Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Aticle(ISSN: 2638-6070) 
Blood Characteristics and Tissue Histology of Nile Tilapia (Oreochromis Niloticus Niloticus) Fed A Diet Containing Cheese Skipper (Piophila Casei) Larvae
Volume 2 - Issue 3Alaa G. M. Osman1,2*, Ali G. Gadel-Rab1, Fatma A. Mahmoud3, Heba S. Hamed4, Mohamed M. Elshehaby1, Ahmed E. Ali1 and Werner Kloas2,5
- 1Department of Zoology, Faculty of Science, Al-Azhar University (Assiut branch), Egypt
- 2Department of Ecophysiology and Aquaculture, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Germany
- 3Department of Zoology, Faculty of Science, Assiut University, Egypt
- 4Department of Zoology, Faculty of Women for Arts, Science and Education, Ain Shams University, Cairo, Egypt
- 5Department of Endocrinology, Institute of Biology, Faculty of Life Sciences, Humboldt University of Berlin, Germany
Received: September 26, 2019; Published: October 15, 2019
*Corresponding author: Alaa Osman, Department of Zoology, Faculty of Science, Al-Azhar University [Assiut branch], 71524 Assiut, Egypt
DOI: 10.32474/SJFN.2019.02.000137
Abstract
A three-month laboratory feeding trial was conducted to evaluate the suitability of cheese skipper larvae [maggots] as an alternative protein source for Nile tilapia (Oreochromis niloticus niloticus) instead of fishmeal. The diets tested were a commercial diet (Diet 1, 0% maggot inclusion) and a maggot diet (Diet 2, 100% maggot inclusion). The values obtained for both treatment groups indicated nutritional adequacy of the diets. Non-significant differences were observed between both treatment groups for nearly all the hematological parameters. A marked increase in the total protein, ALT, AST and triglyceride levels was observed in the blood of fish fed the maggot diet compared to the levels in the blood of fish fed the commercial diet, suggesting health improvement in fish fed the maggot diet. The replacement of fishmeal with maggot meal is acceptable from a growth perspective and in terms of the observed histological architecture. The results show that the maggot diet can be conveniently used as a total replacement for fishmeal in the diet of Nile tilapia.
Abbreviation: Aquaculture; Maggot Diet; Nile Tilapia; Blood Characteristics; Histology
Introduction
Approximately 60% of the fish body is protein, representing a very good source of inexpensive protein for the growing world population. This aspect has led to the development of aquaculture to increase fish production to meet the demand [1]. The aquaculture industry is the fastest growing food production industry in the world, accounting for 50% of all fish consumed by humans [2]. The cost of aquafeed is the main cost factor in aquaculture, accounting for approximately 70% of a fish farming venture [3]. The major protein source and preferred choice in aquafeed is fishmeal due to the high quality of the protein with a nearly balanced amino acid profile [4]. Fishmeal is the most expensive component, leading to an exponential increase in the price of fish feed. Therefore, it was necessary to search for substitute protein sources such as inexpensive plant proteins [e.g., soybean meal, sunflower, cottonseed meal, rapeseed meal] or animal proteins [e.g., shrimp waste, earthworms and insect] [5-8]. These sources have high potential for supplying fish with the protein needed for maximum productivity [9, 10]. Insects have been employed to produce fish feed. Maggot larvae of flies or other insects have the ability to grow on a wide range of substrates and seem to be candidates for the replacement of fishmeal in fish diets [11-13]. Maggot meal has been reported to be highly nutritive, with the crude protein content ranging between 43.9 and 62.4%, lipid content between 12.5 and 21%, and crude fiber content between 5.8 and 8.2% [14-16]. Maggot meal is also rich in phosphorus, trace elements and vitamin B complex.
Blood characteristics are effective and sensitive indices for monitoring physiological changes in fishes. Analysis of blood indices has proven to be a valuable approach for examination of the health status of farmed fish, providing reliable information on metabolic disorders [17] and becoming a basic part of fish health monitoring programmes [18,19]. The ingestion of numerous dietary supplements has measurable effects on blood constituents [20]. According to Maxwell et al. [21], blood parameters are important for assessing the quality and suitability of feed ingredients for farm animals. Alteration in fish histology has been examined to identify changes, if any, in the tissue of fishes fed alternative feeds. The digestive system of teleostean fishes has been widely studied and described morphologically to determine the functions of many specialized anatomical structures in relation to different feeding adaptations [22]. Histological analysis of the digestive system is considered to be a good and immediate indicator of the nutritional status of fish [23,24]. Histological methods used for assessment of different feed effects on the livers and intestines of fishes were reviewed and explained by Raskovic et al. [25]. The intestine and the liver are the most important organs involved in the digestion and absorption of nutrients from ingested food; therefore, monitoring of these organs is considered to be necessary [26] for assessing the effects of ingredients used as raw materials of animal/plant origin. In this study, blood characteristics and tissue histology were used to evaluate the suitability of cheese skipper larvae [maggots] as an alternative protein source for tilapia (Oreochromis niloticus niloticus).
Material and Methods
Experimental design
A total of 120 healthy Nile tilapia (O. niloticus niloticus) were used in the present work. Nile tilapias [average initial weight 28-41 g and length 11-13.4 cm] were collected from the Nile River at Assiut, Egypt. The fish were acclimated in the laboratory for at least 21 days. During acclimatization, the fish were fed a commercial pellet diet twice per day and kept in a recirculation system, ensuring high water quality (dissolved oxygen: 5.6 mg/L, pH: 7.9, total NH3-N: 0.097 mg/L, and temperature: 25.5 °C). The composition of the commercial pellet diet is provided in Table 1. After acclimatization, the fish were divided into two groups. The first group was fed a commercial diet (Diet 1), and the second group was fed a maggot diet (Diet 2) (Table 1). Each group was assessed in triplicate. Experimental tanks were regularly cleaned, and faecal matter was siphoned out daily.
Sources of ingredients and diet preparation
Soybean meal, wheat bran, rice bran, mix oil, premix, dicalcium phosphate, and fishmeal were obtained locally from the market. The maggot meal used for this study was prepared in the laboratory during the experiment using the larval stage of Piophila casei skippers (fly larvae). The larvae were collected from conventionally prepared cheese by the floating method. The homemade cheese was mixed with running water, and the larvae that floated were collected with a sieve. Maggots were harvested, washed, killed in tepid water and dried for 36 hours at 60 °C in an oven. Dried samples were milled using mortar and pestle. The maggot powder was added to the components of the experimental fish food according to the recommended amounts listed in Table 1. Two test diets were formulated. Diet 1 (commercial diet) was formulated with the highest inclusion level of fishmeal and without maggot meal. Diet 2 (maggot diet) was formulated with the highest inclusion level of maggot meal and without fishmeal (Table 1). All dry diet components, including vitamins and mineral mixtures, were thoroughly mixed with oil. Water was added, and the feed was pressed into pellets that were 1 mm in diameter. The wet pellets were dried for 3 days at room temperature and stored at -2 °C until use.
Blood sampling
At the beginning and end of the experiment, 9 fish from each treatment were randomly selected for blood sampling. No anesthetic was applied to the fish, as anesthetics can affect blood parameters. Two samples of peripheral blood were collected by cardiac puncture as described by Osman et al. [27]. The first sample was freshly collected in small glass tubes containing heparin solution [0.2 ml/ml blood] as an anticoagulant. This sample was used for hematological analysis. The second sample was collected and left to coagulate for 15–20 min at 4 °C prior to centrifugation for 20 min at 3,000 rpm to separate the serum. The fresh serum was subjected to biochemical analysis.
Haematological analysis
Whole-blood samples were used for estimation of haemoglobin concentration (Hb), hematocrit(Hct), red blood cell count (RBC), and white blood cell count (WBC) using an automated technical analyzer (Celtic α MEK-6400J/K, TOKYO, JAPAN). The mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration [MCHC] were calculated as described by Dacia and Lewis [28].
MCHC [g/dl] = Hb / Hct × 100
MCH [pg] = Hb / RBC × 10
MCV [mm3] = Hct/RBC × 10
Biochemical analysis
Colorimetric determination of the selected biochemical parameters was performed using a spectrophotometer [Jasco-V530]. The absorbance of the sample was examined at an appropriate wavelength within a range of 340 to 546 nm according to the parameter tested. Commercial diagnostic kits from Biomatrix chemicals were used for assays of total protein content (g/dl) as described by Henry [29], cholesterol (mg/dl) as described by Thomas [30], triglycerides (mg/dl)as described by Friedewald et al. [31], calcium(Ca, mg/dl) as described by Fiereck [32], creatinine and urea (mg/dl) as described by Henry [29], glucose (mg/dl) as described by Trinder [33], and aspartate aminotransferase (AST, U/I)and alanine aminotransferase (ALT, U/I) as described by Reitman [34].
Histological analysis
At the end of the experimental period, three fish from each tank were sacrificed by decapitation. The livers and intestines were immediately dissected, fixed in 10% neutral buffered formalin, processed by conventional methods, sectioned at 3-5-μm thickness using a rotary microtome, and stained with hematoxylin-eosin [35]. PAS staining was also performed on the liver sections to discriminate the PAS-positive reactions caused by the presence of mucopolysaccharides and glycoproteins. The sections were examined under a light microscope (Motic microscope BA310 LED FL) and photographed using a DVC digital camera (HDCE-50 B). Twenty measurements of villus height (μm)were obtained using ImageJ (1.46) software. Baeverfjord and Krogdahl’s [36] method was used to count goblet cells.
Statistical analysis
Data are presented as the means ± standard deviations. The data were analysed by one-way analysis of variance [ANOVA] using a data analysis software system [37]. Means were tested using Fisher’s least significant difference [LSD] test. Two levels of significance were reported; *p<0.05; **p<0.01.
Results
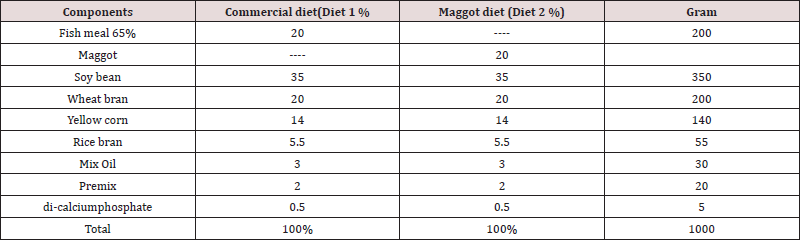
Haematological parameters: Significantly (P<0.05) increased RBC, Hb, and MCH values were recorded in the blood of fish fed the commercial diet and those fed the maggot diet in the final samples compared to the initial blood samples (Table 2) Non-significant (P>0.05) differences were observed in the MCV, MCHC, Hct, and WBC values between the initial and final blood samples from fish fed the commercial diet and those fed the maggot diet (Table 2). Non-significant (P>0.05) differences were observed in the final RBC and WBC values between the blood samples from fish fed the commercial diet and those fed the maggot diet (Table 2). At the same time, the final values of Hb, MCH, MCV, and MCHC were higher in the blood samples from fish fed the commercial diet than in the blood samples from those fed the maggot diet (Table 2). In contrast, the final Hct concentration was higher in the blood samples from fish fed the maggot diet than in the blood samples from those fed the commercial diet (Table 2). The final lymphocyte concentration was lower than the initial lymphocyte concentration in the blood of fish fed the commercial diet. The lymphocyte concentration exhibited a non-significant (P>0.05) increase in the blood of fish fed the maggot diet compared to those fed the commercial diet (Table 2). The neutrophil concentration exhibited a non-significant decrease in the blood of fish fed the maggot diet. The final neutrophil concentration was higher in the blood of fish fed the commercial diet than in the blood of fish fed the maggot diet (Table 2). The monocyte concentration exhibited a marked decrease in the blood of fish fed the commercial diet and those fed the maggot diet. The final monocyte concentration was the same for both diets (Table 2). A significant (P<0.05) increase in the final concentration of eosinophils was observed compared to the initial concentration in the blood of fish fed the commercial diet and those fed the maggot diet. No significant difference was observed in the concentration of eosinophils between the fish fed the commercial diet and those fed the maggot diet (Table 2).
Table 2: Changes in the blood hematological parameters (mean ± SD) in the blood of Nile tilapia Oreochromis niloticus niloticus fed on commercial diet and Maggot diet for 12 weeks.
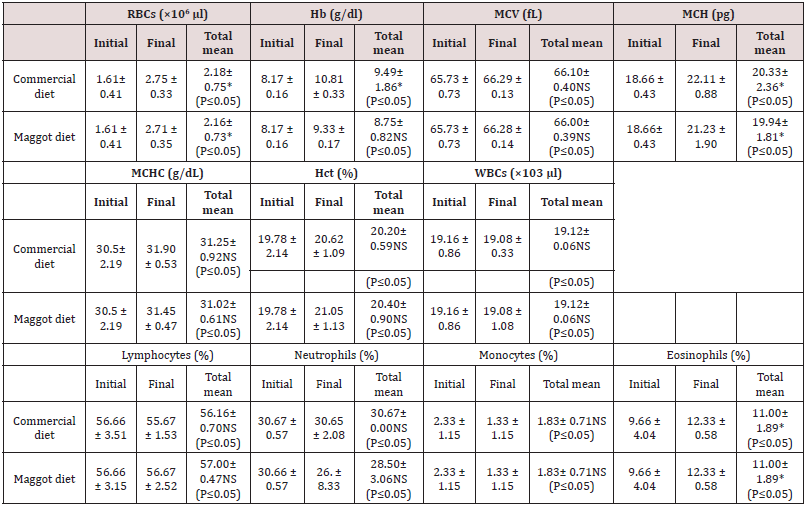
Blood Biochemistry: Significantly (P<0.05) increased final levels of total protein, cholesterol, and AST, compared to the initial levels, were observed in the blood samples from fish fed the commercial diet and those fed the maggot diet. Significantly (P<0.05)reduced final levels of glucose, triglycerides, and creatinine were observed in the blood samples from fish fed the commercial diet and those fed the maggot diet. The calcium level exhibited a significant (P<0.05) increase in the blood of fish fed the commercial diet and a significant (P<0.05)reduction in the blood of fish fed the maggot diet. Non-significant changes were observed in the levels of blood urea and ALT in the blood samples from fish fed the commercial diet and those fed the maggot diet (Table 3). Total protein, triglyceride, creatinine, ALT, and AST levels were significantly (P<0.05) higher in the blood of fish fed the maggot diet (Diet 2) than in the blood of those fed the commercial diet (Diet 1) (Table 3). In contrast, glucose, cholesterol, and calcium concentrations were significantly higher in the blood of fish fed the commercial diet than in the blood of those fed the maggot diet (Table 3). Non-significant changes were observed in the levels of urea and ALT in the blood of fish fed the commercial diet compared to the levels in the blood of those fed the maggot diet (Table 3).
Table 3: Changes in the blood biochemical variables (mean ± SD) in the blood of Nile tilapia fed on commercial diet and Maggot diet for 12 weeks.
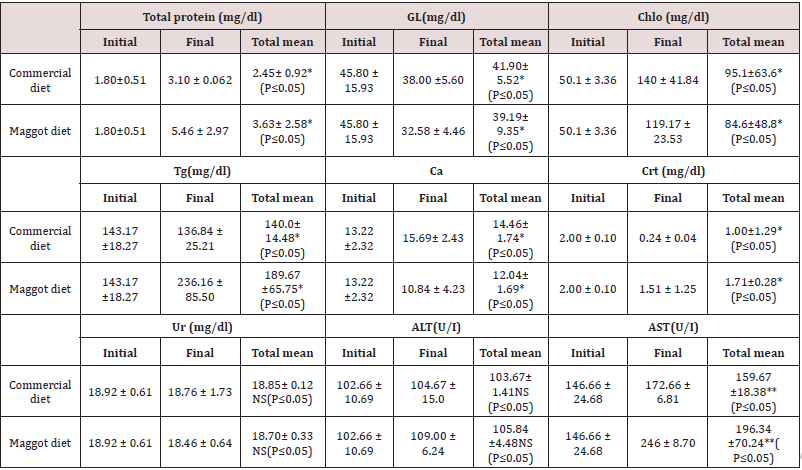
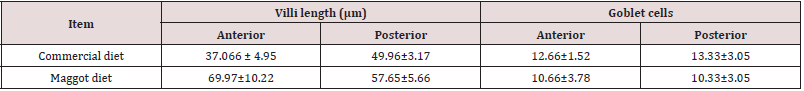
Histological Alterations: The experimental diets used in the present study showed minor impacts on the histological structures of the intestine and liver tissues of Nile tilapia, O. niloticus niloticus. Analysis of the intestinal structures of fish fed the commercial diet and those fed the maggot diet showed normal architecture, with a mucosa, sub-mucosae, a muscular layer and a serosa (Figure 1). The anterior intestine of fish fed the commercial diet exhibited normal architecture, with circular muscles, longitudinal muscles, a serosa and short villi. A large number of small goblet cells were observed (Figure 1). The anterior intestine of fish fed the maggot diet exhibited normal architecture, with longer villi than those observed in fish fed the commercial diet. Small goblet cells were also observed in the intestines of fish fed the maggot diet (Table 4 and Figure 1). The posterior intestine of fish fed the commercial diet showed a narrow lumen and short and weak branched villi with a wide lamina propria. Normal appearance of circular muscles and sub-mucosae were observed. Large numbers of small goblet cells were observed (Table 4 & Figure 2). On the other hand, the posterior intestine of fish fed the maggot diet showed a wide lumen, long and branched villi, a narrow lamina propria, and normal appearance of the muscularis and sub-mucosae. Few goblet cells were observed in these samples (Table 4 and Figure 2).
Figure 1: Light micrograph of the anterior intestine (A) and posterior intestine (B) of Nile tilapia O. niloticus niloticus fed on commercial diet showing; muscularis extena (ME), submucosa (SM), lamina propria (LP), lumen (Lm). C, D, and E are representative micrographs of the intestine of Nile tilapia fed on commercial diet showing Goblet cells (GC), Lymphocytes infiltration (LI), and Blood cell congestion (BC). H and E, stain.
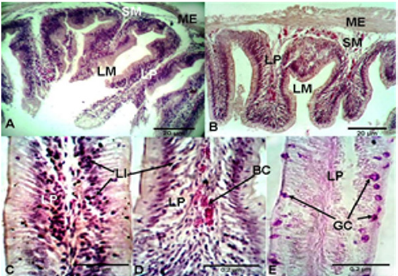
Figure 2: Light micrograph of the anterior intestine (A) and posterior intestine (B) of Nile tilapia O. niloticus niloticus fed on maggot diet showing; muscularis extena (ME), submucosa (SM), lamina propria (LP), lumen (Lm). C, D, and E are representative micrographs of the intestine of Nile tilapia fed on maggot diet showing Goblet cells (GC), and Lymphocytes infiltration (LI). H and E, stain.
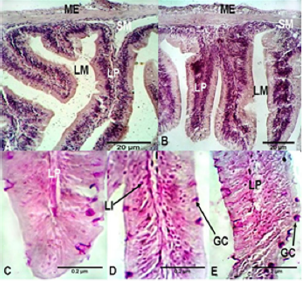
Table 4: Average length of villi and the number of goblet cells in the intestine of fishes fed on commercial diet and Maggot diet for 12 weeks.
The anterior and posterior intestines of fish fed the commercial diet showed detachment at the base of the villi between the circular muscles and villi (Figures 1 & 2). Lymphocyte infiltration in the villi, lamina propria and mucous membrane were observed in the intestines of fish fed the commercial diet. Blood cell congestion was observed in the lamina propria of fish fed the commercial diet. On the other hand, only slight lymphocyte infiltration was observed in the intestines of fish fed the maggot diet (Figures 1 & 2). Histological analysis of the livers of fish fed the commercial diet and those fed the maggot diet showed normal architecture of hepatocytes, with a regular shape and large centrally located nuclei. Large intracytoplasmic vacuoles and blood cell congestion in the sinusoidal blood vessels were observed in the livers of fish fed the commercial diet. Additionally, low glycogen levels were observed in the liver tissues. On the other hand, small intra-cytoplasmic vacuoles were observed in the livers of fish fed the maggot diet, and high glycogen levels were observed.
Discussion
The primary objective in fish nutrition is to provide a nutritionally balanced mixture of ingredients to support the vital functions of fishes at an acceptable cost [38]. Blood parameters are an important tool for monitoring both the nutritional status and health status of fishes [39]. In recent years, increasing attention has been given to haematological studies as an integral part of the examination of the health conditions and productivity of fishes. The effect of maggot meal as a feed supplement on the growth performance indicated that this component was well utilized by O. niloticus niloticus [40]. The same conclusion could be made based on the effects on the haematological and biochemical parameters as well as the results of the histological investigation. Changes in the blood indices of fish as a result of feed have been previously reported [41]. The haematological values obtained for both treated groups indicated the nutritional adequacy of the diets, as the values did not indicate nutritional deficiency [42]. Non-significant differences (P>0.05) were observed among the groups for nearly all the haematological parameters, except RBC, Hb and MCH. These parameters increased markedly after three months of exposure via the blood in Nile tilapia fed the commercial diet and those fed the maggot diet. The fish given the commercial diet exhibited the highest RBC and Hb values, although the values were within the normal ranges. Fish fed the maggot diet exhibited the lowest RBC and Hb values. The differences in the final values of RBC and Hb between the blood of Nile tilapia fed the commercial diet and those fed the maggot diet were non-significant. This finding seems to confirm that the fish were not negatively influenced by the inclusion of maggot meal in the experimental diet. The high values of MCV and MCH observed here, however, may not indicate a serious problem, because the PCV, RBC, WBC, Hb and MCHC in all the treatments were within the normal ranges for healthy fish. The non-significant differences in the evaluated parameters between the two diets implies that maggot meal can successfully replace commercial meal in fish diets. This result is consistent with the reports of other authors who have observed improved performance of fish fed diets containing maggot meal over those solely fed commercial meal. Thus, this finding reflects the nutritive quality and acceptance of this biomaterial [43]. The result also corroborates previous observations that maggot meal, similar to other animal protein sources, is well accepted and utilized by fish [44-46]. Some haematological values observed here appeared to be slightly lower than those reported for tilapia by some authors but were within the acceptable range. Notably, the variations in haematological values within species can be influenced by environmental conditions, sex, age, origin, breeding system, and feeding, among other factors [47]. There was no significant difference in WBC in fish fed the commercial diet and those fed the maggot diet. For both diets, the WBC decreased from an initial value of 19.16 × 103/μl to a final value of 19.08 × 103/μl. The values of WBC recorded here were within the range reported by Bittencourt et al. [45] for healthy Nile tilapia. These results indicate that the fish were healthy. Reduction in the WBC value to below the normal range [which is not the case here] is an indication of allergic conditions and can be harmful to fish because these cells play an important role in the innate immune system [48, 49]. Lymphocytes produce antibodies that provide defence against infection. Lymphocytes represented the highest proportion of the WBC in the blood of fish in this study. This finding is in contrast with the WBC composition in most livestock, with neutrophils exhibiting the highest proportion. Neutrophils were in the second most abundant, representing approximately 30% of the total WBC in the blood of Nile tilapia fed the commercial diet and those fed the maggot diet. At the end of the experiment, the percentage of lymphocytes was high in the blood of fish fed the maggot diet, while the percentage of neutrophils was high in the blood of fish fed the commercial diet. This finding can explain the constant percentage of monocytes and eosinophils in the blood of Nile tilapia fed the commercial diet and those fed the maggot diet. The concentration of monocytes in the blood of Nile tilapia in this study was within the normal range. The monocyte concentration in the blood of Nile tilapia fed the commercial diet and those fed the maggot diet ranged from 1.3% to 2.3%. This finding was consistent with the results of Kelly [50], who reported that monocytes constitute less than 10% of the total WBC in animals of all species. Basophils were not observed in the blood of fish in this study. This finding is similar to the results obtained in most livestock. Kelly [50] reported that basophils rarely occur in the blood of all species of livestock. Biochemical parameters vary among species and can be influenced by many biotic and abiotic factors, such as water temperature, seasonal pattern, food, age and sex [18]. A marked increase in the total protein level was observed in the blood of fish fed the maggot diet compared to that in the blood of fish fed the commercial diet. The low plasma protein level observed in Nile tilapia fed the commercial diet may be a consequence of decreased protein absorption by the relatively short villi observed in these fish. These results suggested that fish health was improved when the fish were fed the maggot diet. Although there was an increase in serum protein levels, the increased levels of ALT and AST suggest protein catabolism at the high dietary protein levels present in the maggot diet [51,52]. The increase in ALT and AST activities observed in Nile tilapia fed the maggot diet may reflect the use of excess hydrocarbons from amino acids to meet energy demands. Similar responses were observed in Oncorhynchus mykiss for ALT [53] and in Rhamdia quelen for AST and ALT [54]. Blood glucose levels may vary according to season and water temperature and may decrease with increasing ages and sizes of fishes [55]. Plasma glucose levels in fishes increase during stress, probably due to the action of catecholamine on stored glycogen in liver and other tissues [56]. Here, elevation of plasma glucose was not observed in either feeding group. The level of glucose was lower in the blood of fish fed the maggot diet than in the blood of those fed the commercial diet. In this study, cholesterol concentrations increased from 50.1 to 140 in fish fed the commercial diet (Diet 1), while in fish fed the maggot diet (Diet 2), cholesterol concentrations increased to only 119.17. The cholesterol concentration in the blood of fish fed the commercial diet was higher than that in the blood of fish fed the maggot diet due to the high proportion of fat in the chemical composition of the feed. Because glucose and cholesterol levels were within the normal range, possibilities of anorexia, diabetes, liver dysfunction and malabsorption of fat, which are symptoms of abnormal glucose and glucose levels in the blood [57], were ruled out. The triglyceride levels in the blood of fish fed the maggot diet were significantly higher and nearly twice those found in fish fed the commercial diet. Similar results were obtained in Liza klunzingeri by Mohammadizadeh et al. [58]. The triglyceride levels increased significantly due to the increase in protein levels in the maggot diet, which may be because the muscle is a pivotal compartment that is directly linked to amino acid turnover. The level of calcium was significantly low in the blood of fish fed the maggot diet. The creatinine level was high in the blood of fish fed the maggot diet. The urea level exhibited non-significant changes in the blood of fish fed the commercial diet and those fed the maggot diet during the experimental period. The histological structure of the fish digestive system has been well documented [59,60]. Examination of the intestinal histology of aquaculture species is important for understanding pathological alterations related to infectious diseases or promoted by nutritional sources [61]. Although fish histological studies provide much information regarding the gastro-intestinal tract [62], further information is needed regarding morphological adaptations to variations in diet nutrients, which could affect diet formulation. Histological analysis of the digestive system is considered to be a good method to determine the nutritional status of fishes [23,24], and identification of the structural variations is useful for studies on nutritional development [63,64]. Histopathological changes in the intestine may vary depending on the species and feed used in the experiments [65]. The flexibility of the piscine gastro-intestinal tract for adaptation to food availability has been well studied [65,66]. In the present study, the intestines of fish fed the commercial diet exhibited normal architecture, with short villi, a wide lamina propria, a large number of small goblet cells, lymphocyte infiltration, and blood congestion. In the case of Nile tilapia fed the maggot diet, the anterior intestine exhibited normal architecture, with long villi, a narrow lamina propria, few small goblet cells, and slight lymphocyte infiltration and blood cell congestion. The histological alteration observed in the intestines of fish fed the commercial diet, that is, shortening of the villi, widening of the lamina propria, lymphocyte infiltration, blood cell congestion, and increasing number of goblet cells, was previously identified as enteritis [67,37]. The same changes were also observed in the intestines of salmonids fed full-fat diets [68,67]. Generally, widening of the lamina propria was accompanied by profound infiltration of a mixed population of inflammatory cells such as lymphocytes, neutrophilic granulocytes, macrophages, and eosinophilic granular cells [67,68]. Similar results were observed when fishmeal was replaced with sunflower meal in the feed of sharpsnout sea bream (Dyploduspuntazzo Cetti) [69]. Villus length is a useful histological parameter that can be monitored not only in experiments regarding the replacement of fishmeal but also in the evaluation of different types of commercial feed.
Based on the present results, we can conclude that the selected commercial diet is rich in fat and proteins of plant origin compared to the maggot diet, which is a 100% animal protein product. An increase in villus length is associated with an increase in the surface area for absorption of nutrients [70]. The long villi found in fish fed the maggot diet indicate high efficiency in the absorption process [24,71]. This high efficiency was evidenced by the improved growth performance of fish fed this diet [12]. This finding shows that the maggot diet promoted an increase in villus length in these fish. A decrease in villus length leads to reduced surface area for nutrient absorption [71], which may also explain the poor condition of the liver observed in fish fed the commercial diet. The number of goblet cells could vary with feeding habits or starvation [72]. A higher number of goblet cells was observed in the intestines of fish fed the commercial diet than in those of fish fed the maggot diet. Goblet cells are associated with the immune system and act through the mucus as a lubricant. The increase in the number of goblet cells may be an indication of increased irritation [71], as these cells produce the mucus lining the brush border. This mucus serves as a lubricant, providing protection against chemical and mechanical damage. The increase in goblet cell number may also be an immune response against anti-nutrients [73]. The liver is of significant importance for nutrition and homeostasis in fishes. The liver of O. niloticus niloticus fed the commercial diet showed normal architecture, with normal hepatocytes and blood sinusoids. Large intra-cytoplasmic vacuoles and blood cell congestion in the sinusoidal blood vessels were observed. Low glycogen levels were recorded. Additionally, a normal architecture was observed in the liver of Nile tilapia fed the maggot diet, with normal hepatocytes and blood sinusoids, small intracytoplasmatic vacuoles, and high glycogen content. In conclusion, the haematological and blood biochemical analyses suggest improvement of fish health upon dietary administration of maggotsupplemented feed. The obtained results showed no negative effect on the histology of the visceral organs of Nile tilapia fed the maggot diet, suggesting that this diet is essentially good in terms of growth and utilization [72-74]. The replacement of fishmeal with maggot meal is acceptable from a growth perspective and in terms of the observed histological architecture. Thus, maggot meal could be an alternative animal protein source in fish diets to lower the production cost of fish diets. In future experiments in the area of fish nutrition, the histological status of the intestine should always be considered. This analysis should provide additional information regarding the state of this organ if any of the mentioned methods are used [75,76]. These methods are valuable in field experiments as well as in the laboratory. Maggots are a good alternative protein source for O. niloticus niloticus. However, further studies with tilapia and other fish species are needed to validate this conclusion.
Acknowledgement
The first author is grateful for the continuous support from the Alexander von Humboldt Foundation.
References
- Idowu EO, Afolayan EB (2013) The Effects of Supplementing of Fish eal With Maggots at Varying Levels in the Diet of Clarias gariepinus. Int Arch App Sci Technol 4(4): 41-47.
- Thorarinsdottir RI, Jokumsen A, Bjornsson BT, Torrissen O (2011) Local raw materials for production of fish feed for aquaculture. Nordic Innovation Centre Project no. 10102.
- Sogbesan OA, Ajuonu ND, Ugwumba AAA, Madu CT (2005) Cost benefits of maggotmeal as supplemented feed in the diets of Clarias gariepinus heterobranchus longifilis hybrid fingerlings in outdoor concrete tanks. Journal of Scientific and Industrial Studies 3(2).
- Gatlin DM, Barrows FT, Brown P, Dabrowski K (2007) Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquaculture Research 38: 551-579.
- Fagbenro OA, Davies SJ (2013) Uses of high percentage of soy protein concentrate as fishmeal substitute in practical diets for African catfish, Clarias gariepinus (Burchell, 1822). Growth feed utilization and digestibility J Appl Aqua 16 (1): 113-124.
- Ayola BE, (2010) Effects of feeding on ammonium excretion and growth of the African catfish (Clarias gariepinus) fry. J Anim Sci 48 (3): 106-112.
- Omotoyin BO (2006) Haematological changes in the blood of Clarias gariepinus (Burchell 1822) juvenile fed poultry litter. J Rural Development 18: 11-22.
- Olaniyi CO, Salau BR (2013) Utilization of maggot meal in the nutrition of African catfish. African Journal of Agricultural Research. 8(37): 4604-4607.
- Hasting WH (1976) Nutritional Requirements and Field Technology in advances in aquaculture paper presented at the F.A.O. Technical Conference on Aquaculture, Kyoto Japan 75.
- Nwanna, L. Oishi, C (2008) Use of phytase to improve the digestibility of alternative feed ingredients by Amazon tambaqui, Colossoma macropomum Science Asia 34: 353-360.
- Ogunji JO, Kloas W, Wirth M, Schulz C, Rennert B (2006) Housefly Maggot Meal (Magmeal): An Emerging Substitute of Fishmeal in Tilapia Diets. 10: 11-13.
- Makinde Olayinka J (2015) Maggot Meal: A Sustainable Protein Source for Livestock Production-A Review. Advances in Life Science and Technology 31: 2224-7181.
- Ezewudo B I, Monebi CO Ugwumba AAA (2015) Production and utilization of Musca domestica maggots in the diet of Oreochromis niloticus (Linnaeus, 1758) fingerlings. African Journal of Agricultural Research 10(23): 2363-2371.
- Awoniyi T, Aletor V, Aina J (2003) Performance of broiler-chickens fed on maggot meal in place of fishmeal. Int J Poult Sci 2(4): 271-274.
- Fasakin EA, Balogun A M, Ajayi OO (2003) Evaluation of full-fat and defatted maggot meals in the feeding of Clariid catfish Clarias gariepinus Aquaculture Research 34: 733-738.
- Ajani EK, Nwanna LC, Musa BO (2004) Replacement of fishmeal with maggot meal in the diets of Nile tilapia, Oreochromis niloticus. World Aquaculture 35(1): 52-54.
- Bahmani M, Kazemi R, Donskaya P (2001) A comparative study of some hematological features in young reared sturgeons(Acipenserpersicus and Husohuso). Fish physiology and biochemistry 24: 135-140.
- Jawad L, Al-Mukhtar M, Ahmed H (2004) The relationship between haematocrit and some biological parameters of the Indian shad, Tenualosailisha (Family Clupeidae). Animal Biodiversity and conservation 27: 47-52.
- Svobodova Z, Vykusova B (1991) Unified methods of haematological examination of fish. Research Institute of Fish Culture and Hydrobiology Vodnany Methods 20: 30-31.
- Animashahun RA, Omoikhoje SO, Bamgbose AM (2006) Haematological and biochemical indices of weaner rabbits fed concentrates and Syndrellanodiflora forage supplement. Proc. 11th Annual Conference of Animal Science Association of Nigeria. Institute of Agricultural Research and training Nigeria, p. 29-32.
- Maxwell M, Robertson G, Spence S, Mc Corquodale C (1990) Comparison of haematological values in restricted and adlibitum-fed domestic fowls: Red blood cell characteristics. British Poultry Science 31: 407-413.
- Cataldi E, Cataudella S, Monaco G, Rossi A (1987) A study of the histology and morphology of the digestive tract of the seabream, Sparus aurata. Journal of Fish Biology 30: 135-145.
- Hall K, Bellwood DR (1995). Histological effects of cyanide, stress and starvation on the intestinal mucosa of Pomacentrus coelestis, a marine aquarium fish species. Journal of Fish Biology 47: 438-454.
- Caballero MJ, Izquierdo MS, Kjørsvik E, Montero D, Socorro J, et al. (2003) Morphological aspects of intestinal cells from gilthead seabream(Sparusaurata) fed diets containing different lipid sources. Aquaculture 225: 325-340.
- Raskovic B, Stankovic MB, Markovic ZZ, Poleksic VD (2011) Histological methods in the assessment of different feed effects on liver and intestine of fish. Journal Agriculture Science 56: 87-100.
- Roberts RJ (1989) Fish Pathology. Baillière Tindall London.
- Osman AG, Abd El Reheema, AM Moustafa MA, Mahmoud UM, Abuel-Fadld KY et al. (2011) In Situ Evaluation of the Genotoxic Potential of the River Nile: I. Micronucleus and Nuclear Lesion Tests of Erythrocytes of Oreochromis niloticus niloticus (Linnaeus, 1758) and Clarias gariepinus (Burchell, 1822). Toxicological and Environmental Chemistry 93: 1002-1017.
- Dacie and Lewis 2002. Practical Haematology. (7th Edn).
- Henry RJ (1964) Clinical Chemistry. New York, Harper and Row publish. integrated aquaculture of fish and red seaweeds in Northern Portugal. Aquaculture, pp. 252.
- Thomas L (1992). Enzymatic Colorimetric Method to Determine the Cholesterol. Lab and Diagnosis, 4 D.
- Friedwold WT, Levy RI, Fredickson OS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of preperative ultracentrifuge. clin Chem 18: 499-502.
- Fiereck EA (1964) Appendex. Normal Values. In: Fundimentals of Clinical Chemistry. Nw Tietz Editor Saunders Philadelphia, pp. 1208.
- Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Annal Clin Biochem 6: 24-27.
- Reitman S, Frankel S (1957) A colorimetric method for determination of serum glutamic oxaloacetic and glutamic pyrovic transaminases. Amer J Clin Path 28: 56-59.
- Van den Ingh T, Olli J, Krogdahl Å (1996) Alcohol-soluble components in soybeans cause morphological changes in the distal intestine of Atlantic salmon, Salmosalar L. Journal of Fish Diseases 19: 47-53.
- Baeverfjord G, Krogdahl Å (1996) Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmosalar L.,distal intestine: a comparison with the intestines of fasted fish. Journal of Fish Diseases 19: 375-387.
- Aslaksen MA, Kraugerud OF, Penn M, Svihus B, Denstadli V, et al. (2007). Screening of nutrient digestibilities and intestinal pathologies in Atlantic salmon, Salmosalar, fed diets with legumes, oilseeds, or cereals. Aquaculture 272(1-4): 541-555.
- NRC, NRC (1993) Nutrient requirements of fish. National Academy Press Washington DC, USA.
- Hrubec TC, Cardinale JL, Smith SA (2000) Hematology and plasma chemistry reference intervals for cultured tilapia (Oreochromis hybrid). Veterinary Clinical Pathology 29: 7-12.
- Sayed AE, Mekhamar MI, Gadel-Rab AG, Osman AGM (2015) Evaluation of Growth Performance of Nile Tilapia Oreochromis niloticus niloticus Fed Piophila casei Maggot Meal (Magmeal) Diets. American Journal of Life Sciences, 3(6-1): 24-29.
- Kilgour OFG (1987) Mastering Nutrition. Macmillan Education Limited, London.
- Church JP, Judd JT, Young CW, Kelsay JL (1984) Relationships among dietary constituents and specific serum clinical components of subjects eating self-selected diets. The American Journal of Clinical Nutrition 40: 1338-1344.
- Ogunji JO, Wirth M (2001) Alternative protein sources as substitutes for fish meal in the diet of young Tilapia, Oreochromis niloticus (Linn.). Israeli Journal of Aquaculture Bamidgeh 53: 34-43.
- Alegbeleye WO, Anyanwu DF, Akeem AM (2003) Effect of varying dietary protein levels on the growth and utilization performance of catfish, Clarias gariepinus. Proceedings of the 4th Annual Conference of Nigerian Association of Aquatic Science Ibadan Nigeria 51-53.
- Bittencourt E, Needleman A, Gurtin ME, Van der Giessen E (2003) A comparison of nonlocal continuum and discrete dislocation plasticity predictions. Journal of the Mechanics and Physics of Solids 51(2): 281-310.
- Okore OO, Ekedo CM, Ubiaru PC, Uzodinma K (2016) Growth and haematological studies of African catfish (Clarias gariepinus) juveniles fed with Housefly larva (Musca dometica) as feed supplement. International Journal of Agriculture and Earth Science 2: 2489-0081.
- McCarthy D, Stevenson J, RobertsM (1973) Some blood parameters of the rainbow trout (Salmogairdneri Richardson). Journal of Fish Biology 5: 1-8.
- Dalmo R, Ingebrigtsen K, Bøgwald J (1997) Non-specific defense mechanisms in fish, with particular reference to the reticuloendothelial system (RES). Journal of Fish Diseases 20: 241-273.
- Ahamefule F, Obua B, Ukweni I, Oguike M (2008) Haematological and biochemical profile of weaner rabbits fed raw or processed pigeon pea seed meal-based diets. African Journal of Agricultural Research, 3(4): 315-319.
- Kelly D (1979). Motion and vision. II. Stabilized spatio-temporal threshold surface.
- Ballantyne J S (2001) Amino acid metabolism. Fish Physiology 20: 77-107.
- Stone DA, Allan GL, Anderson AJ (2003) Carbohydrate utilization by juvenile silver perch, Bidyanus bidyanus (Mitchell) II. The protein-sparing effect of wheat starch-based carbohydrates. Aquaculture Research, 34: 109-121.
- Sánchez-Muros MJ, Gárcia-Rejón L, Gárcia-Salguero L, de la Higuera M, Lupiánes, JA (1998) Long-term nutritional effects on the primary liver and kidney metabolism in rainbow trout. Adaptive response to starvation and high-protein, carbohydrate-free diet on glutamate dehydrogenase and alanine aminotransferase kinetics. Biochemestry cell Biolology 30: 55-63.
- Melo JFB, Lundstedt LM, Metón I, Baanante IV ( 2006) Effects of dietary levels of protein on nitrogenous metabolism of Rhamdiaquelen (Teleostei: Pimelodidae). Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology 145: 181-187.
- Coz Rakovac R, Strunjak Perovic I, Hacmanjek M Lipej Z (2005) Blood chemistry and histological properties of wild and cultured sea bass (Dicentrarchus labrax) in the North Adriatic Sea. Veterinary Research Communications 29: 677-687.
- Pottinger T, (1998) Changes in blood cortisol, glucose and lactate in carp retained in anglers’ Journal of Fish Biology 53: 728-742.
- Bush B (1991) Interpretation of laboratory results for small animal clinicians. lackwell Scientific Publications London 32-67.
- Mohammadizadeh M, Afkhami M, Kazem DB, Maryam E, khazaali A, etal. (2012) Determination of some biochemical values in the blood of Liza klunzingeri from the coastal water of the Persian Gulf. African Journal of Biotechnology 11: 3022-3025.
- Smith LA (1981) A revision of the Liasis olivaceus species-group (Serpentes: Boidae) in Western Australia. Records of the Western Australian Museum 9: 227-233.
- Domeneghini C, PannelliStraini R, Veggetti A (1998) Gut glycoconjugates in Sparusaurata L. (Pisces, Teleostei). A comparative histochemical study in larval and adult ages. Histology and Histopathology 13: 359-372.
- Gargiulo AM, Ceccarelli P, Dall'aglio C, Pedini, V (1997) Ultrastructural study on the stomach of Tilapia spp (Teleostei). Anatomy histology and embryology 26: 331-336.
- Calzada A, Medina A, Canales M (1998) Fine structure of the intestine development in cultured sea bream larvae. Journal of Fish Biology 53: 340-365.
- Rotta, M (2003) Aspectos gerais da fisiologia e estrutura do sistemadigestivo dos peixesrelacionados à Corumba, Embrapa Pantanal, 48.
- Dayal R, Srivastava PP, Lakra WS, Bhatnagar A, Chowdhary SA, et al. (2013) Histological changes in the intestine of channastriatus grow-outs fed with different fat sources: Effects of dietary manipulation. Asia Journal of Experimental Marine Biology and Ecology 4: 61-566.
- Refstie S, Førde-Skjærvik O, Rosenlund G, Rørvik KA (2006) Feed intake, growth, and utilization of macronutrients and amino acids by 1-and 2-year old Atlantic cod (Gadusmorhua) fed standard or bioprocessed soybean meal. Aquaculture 255: 279-291.
- Uran PA, Goncalves AA, Taverne-Thielem JJ, Schrama JW, Verreth JAJ, etal. (2008) Soybean meal induces intestinal inflammation in common carp (L). Fish and Shellfish Immunology 25: 751-760.
- Van den Ingh T, Krogdahl Å, Olli J, Hendriks H (1991) Effects of soybean-containing diets on the proximal and distal intestine in Atlantic salmon (Salmosalar): a morphological study. Aquaculture 94: 297-305.
- Bakke McKellep A, Press CM, Baeverfjord G Krogdahl Å (2000) Changes in immune and enzyme histochemical phenotypes of cells in the intestinal mucosa of Atlantic salmon, Salmosalar L., with soybean meal-induced enteritis. Journal of Fish Diseases 23(2): 115-127.
- Nogales Mérida S, Tomás-Vidal A, Martínez-Llorens S, Jover Cerdá M (2010) Sunflower meal as a partial substitute in juvenile sharp snout sea bream (Diploduspuntazzo) diets: amino acid retention, gut and liver histology. Aquaculture 298: 275-281.
- Aanyu M, Ondhoro CC, Ganda E, Kato D (2014) Intestine histology, nutrient digestibility and body composition of Nile tilapia (Oreochromis niloticus) fed on diets with both cotton and sunflower seed cakes. African Journal of Biotechnology 13(37): 3831-3839.
- da Silva MR, Natali MRM, Hahn NS (2012) Histology of the digestive tract of Satanopercap appaterra (Osteichthyes, Cichlidae). Acta Scientiarum Biological Sciences 34: 319-326.
- Da Silva SS, Anderson TA (1995) Fish nutrition in aquaculture. Chapman and Hall London p 31.
- Marchetti L, Capacchietti M, Sabbieti M, Accili, D (2006) Histology and carbohydrate histochemistry of the alimentary canal in the rainbow trout Oncorhynchus mykiss. Journal of Fish Biology 68: 1808-1821.
- Fagbenro OA, Davies SJ (2013) Uses of high percentage of soy protein concentrate as fishmeal substitute in practical diets for African catfish, Clarias gariepinus (Burchell, 1822): Growth, feed utilization and digestibility. J Appl Aqua 16 (1).
- Najim S, Al-Noor S, Basim M Jasim (2014) Effects of fish meal replacement with fish biosilage on some haematological and biochemical parameters in common carp Cyprinus carpio fingerlings. International Journal of Research in Fisheries and Aquaculture 4: 112-116.
- Svobodova Z, Vykusova B (1991) Unified methods of haematological examination of fish. Research Institute of Fish Culture and Hydrobiology Vodnany Methods 20: 30-31.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...