Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Article(ISSN: 2638-6070) 
Biodegradable Emulsified Films Based on Gleditsia Caspica (Persian Honey Locust) Galactomannan as a New Source of Biopolymer: Effect of Plant Oils on Microstructure and Physicochemical Aspects
Volume 4 - Issue 4Rasoul Niknam1*, Mohammad Mousavi2 and Hossein Kiani1*
- 1Department of Food Science and Technology, Bioprocessing and Biodetection Lab (BBL), University of Tehran, Iran
- 2Department of Food Science and Technology, College of Agriculture and Natural Resources, University of Tehran, Iran
Received:September 1, 2022; Published:September 19, 2022
*Corresponding author:Rasoul Niknam and Hossein Kiani, Bioprocessing and Biodetection Lab (BBL), Department of Food Science and Technology, College of Agriculture and Natural Resources, University of Tehran, Karaj, Iran
DOI: 10.32474/SJFN.2022.04.000191
Abstract
The production of biodegradable films based on newly introduced galactomannan (Gleditsia caspica galactomannan) were investigated in current research. Also, the effect of adding two plant oils (olive and sunflower oils) on various aspects of obtained films were scrutinized. Availability of plant oils in the formulation of films remarkably elevated the film thickness, OP, SAB and opacity values. Contrarily, existence of oils significantly reduced the values of moisture content, moisture absorption, WVP, Tm, Tg and UTS. The XRD patterns indicated that all of the samples had amorphous (none crystalline) structure. The results of SEM images indicated that adding plant oils to the formulation of films led to rougher surfaces. Depending on the characteristics of food formulation in which the produced film is to be used in its packaging, a film with appropriate characteristics (for example incorporated or not incorporated with plant oils) can be selected.
Keywords:Biodegradable Films; Emulsified Films; Galactomannan; Gleditsia caspica; Persian honey locust; plant oils
Introduction
Utilizing plastics in food packaging has elevated significantly in current decade. At the same time, environmentalists are increasingly concerned that plastics are not biodegradable and pollute the environment. For this reason, researchers are looking for materials that, while natural and biodegradable, have acceptable properties for use in food packaging. Obviously, it is not possible to create properties that are fully compatible with plastics, but the use of plastics can be minimized. Consumers of food products have been looking for biodegradable packaging in recent years because they understand the importance of protecting the environment. It should be noted that in some countries, the process of food industry has progressed to the point where even the packaging of the product can be eaten. If the manufacturers of food industry want to attract consumers in a competitive market, must cover their demand for the utilization of edible or biodegradable materials in food packaging [1-3]. Edible or biodegradable films have numerous advantages particularly preventing loss of moisture or aromas, not having adverse chemical reaction, being environmentally friendly and not having microbial contamination that is known as spoilage, that convinces the researchers to work on them and introduce these alternatives to food industries [4,5].
Hydrocolloids are acceptable choice for the construction of these edible and biodegradable films. Hydrocolloids are divided into two classes of proteins and polysaccharides. The use of hydro-colloids extracted from plant seeds has become particularly important in the production of films due to their availability, acceptable cost and ease of extraction. Researchers have done extensive research in current decade on the use of this type of hydrocolloid in the formulation of packaging films like the films based on chia seed mucilage [6], Plantago major seed [7] and basil seed gums [8]. Between the biopolymer sources extracted from plant seeds, galactomannans are of particular importance. Galactomannans are polysaccharides that utilized as stabilizing, emulsifying, thickening and foaming agents in food products [9,10]. Investigation of the results obtained from former studies has shown that various factors such as the mannose to galactose ratio (M/G) as well as the structure of galactomannan have a remarkable role in the ability of galactomannans to construct biodegradable films [11,12]. Production of biodegradable films based on galactomannans extracted from plant seeds like Gleditsia triacanthos [13], Delonix regia [3], Arenga Pinnata [14] and spent coffee [15] are being popular in recent years. Acceptable results obtained from these studies demonstrate that galactomannans can be considered as suitable choices for the construction of edible and biodegradable films. If these galactomannans are easily accessible (like plant seed-based galactomannans) and can exhibit acceptable properties, food packaging costs can be expected to be greatly reduced over the coming years by further research and modification.
Plants known as Gleditsia has been utilized for many years to treat diseases particularly headaches. This treatment is mostly performed in China and Japan. In recent years, numerous types of this genus are comprehensively investigated such as Gleditsia japonica [16], Gleditsia sinensis Lam. [17] and Gleditsia triacanthos [18]. Obtained outcomes from former studies represented that, these plants (particularly seeds) have alkaloids, phenols, flavonoids and galactomannan (as the main carbohydrate). Among the numerous varieties of Gleditsia, there is a vital species known as Gleditsia caspica (Persian honey locust) that cultivated in northern part of Iran (called Lilaki in Persian). Similar to other types of Gleditsia, seeds of Gleditsia caspica, in addition to having carbohydrate and protein as major constituents, also contain bioactive components [19]. The obtained galactomannan from the seeds of Gleditsia caspica had 83.45% carbohydrate and also 0.94% protein. Also, M/G ratio is 1.95 which is near to value delineated for guar gum as a commercial galactomannan [20]. Additionally, the obtained galactomannan has some bioactive compounds particularly flavonoids and phenolics which could be affiliated with its functional features. These features could be considered as a positive point in producing films based on this biopolymer [20,21,22].
Appearance of some weak properties in biodegradable films based on hydrocolloids has led researchers to add materials to it that can solve these problems to some extent. One of these effective ingredients is plant oils. Adding plant oils to the formulation of biodegradable films improves the performance of these films in food packaging. For example, the addition of these oils reduces the release of moisture from the film structure, thus preventing it from drying out, tearing and reducing its elasticity [7,23]. Olive and sunflower oils are known to be the main plant oils utilized in food products. These oils vary in fatty acid composition and conformation, and when the oils are incorporated with galactomannan, these may be resulted in various film characteristics. Further research on the effect of these oils on galactomannan composite film aspects is vital to understand the behaviour of the films during the utilization stage [24]. Incorporation of plant oils further improve the properties of the films produced. For example, in films based on gellan gum [25], chitosan [25] containing thyme oil, Arabic gum containing cinnamon oil [26] and gum karaya containing oregano oil [27], various aspects of films were enhanced which made them more acceptable for utilizing in food packaging. According to the previous results of studies, there was no research on films based on this new source of galactomannan. So, the main goal of current study was to produce films based on Gleditsia caspica galactomannan. In addition, the effect of plant oils (sunflower and olive oils) on numerous features of biodegradable films were also comprehensively evaluated.
Material and Methods
Raw Material and Chemicals
Gleditsia caspica seeds and plant oils (sunflower oil and olive oil) were acquired from local market in Tabriz, Iran. Other materials were analytical grade and purchased from Merck.
Galactomannan Extraction from Gleditsia caspica Seed (Gg)
To accomplish the galactomannan extraction procedure, the method suggested by Niknam et al. 2021 [20] was utilized. A suitable extractor was utilized to separate the mucilage from the seeds. The extracted mucilage was blended with ethanol (96%) to precipitate galactomannan. After precipitation and purification processes, the filtered galactomannan was putted in an oven (50℃ for 17 hours) for drying procedure and at last, completely powdered.
Preparation of Emulsified Films
Gleditsia caspica galactomannan (GG) powder (1.5g) in were dissolved in 80 mL deionized water (82°C for 1.45h) to proper hydration. After that, 0.5g glycerol w/w of hydrocolloid was added to the solution and stirred. Subsequently, solutions were cooled till 55°C and kept in mentioned temperature. Simultaneously, 0.3 mL Tween 80 was added to 20 mL distilled water and homogenized for a while at special condition. After that, 0.3 and 0.5 mL of each plant oil (Sunflower oil (SFO) and olive oil (OO) was added to the solution (distilled water and Tween 80). At last, the solution was homogenized at 20000 rpm for 6 min. Two solutions were mixed properly (50°C for 1.15h). Then, each of the films were incubated at 42°C for 16h. Dried films were peeled from the casting surfaces and stored at room temperature for further experiments. The films were named from A to D for films with no oil (control film (A)) and those containing 0.3 v/v OO (B), 0.5 v/v OO (C), 0.3 v/v SFO (D) and 0.5 v/v SFO (E) [28].
Film Thickness
This parameter in produced films were evaluated by applying a digital micrometer. The average value obtained from ten random locations of each film sheet was considered as thickness of films [29].
Moisture Content (MC)
0.5 g from each of the conditioned films were dried in an oven (100℃) till constant weight was reached. This parameter was computed by Eq (1):
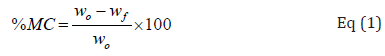
that W0 and Wf indicated sample weight before and after drying [30].
Moisture Absorption (MA)
Produced samples were first conditioned. After weighing, they were conditioned (room temperature) in a desiccator containing Nacl saturated solution. This solution makes a relative humidity of 97 ± 1%. Weight of the samples was recorded every 24h, then, 48h and at last 72h till constant weight was reached. This parameter was computed by Eq (2):
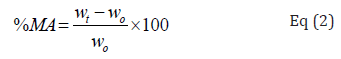
that Wt and W0 demonstrated the weights of the film after time t and primary weight of the film [31].
Water Vapor Permeability (WVP)
WVP was computed by the standard ASTM method E96. Unique cups (with special diameter and depth) were utilized to compute this parameter in samples. Cups containing conditioned samples were weighed every 24h, then, 48h and at last 72h and water vapor transport was computed by the weight gain of the cup. Alterations in the weight of the cup were recorded as a function of time. Slopes were computed by linear regression (weight changes vs. time). This parameter (gm-1 day-1 Pa-1) was computed as:
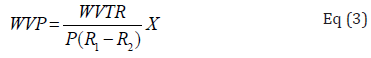
that P indicated the saturation vapour pressure of water (Pa) at room temperature, R1 represented the RH in the desiccators (0.97), R2, the RH in the cup (0) and X showed the film thickness (m).
Oxygen Permeability (OP)
This parameter for the samples was computed by an automated oxygen permeability testing machine (PBI Dansensor, Denmark) at 26℃ and 0% RH [32].
XRD Pattern
An X-ray diffractometer (Phillips, Netherlands) at a voltage and current of 40 kV and 40 mA was used to evaluate the degree of crystallinity in films. The pattern was recorded with 2θ ranging from 10° to 80° [33].
DSC Analysis
The thermal features of the conditioned films were scrutinized by differential scanning calorimetry (DSC) (TA Q600, USA) at a rate of 10°C/min between -50 and 250°C under a nitrogen flow [20].
SEM Imaging
A scanning electron microscope (SEM, MlRA3, TESCAN, Czech Republic) was applied to scrutinize the microstructure of surface of the samples [34].
Opacity of the Films
This parameter was scrutinized after placing film samples in a UV-Visible spectrophotometer (Biomate5, Thermo, USA), and the absorption spectrum of the film was recorded at wavelengths from 400 to 800 nm [35].
Mechanical Features
Ultimate tensile strength (UTS, MPa) and strain at break (SAB, %) were scrutinized utilizing a tensile machine (506B1, Cometech, Taiwan) and computed by ASTM standard method D882-02 [36].
Statistical Analysis
Obtained data was analyzed by ANOVA and Duncan test (p< 0.05) in SPSS 21 software.
Results and Discussion
Film Thickness (FT)
Measuring this parameter in produced films is important because it is effective in other properties of the film and also have a vital role in the marketability of the product. By the way, most of the biodegradable films produced are thin in thickness and the manufacturer is not encouraged to use them in packaging of food products. So having proper thickness is one of the most important issues in evaluating the efficiency of films [28]. The obtain value for this parameter is mostly attributed to the drying process and composition of film forming solution [37]. Table 1 demonstrated the FT values of samples based on GG and plant oils. The obtained value for control sample (0.0511 mm) was near to the value reported for film containing Gleditsia triacanthos galactomannan (0.052 mm) [13] and Delonix regia galactomannan (0.050 mm) [3] and higher than value reported for film based on Cassia grandis galactomannan (0.0310 mm) [39]. By adding SFO and OO to the films, the FT values significantly raised from 0.0511 mm for sample A (control sample) to 0.0821 (sample B), 0.0994 (sample C), 0.0731 (sample D) and 0.0945 (sample E). In addition, FT value also increased by elevating plant oil concentration in the film formulation. This elevation in FT might be affiliated with structural alterations in the film matrix [24]. The same result was also reported in films based on Plantago major seed gum [28] and mesquite seed gum [38], Kappa-carrageenan [24] which were incorporated with plant oils especially maize, olive, canola and palm fruit oils. As can be seen in Table 1, samples containing olive oil had relatively higher FT values in comparison with those containing sunflower oil which was not statistically significant in high concentrations (0.5% v/v) (p<0.05). This difference could be affiliated with the structure and composition of galactomannan and oil and the produced interaction between biopolymer and oil [30].
MC and MA
Determining moisture content of films is of particular importance in assessing their quality. Sometimes having high moisture content is a positive point and sometimes it is a negative point. Depending on what kind of product is going to be packed by the film, it will indicate whether high moisture content is positive or negative [40]. Many factors including the amount of plasticizer, degree of hydrophobicity and existence or absence of some components especially plant oils have vital effects in MC value [28]. The MC values of samples containing GG and plant oils are demonstrated in Table 1. As can be seen, by adding plant oils to the formulation of films, the MC values of samples significantly decreased from 30.70% for control sample to 27.55, 25.30, 24.11 and 21.59% for samples B to E, respectively. This diminish in values might be affiliated with reduction in hydroxyl groups (OH) which reacted with oil molecules (Polysaccharide-oil interactions would diminish the OH group accessibility) [28]. Additionally, with rising plant oil concentration, MC values diminished. Same result was reported in films based on Plantago major seed gum [28], Kappa-carrageenan [24], Lepiduim perfoliatum seed gum [31], [30] and guar gum [41] which were incorporated with various plant oils. Samples incorporated with sunflower oil had lower MC values compared with those containing olive oil which could be affiliated with their hydrophobicity and conformation. The obtained MC value for control sample (30.70%) was lower than values reported for films based on fenugreek galactomannan (32.60%) Rashid et al. 2019 and chia seed mucilage (32%) [6] and higher than values reported for films based on Delonix regia galactomannan (11%) [3], Cassia grandis galactomannan (29.33%) [39], Salvia macrosiphon seed gum (23.73%) [42], guar gum (as a known commercial galactomannan) [41] and Plantago major seed gum (22.46%) [7].
A vital factor that affected the MA value is availability of plasticizer and amount of it in the formulation of film [28]. The obtained MA values of samples are represented in Table 1. As can be seen, by adding plant oils to the formulation of films, the MA values were remarkably diminished from 39.11% for control sample (A) to 35.20, 33.70, 30.60 and 28.20% for samples B to E. Also, the MA values of samples incorporated with olive oil were higher than those containing sunflower oil which could be affiliated with the composition of oil, interaction of oil – polysaccharide and structure of utilized oil [41]. The absence of oil in control film formulation allows glycerol to react more with the water molecules existed in the film formulation and absorb more moisture, but when oil is added to the film formulation, this possibility is less. Because oil molecules react with water molecules and prevent them from reacting with glycerol which is mainly affiliated with the hydrophobicity nature of plant oils [43]. The same result was reported in film based on Plantago major seed gum [9], Kappa-carrageenan [26], Lepiduim perfoliatum seed gum [31], kefiran [39], tara gum [44] and guar gum [41] which were incorporated with various plant oils. Also, the MA value of control film was lower than value reported for film based on Lepiduim perfoliatum seed gum (152.6%) [31] and higher than value reported for film based on Plantago major seed gum (35.44%) [28].
WVP and OP
Extension of shelf life in food products is being important for manufacturers in recent years which is mainly affected by utilizing proper films for packaging the products in order to decrease the rate of spoilage. WVP which is the indicator of water transfer between food and air, is an important parameter to evaluate the useability of produced film in packaging of food products which are sensitive to spoilage [31]. It must be mentioned that some parameters including extraction process of biopolymer (especially drying process) and the composition of film forming solution (especially the amount of glycerol as plasticizer) which indicated the hydrophilic – hydrophobic ratio of film constituents had vital effects on WVP value [9]. Plasticizers especially when used in high concentration, favor the adsorption of water molecules, that is mostly affiliated with predisposition of plasticizers to construct hydrogen bonds, elevating WVP values [45]. Obtained WVP values of samples are demonstrated in Table 1. As can be understood, the WVP values of films remarkably decreased from 20.17 × 10-9 g m-1 s-1 Pa-1 for sample A (control sample) to 18.54, 15.11, 16.30 and 12.29 × 10-9 g m-1 s-1 Pa-1 by adding plant oils to the film formulation. This reduction could be affiliated with the decrease in hydrophilic portion of the film because of existence of oil [45]. The same result was reported in film based on Plantago major seed [28, Kappa-carrageenan [24], Lepiduim perfoliatum seed gum [31], kefiran [30], tara gum [44] and guar gum [41] which were incorporated with various plant oils. In addition, samples incorporated with olive oil had higher WVP values compared with those containing sunflower oil which was in line with the results reported in MC and MA section. The obtained value for control sample (20.17 × 10-9 g m-1 s-1 Pa-1) indicated the weak potential of film based on GG to be used in food packaging because in most cases, the manufacturers want to use films with low WVP. By adding oils to the film formulations, the WVP values decreased and become more acceptable for utilization in food packaging. Therefore, utilizing plant oils has positive effect in this case. WVP value of control sample was higher than values reported for films based on Gleditsia triacanthos galactomannan (9.30 × 10-9 g m-1 s-1 Pa-1 [45], Delonix regia galactomannan (9.35 × 10-9 g m-1 s-1 Pa-1) [3], Plantago major seed gum (12.77 × 10-9 g m-1 s-1 Pa-1) [28] and Cassia grandis galactomannan (5.60 × 10-9 g m-1 s-1 Pa-1) [39].
One of the common phenomena in food products is oxidation, which has a direct effect on organoleptic aspects of the product, including its taste, color and odor. The factor affecting oxidation is oxygen. Therefore, it is necessary to evaluate the amount of oxygen permeability in the produced films to select a film that is suitable for the product conditions. Generally, films with lower OP values are more suitable than those having higher values [32]. The OP values of samples based on GG which were incorporate with plant oils are represented in Table 2. As can be understood, the OP value of samples significantly increased (from 0.1944 × 10-12 g m (Pa s m2)-1 for sample A to 0.2128, 0.2511, 0.2385 and 0.2645 × 10-12 g m (Pa s m2)-1 for samples B to E by adding plant oils to the film formulation. This could be affiliated with the hydrophobicity of oil [24]. In addition, OP value for samples incorporated with olive oil was higher than those containing sunflower oil. It can be concluded that if the film is going to be used in a product which needs low OP, the films incorporated with plant oils especially sunflower oil must be utilized in the packaging of it. The obtained OP value for sample A (control sample) was near to the value reported for film based on Gleditsia triacanthos galactomannan (20.4 × 10-12 g m (Pa s m2)-1) [45].
Opacity
The transparency of the produced films can have a vital role in marketing of product because these films are used in the packaging of the product and consumers buying these products, first, see the packaging of the product and pay attention to the appearance of the product before checking the quality [46]. Opacity is attributed to the amount of light that passes the film. As more light passes through, the value of current parameter diminishes in film and this barrier can be vital to control quantity of light in food products [47]. The obtained values for opacity of samples are demonstrated in Table 2. As can be seen, the opacity of samples elevated from 11.34% (for sample A as control sample) to 13.57, 14.95, 12.35 and 13.02% for samples B to E, respectively by adding plant oils to the formulation of films. This could be affiliated with the increase in strength of constructed network in the film forming solution due to the existence of oil. Also, this elevation in opacity is doubtless due to an elevation in light-scattering [44]. Additionally, by elevating the concentration of oil in formulation, the opacity of samples increased. The same result was reported in films based on tara gum (as a commercial galactomannan) [44], Kappa-carrageenan [24], Lepiduim perfoliatum seed gum [31] and mesquite seed gum [38] which were incorporated with plant oils. Also, samples incorporated with olive oil had higher opacity values compared with those containing sunflower oil. This could be affiliated with the composition of utilized oil (especially fatty acids) [48]. The obtained opacity value for control sample was near to the values reported for films based on Gleditsia triacanthos galactomannan (11.30%) [45] and Cassia grandis galactomannan (11.83%) [39] and higher than value reported for films based on Delonix regia galactomannan (9.77%) [3]. Also, the obtained value was lower than value delineated for film based on Lepiduim perfoliatum seed gum (17.08%) [31].
Mechanical Properties
The ultimate goal of researchers is to produce biodegradable films that have good UTS and SAB (especially in storage and transportation stages) which might be utilized in packaging of food products [28]. A property that have vital effect on mechanical features is the M/G (mannose to galactose ratio) in galactomannan in which those with higher ratio constructed stronger and more flexible films compared with those with lower values [39]. Two main parameters including UTS, and SAB were used to evaluate the mechanical aspects of various films. Higher values of these parameters indicated the mechanical quality of produced films [31,44]. The obtained UTS and SAB values for samples are demonstrated in Table 2. As can be understood, the UTS values decreased from 30.50 MPa for sample A as control sample to 26.11, 22.45, 28.35 and 24.11 MPa for samples B to E which were incorporated with plant oils. This diminish could be affiliated with the interrupt polymeric network by adding oil. In other words, adding oil resulted in changing the polymer – polymer strong interaction to polymer – oil weak bond. The same result was reported in film based on Plantago major seed gum [28], Kappa-carrageenan [24], Lepiduim perfoliatum seed gum [31], kefiran [30], tara gum (Ma et al. [44] and guar gum [41] which were incorporated with various plant oils. Also, samples incorporated with sunflower oil had higher UTS values compared with those containing olive oil which might be affiliated with the composition (especially fatty acids) and structure of utilized oil [28]. The obtained value for UTS of control sample was lower than values delineated for films based on Delonix regia galactomannan (69.04 MPa) [3] and higher than values delineated for films based on Plantago major seed gum (23.41 MPa) [7], kappa carrageenan (26.29 MPa) [23], guar gum (11.73 MPa) [41] and Salvia macrosiphon (16.35 MPa) [42].
Contrarily, by adding plant oils to the formulation of films, the SAB values of samples elevated from 35.17% for sample A as control sample to 44.25, 49.25, 49.80 and 51.37% for samples B to E. The same result was reported in film based on Plantago major seed gum [28], Kappa-carrageenan [24], Lepiduim perfoliatum seed gum [31], kefiran [30], tara gum (Ma et al. [44] and guar gum [41] which were incorporated with various plant oils. Similar to UTS, the samples incorporated with sunflower oil had higher SAB values in comparison with those containing olive oil which might be connected with plasticizing effect of oils [28]. The obtained value of SAB for control sample was higher than values reported for films based on Delonix regia galactomannan (3.75%) [3], Cassia grandis galactomannan (18.06%)) [37] and Plantago major seed gum (14.93%) [28] and lower than values delineated for films based on kappa carrageenan (36.46%) [23] and guar gum (39.65%) [41].
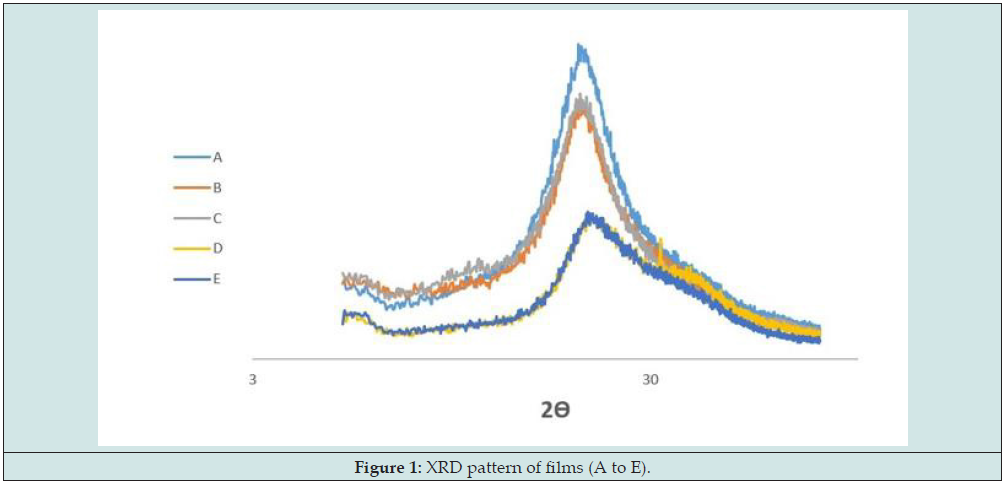
XRD Analysis
The obtained XRD patterns for samples are represented in Figure 1. As can be seen, all of the films (incorporated or not incorporated with plant oils) had amorphous structure, showing broad-ranging peak about 2Ɵ = 25.37° (which is the indicator of biopolymers) and no crystallinity was observed (which could be detected by sharp peaks) in samples. Same result was delineated in films based on Plantago major seed gum [7], Kappa-carrageenan [26], Lepiduim perfoliatum seed gum [31], kefiran [30], tara gum [44] and guar gum [41] which were incorporated with various plant oils.
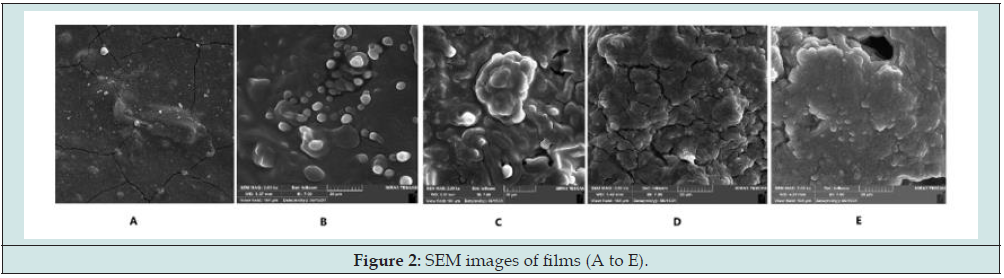
SEM
The images obtained by SEM are demonstrated in Figure 2. As can be noticed, the control sample (A) had compact, smooth and homogeneous structure but adding plant oils to the formulation of films led to rougher surface samples, indicating that oil droplets were embedded and aggregated in the polymer matrix (B to E). Between the samples incorporated with plant oils, those containing sunflower oil had more smooth, compact and uniform physical appearance structure in comparison with samples containing olive oil. This might be affiliated with the quality of distribution of oil droplets and stability of the produced emulsion during casting which had vital effect on phase separation rate [28]. The performance of oil droplets during homogenization and drying is different that led to various compatibility with utilized biopolymer matrix and at last, resulted in formation of diverse dried film structures [31]. The same result was reported in films based on Plantago major seed gum [7], Kappa-carrageenan [24], Lepiduim perfoliatum seed gum [31], kefiran [30], tara gum [44] and guar gum [41] which were incorporated with various plant oils.
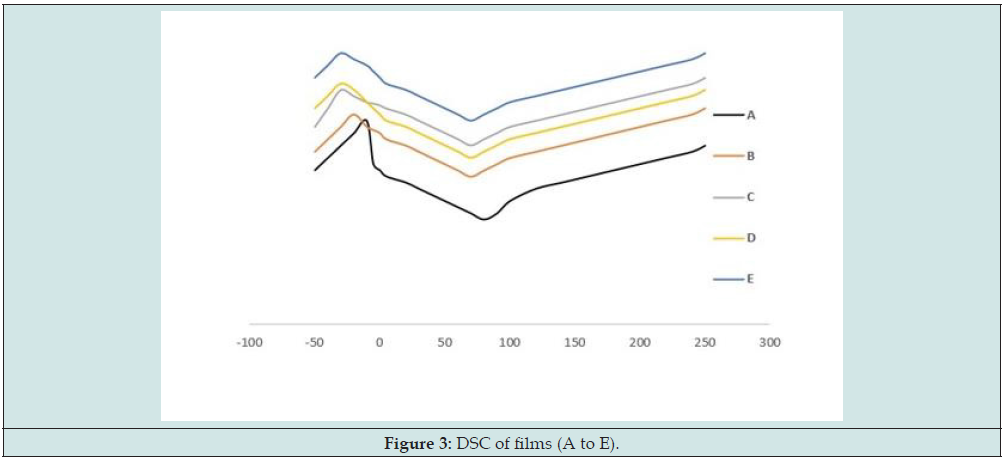
DSC
The obtained DSC graphs for samples A to E are represented in Figure 3. As can be understood, Tm, the temperature of the melting, diminished from 89.32℃ for sample A (control sample) to 65.80, 58.45, 63.11 and 55.39℃ for samples B to E which were incorporated with plant oils. This remarkable decrease in Tm might be affiliated with reduction in the number of hydroxyl groups in the biopolymer structure that can form strong hydrogen bonds (intermolecular forces) between polymer chains. Also, existence of plant oils in the formulation let to higher rate of mobility in polymer – polymer chains [28]. Between the samples incorporated with oils, those containing olive oil had higher Tm values compared with others. The glass transition temperature (Tg) decreased from -17℃ for sample A (control sample) to -25, -37, -33 and -39℃ for samples B to E which were incorporated with plant oils. This remarkable diminish in Tg may be affiliated with elevation of free volume and mobility of the polymer matrix, altering the physical form of GG based samples [28]. The same result was reported in films based on Plantago major seed gum [28], Kappa-carrageenan [24], Lepiduim perfoliatum seed gum [31], kefiran [30], tara gum [44] and guar gum [41] which were incorporated with various plant oils [49-53].
Conclusion
The introduction of new hydrocolloid sources for the production of biodegradable films has become one of the main concerns of researchers in recent years. The properties of biodegradable films based on galactomannan derived from Gleditsia caspica seed as a novel source of galactomannan have not been investigated to date and the aim of this study was to determine whether films based on this hydrocolloid can be used in food packaging or not. The addition of plant oils to the formulation of these films was done to produce a variety of films to be used in as film in packaging of food products depending on the type and aspects of food product. Between the utilized plant oils, films incorporated with olive oil had higher film thickness, moisture content, moisture absorption, WVP, OP, opacity, Tm and Tg. On the other hand, samples containing sunflower oil had higher UTS and SAB values compared with those incorporated with olive oil. Therefore, when enhanced mechanical properties is needed, sunflower oil could be more acceptable than olive oil in producing biodegradable films. The difference between samples might be affiliated with the interaction between the galactomannan and utilized plant oil, amount of plasticizer, the structure and composition (especially fatty acids and degree of hydrophobicity of plant oils) of biopolymer and oils. The obtained images from SEM indicated that samples incorporated with sunflower oil had more smooth and compact conformation compared with films containing olive oil which might be associated with the stability of produced emulsion during casting. At last, it can be suggested that biodegradable films based on GG incorporated or not incorporated with plant oils had various properties and could be utilized in packaging of food products.
References
- Niknam R, Ghanbarzadeh B, Ayaseh A, Adun P (2019) Comprehensive study of intrinsic viscosity, steady and oscillatory shear rheology of Barhang seed hydrocolloid in aqueous dispersions. Journal of Food Process Engineering 42(4): 13047.
- Basch C, Jagus R, Flores S (2013) Physical and antimicrobial properties of tapioca starch-HPMC edible films incorporated with nisin and/or potassium sorbate. Food and Bioprocess Technology 6(9): 2419-2428.
- Rodriguez-Canto W, A Cerqueira M, Chel-Guerrero L, M Pastrana L, Aguilar-Vega M (2020) Delonix regia galactomannan-based edible films: Effect of molecular weight and k-carrageenan on physicochemical properties. Food Hydrocolloids 103: 105632.
- Beikzadeh S, Khezerlou A, Jafari SM, Pilevar Z, Mortazavian AM (2020) Seed mucilages as the functional ingredients for biodegradable films and edible coatings in the food industry. Advances in Colloid and Interface Science 280: 102164.
- Niknam R, Ghanbarzadeh B, Ayaseh A, Rezagholi F (2018) The effects of Plantago major seed gum on steady and dynamic oscillatory shear rheology of sunflower oil-in-water emulsions. Journal of Texture Studies 49(5): 536-547.
- Dick M, Haas Costa T, Gomaa A, Subirade M, De Oliveira Rios A, et al. (2015) Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydrate Polymers 130: 198-205.
- Niknam R, Ghanbarzadeh B, Ayaseh A, Rezagholi F (2019) The hydrocolloid extracted from Plantago major seed: Effects on emulsifying and foaming properties. Journal of Dispersion Science and Technology 41(5): 667-673.
- Majdinasab M, Niakousari M, Shaghaghian S, Dehghani H (2020) Antimicrobial and antioxidant coating based on basil seed gum incorporated with Shirazi thyme and summer savory essential oils emulsions for shelf-life extension of refrigeration of chicken fillets. Food Hydrocolloids 108: 106011.
- Niknam R, Mousavi M, Kiani H (2021) A new source of galactomannan isolated from Gleditsia caspica (Persian honey locust) seeds: Extraction and comprehensive characterization. Journal of Food Processing and Preservation 45(10): 15774.
- Memis S, Tornuk F, Bozkurt F, Durak Z (2017) Production and characterization of a new biodegradable fenugreek seed gum based active nanocomposite film reinforced with nanoclays. International Journal of Biological Macromolecules 103: 669-675.
- Liu W, Gu J, Huang C, Lai C, Ling Z, Yong Q (2021) Fabrication of hydrophobic and high-strength packaging films based on the esterification modification of galactomannan. International Journal of Biological Macromolecules 167: 1221-1229.
- Niknam R, Ghanbarzadeh,B, Ayaseh A, Rezagholi F (2020) Barhang (Plantago major ) seed gum: Ultrasound-assisted extraction optimization, characterization and biological activities. Journal of Food Processing and Preservations 44(10): 14750.
- Cerqueira M, WS Souza, Teixeira J, A Vicente A (2010) Seed extracts of Gleditsia triacanthos: Functional properties evaluation and incorporation into galactomannan films. Food Research International 43(8): 2031-2038.
- Tarigan J, Nainggolan I, Kaban J (2018) The physicochemical and antibacterial properties of galactomannan edible film of Arenga Pinnata incorporated with Zingiber officinale essential oil. Asian Journal of Pharmaceutical and Clinical Research 11(12): 138-142.
- Coelho G, Batista M, Avila A, Franca A, Oliveira L (2021) Development and characterization of biopolymeric films of galactomannans recovered from spent coffee grounds. Journal of Food Engineering 289: 110083.
- Harauchi Y, Kajimoto T, Ohta E, Kawachi H, Imamura-Jinda A, et al. (2017) Prenylated purine alkaloids from seeds of Gleditsia japonica. Phytochemistry 143: 145-150.
- Jian H, Cristhian C, Zhang W, Jiang J (2011) Influence of dehulling pretreatment on physicochemical properties of Gleditsia sinensis Lam. gum. Food Hydrocolloids 25(5): 1337-1343.
- Zhang JP, Tian XH, Yang YX, Liu QX, Wang Q, et al. (2016) Gleditsia species: An ethnomedical, phytochemical and pharmacological review. Journal of Ethnopharmacology 178: 155-171.
- Usama Shaheen, Ehab A Ragab, Ashraf N Abdalla, Ammar Bader (2018) Triterpenoidal saponins from the fruits of Gleditsia caspica with proapoptotic properties. Phytochemistry 145: 168-178.
- Niknam R, Mousavi M, Kiani H (2021) Intrinsic viscosity, steady and oscillatory shear rheology of a new source of galactomannan isolated from Gleditsia caspica (Persian honey locust) seeds in aqueous dispersions. European Food Research and Technology 247(10): 2579-2590.
- Niknam R, Mousavi M, Kiani H (2020) New studies on galactomannan extracted from Trigonella foenum-graceum (fenugreek) seed: Effect of subsequent use of ultrasound and microwave on the physicochemical and rheological properties. Food and Bioprocess Technology 13(5): 882-900.
- Gupta SK, Kalaiselvan V, Srivastava S, Saxena R, Agrawal SS (2010) Trigonella foenum-graecum (Fenugreek) protects against selenite-induced oxidative stress in experimental cataractogenesis. Biological Trace Element Research 136(3): 533-542.
- Shojaee-Aliabadi S, Hosseini H, Mohammadifar MA (2013) Characterization of antioxidant antimicrobial kappa-carageenan films containing Satureja hortensis essential oil. International Journal of Biological Macromolecules 52(1): 116-124.
- Nur Fatin Nazurah R, Nur Hanani ZA (2017) Physicochemical characterization of kappa-carrageenan (Euchema cottoni) based films incorporated with various plant oils. Carbohydrate Polymers 157: 1479-1487.
- Zhang X, Liu D, Jin T, Chen W, He Q, et al. (2021) Preparation and characterization of gellan gum- chitosan polyelectrolyte complex films with the incorporation of thyme essential oil nanoemulsion. Food Hydrocolloids 114: 106570.
- Xu T, Gao C, Feng X, Huang M, Yang Y, et al. (2019) Cinnamon and clove essential oils to improve physical, thermal and antimicrobial properties of chitosan-gum Arabic polyelectrolyte complexed films. Carbohydrate Polymers 217: 116-125.
- Cao T, Song K (2019) Effects of gum karaya addition on the characteristics of loquat seed starch films containing oregano essential oil. Food Hydrocolloids 97: 105198.
- Niknam R, Ghanbarzadeh B, Ayaseh A, Hamishehkar H (2019) Plantago major seed gum based biodegradable films: Effects of various plant oils on microstructure and physicochemical properties of emulsified films. Polymer Testing 77: 105868.
- Khodaei D, Oltrogge K, Hamidi Esfahani Z (2020) Preparation and characterization of blended edible films manufactured using gelatin, tragacanth gum, and Persian gum. LWT 117: 108617.
- Ghasemlou F, Khodaiyan F, Oromiehie A, Yarmand MS (2011) Characterization of edible emulsified films with low affinity to water based on Kefiran and oleic acid. International Journal of Biological Macromolecules 49: 378-384.
- Seyedi S, Koocheki A, Mohebbi M, Zahedim Y (2015) Improving the physical and moisture barrier properties of Lepidium perfoliatum seed gum biodegradable film with stearic and palmitic acids. International Journal of Biological Macromolecules 77: 151-158.
- Rao MS, Kanatt SR, Chawla SP, Sharma A (2010) Chitosan and guar gum composite films: Preparation, physical, mechanical and antimicrobial properties. Carbohydrate Polymers 82(4): 1243-1247.
- Liu F, Chang W, Chen M, Xu F, Ma J, Zhong F (2020) Film-forming properties of guar gum, tara gum and locust bean gum. Food Hydrocolloids 98: 105007.
- Zhang C, Wang Z, Li Y, Yang Y, Ju X, et al. (2019) The preparation and physicochemical characterization of rapeseed protein hydrolysate-chitosan composite films. Food Chemistry 272: 694-701.
- Tongnuanchan P, Benjakul S, Prodpran T (2013) Physico-chemical properties, morphology and antioxidant activity of film from fish skin gelatin incorporated with root essential roots. Journal of Food Engineering 117(3): 350-360.
- Standard test method for tensile properties of thin plastic sheeting D882-02, In Annual book of ASTM. American Society for Testing and Materials, Philadelphia, PA, USA.
- Galus S, Lenart A (2013) Development and characterization of composite edible films based on sodium alginate and pectin. Journal of Food Engineering 115(4): 459-465.
- Rodrigues D, P Cunha A, S Brito E, Azeredo H, Gallao M (2016) Mesquite seed gum and palm fruit oil emulsion edible films: Influence of oil content and sonication. Food Hydrocolloids 56: 227-235.
- Albuquerque P, A Cerqueira M, A Vicente A, A Teixeira J, Cunha M (2017) Immobilization of bioactive compounds in Cassia grandis galactomannan-based films: Influence on physicochemical properties. International Journal of Biological Macromolecules 96: 727-735.
- Beikzadeh S, Khezerlou A, Jafari SM, Pilevar Z, Mortazavian AM (2020) Seed mucilages as the functional ingredients for biodegradable films and edible coatings in the food industry. Advances in Colloid and Interface Science 280: 102164.
- Aydogdu A, J Radke C, Bezci S, Kirtil E (2020) Characterization of curcumin incorporated guar gum/orange oil antimicrobial emulsion films. International Journal of Biological Macromolecules 148: 110- 120.
- Davachi S, Shekarabi A (2018) Preparation and characterization of antibacterial, eco-friendly edible nanocomposite films containing Salvia macrosiphon and nanoclay. International Journal of Biological Macromolecules 113: 66-72.
- Torrieri E, Cavella S, Masi P (2015) Effect of rosemary oil and an emulsion of essential oils on structure and physical properties of chitosan film. Chemical Engineering Transactions 43: 25-30.
- Ma Q, D Hu, H., Wang L (2016) Tara gum edible film incorporated with oleic acid. Food Hydrocolloids 18: 257-261.
- Cerqueira M, WS Souza, BA Teixeira J, A Vicente A (2013) Utilization of galactomannan from Gleditsia triacanthos in polysaccharide-based films: Effects of interactions between film constituents on film properties. Food and Bioprocess Technology 6: 1600-1608.
- Almeida LBS, Figueiredo EAT, Dias FGB, Santos FMS, Fernandes BD, et al. (2021) Antimicrobial properties of chitosan and galactomannan composite coatings and physical properties of films made thereof. Future Foods 3: 100028.
- Kurt A, Toker O, Tornuk F (2017) Effect of xanthan and locust bean gum synergistic interaction on characteristics of biodegradable edible film. International Journal of Biological Macromolecules 102: 1035-1044.
- Amalraj A, T Haponiuk J, Thomas S, Gopi S (2020) Preparation, characterization and antimicrobial activity of polyvinyl alcohol/gum Arabic/chitosan composite films incorporated with black pepper essential oil and ginger essential oil. International Journal of Biological Macromolecules 151: 366-375.
- Mohite AM, Chandel D (2020) Formulation of edible films from fenugreek mucilage and taro starch. SN Applied Sciences 2: 1900.
- ASTM E96-95, Annual Book of ASTM, American Society for Testing and Materials, Philadelphia, PA, USA.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...