W, Huan XD, Cao Q, Yang J (2015) Cardioprotective effect of epigallocatechin-3-gallate against myocardial infarction in hypercholesterolemic rats. Exp Ther Med 9(2): 405-410.
- Deka A, Vita JA (2011) Tea and cardiovascular disease. Pharmacol Res 64(2): 136-145.
- da Silva Ferreira V, Sant’Anna C (2017) Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J Microbiol Biotechnol 33(1): 20.
- Khanra S, Mondal M, Halder G, Tiwari O, Gayen K (2018) Downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: A review. Food and bioproducts processing 110: 60-84.
- Miazek K, Ledakowicz S (2013) Chlorophyll extraction from leaves, needles and microalgae: A kinetic approach. International Journal of Agricultural and Biological Engineering 6(2): 107-115.
- Park HS, Im NG, Kim KH (2012) Extraction behaviors of caffeine and chlorophylls in supercritical decaffeination of green tea leaves. LWT-Food Science and Technology 45(1): 73-78.
- Humphrey AM (2004) Chlorophyll as a color and functional ingredient. Journal of Food Science 69(5): 422-425.
- Simon O, Helliwell S (1998) Extraction and quantification of chlorophyll a from freshwater green algae. Water Resource 32(7): 2220-2223.
- Hung SM, Hsu BD, Lee S (2014) Modelling of isothermal chlorophyll extraction from herbaceous plants. Journal of Food Engineering 128: 17-23.
- Hu J, Webster D, Cao J, Shao A (2018) The safety of green tea and green tea extract consumption in adults–Results of a systematic review. Regul Toxicol Pharmacol 95: 412-433.
- Rowan KS (1989) Photosynthetic pigments of algae. Cambridge University Press, Cambridge, UK.
- Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: Measurement and characterization by UV‐VIS spectroscopy. Current protocols in food analytical chemistry 1: F4. 3.1-F4. 3.8.
- Porra R, Thompson W, Kriedemann P (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta (BBA)-Bioenergetics 975(3): 384-394.
- Reichardt C (2003) Solvents and Solvent Effects in Organic Chemistry. Wiley-VCH Publishers, Weinheim, Germany, 3rd ed.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




