Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-6070
Research Article(ISSN: 2638-6070) 
Bacterial and Parasitic Load of Salad Vegetables (Cabbage and Lettuce) Sold in a Market in Southern Nigeria
Volume 4 - Issue 5Iyevhobu Kenneth O1,2,3*, Omolumen Lucky E4, Babatope Isaac O5, Aliemhe Charity A6, Ken Iyevhobu Benedicta A3 and Oseni David I3,4
- 1Department of Public Health, National Open University of Nigeria, Uromi Community Study Centre, Nigeria
- 2Lassa Fever Enable Study CEPI/ISTH Irrua, Nigeria
- 3St. Kenny Research Consult, Ekpoma, Nigeria
- 4Department of Chemical Pathology, Faculty of Medical Laboratory Science, Ambrose Alli University, Nigeria
- 5Department of Haematology, Irrua Specialist Teaching Hospital, Nigeria
- 6Department of Biological Sciences, School of Applied Science, Auchi Polytechnic Auchi, Nigeria
Received: January 02, 2023 Published: February 14, 2023
*Corresponding author:Iyevhobu KO, Department of Public Health, National Open University of Nigeria, Uromi Community Study Centre, Uromi, Edo State, Nigeria
DOI: 10.32474/SJFN.2023.04.000197
Abstract
This project aimed at establishing the presence of bacteria and parasitic ova, cysts, larvae and trophozites on salad vegetables (cabbage and lettuce) sold in Iruekpen Market. Samples were collected from different traders in the Iruekpen market. The samples were analysed using standard microbiological methods. A total number of 8 samples of two different types of salad vegetables (Cabbage and Lettuce) were examined for parasites (protozoa and geohelminths) of which 5 (62.5%) were found positive for different parasite forms. The parasites encountered include Ascaris lumbricoides, Entamoeba histolytica, Trichuris trichiura and Strongyloides stercoralis. These percentages suggest a high risk of human infection, since parasites which exist in association with these vegetables are capable of infecting human; especially Ascaris lumbricoides, Entamoeba histolytica and Trichuris trichuria are highly prevalent from both farms and markets. The six bacteria species isolated and identified from the salad vegetable samples are Staphylococcus aureus, Proteus spp, Bacillus subtilis, Salmonella spp, Pseudomonas aeruginosa and Escherichia coli. were organisms isolated and identified as contaminant of vegetable samples. Total aerobic mesophilic count was highest in lettuce (4.52 x 106cfu/g) while the Total coliform count was highest in lettuce (3.66 x 106cfu/g) and lowest in cabbage (2.04 x 106cfu/g). From this study, some of the isolated organisms have serious public health risk while others encourage spoilage of the vegetables. High numbers of these microorganisms in raw consumed and salad produce would lead to the consumer’s illness with attendant symptoms and consequences of the particular or combined microbial presence. It is therefore necessary and important for regulatory authorities in conjunction with the government to formulate a technique or safe way of producing, handling, processing, storing and retailing leafy vegetables especially in developing countries such as Nigeria.
Keywords:Salad; Vegetable; Microbes; Bacterial; Parasite; Market
Introduction
Vegetable salad is a very common food accompaniment in Nigeria. Salad is a form of food made primarily of a mixture of raw vegetables and or fruits [1]. Common vegetables used in salad include cucumber, pepper, tomatoes, onions, red onions, cabbages, carrots, spring onions, and radishes. Other ingredients such as olives, mushrooms, hard-boiled eggs, cheese, meat or seafood are sometimes added to salads. There are reports that a large number of vegetables can serve as good sources of antioxidants and phytonutrients, and have health protecting properties, to improve human well-being [2]. However, salads containing raw vegetables may be unsafe, mainly because of the environment under which they are prepared and consumed Taban and Halkman et al., 2011.
The recent increase in awareness of the health benefits of vegetables has resulted in increased consumption. Insufficient consumption of fruit and vegetable contributes to poor health and increases the risk of non-communicable diseases [3]. Consumption of vegetables as part of a diet contributes to weight loss [4] reducing the risk of obesity, a risk factor for non-communicable diseases. Because of their health benefits, WHO and FAO in 2003 launched a global initiative to promote the consumption of fruits and vegetables [3]. Vegetables are essential for human health and well-being, and they form a major component of healthy diet in every family. They are highly beneficial for maintenance of health and prevention of diseases. Vegetables contain valuable nutritional factors, which can be successfully utilized to build up and repair the body. They are valued mainly for their high carbohydrate, vitamins, minerals, and fibre contents. Joint FAO/ WHO Expert Consultation on diet, nutrition and the prevention of chronic diseases, has recommended the intake of a minimum of 400g of vegetables and fruits per day for the prevention of chronic diseases such as heart disease, cancer, diabetes and obesity, as well as for the prevention and alleviation of several micronutrient deficiencies, especially in less developed countries [5]. Consumption of raw vegetables and salads is a common practice, as they retain natural flavor and preserve heat labile nutrients. They are vital energy contributors that are depended upon by all levels of human as food supplement or nutrient. The extent of contamination depends on several factors that include, among others, use of untreated wastewater and water supplies contaminated with sewage for irrigation [6].
[7] reported that bacterial contamination results from various unsanitary cultivation and marketing practices. In another study, [8] reported that bacterial contamination of salad vegetables was linked to the fact that they are usually consumed without any heat treatment. These vegetables can become contaminated with pathogenic microorganisms during harvesting, through human handling, harvesting equipments, transport containers, wild and domestic animals. Pathogens from the human and animal reservoir as well as other environmental pathogens can be found at the time of consumption. Although spoilage bacteria, yeasts and mould dominate the micro flora on raw fruits and vegetable, the occasional presence of pathogenic bacteria, parasites and viruses capable of causing human infections has also been documented [9].
Every year, millions of individuals become ill from food borne diseases and in those salads vegetables may be sources of pathogen transmission [10]. In recent years, the occurrence of antibiotic resistant strains of a number of pathogenic bacteria has emerged as another health concern all over the world [11]. Several outbreaks of intestinal parasitic infections epidemiologically associated with raw vegetables have been reported from developed and developing countries. Although raw vegetables (Salad) are important part of human diet, there is more chance of microbial infection in human being through consumption of salad, contaminated with various microorganisms. The consumption of raw vegetables without proper washing is an important route in the transmission of parasitic diseases also [12]. Salads are considered as a high-risk food because they do not require any heating or standard washing prior to consumption. Microbial infection may cause a serious health problem. This project was aimed at establishing the presence of bacteria and parasitic ova, cyts, larvae and trophozoites on salad vegetables (cabbage and lettuce) sold in Iruekpen market.
Materials and Methods
Study Area and Population
This study was carried out in Iruekpen town, a semi urban town located in Esan West Local Government Area of Edo State Nigeria with longitude 6.13oE and latitude 6.73oN having a population of about 61,870 people. Iruekpen is located in Eguare- Egoro off Benin/Auchi express road in ward 6. The town is bounded to the North by Ujemen, to the south by Orua, to the east by Ozalla and west by Ehor. The town is governed by ‘Edionwere’ in the community. The town is a clan because it is a part of Ekpoma. Iruekpen has 12 quarters namely; Idumegbeide, Idumoza, Idumebhor, Idumebo, Idumemalua, Idumeke, Ikhine, Abia, Idumogo, Ogbomoide, Ughodin, Ebhokpe (NPC, 2007).
Materials
The following materials and apparatus were used for the analysis: normal saline, iodine solution, saturated salt solution, formol ether, distilled water, binocular microscope with camera, centrifuge, weighing balance, measuring cylinder, test tubes, microscope slides, cover slips, sieves and beakers.
Sample Collection
Samples were collected from different traders in Iruekpen market. The samples collected were cabbage (Brassica oleracea) and lettuce (Lactuca sativa). These were collected randomly from different locations and vendors in Iruekpen Markets. They were then placed in polythene bags and labeled with date of collection, name of the sample, time and place of collection and taken to the laboratory for analysis.
Examination/Analysis of Samples
Microbial Analysis: Samples of cabbage and lettuce (10g each) were washed with 90ml of sterile distilled water separately and the water drained after washing was collected in a sterile beaker. Serial dilution was carried out in a test tube using 1ml of the wash water to 9ml of distilled water from 10-1 – 10-6 for each of the sample. Dilution 10-4 – 10-6 was used, 1ml of each dilution was placed in the middle of the petri dish. Agar was prepared using manufacturers description. Agar used was Nutrient and MacConkey. It was allowed to cool down to body temperature and 20ml was poured in each of the petri dish containing the dilution, it was rocked gently on the working bench and allowed to solidify and was placed upside down in an incubator at 370C for 24 hours. After 24 hours, the total number of heterotrophic and coliform count were determined as a colony forming unit per ml (cfu/ml) of samples. Total number of microbe per gram of salad was calculated accordingly. Pure colonies were isolated using streaking method on a nutrient agar and was incubated for 24 hours at 370C. Gram staining was carried out on each of the colonies. Slant was made using nutrient agar of reduced concentration and each of the colonies were placed on each slant and it was incubated at 370C for 24 hours for further biochemical test.
Motility Test: This test is performed to distinguish the motile organisms from the non-motile ones. Nutrient agar of reduced concentration was used. With the aid of a straight wire loop, a colony of the test organism was inoculated and stabbed on a semi solid nutrient agar. It was incubated at 370C for 24 hours. After inoculation, spikes were looked out for, presence of spikes indicated motile organism while absence of spikes indicated non motile organism.
Detection of Parasites
Macroscopy: The unwashed vegetable salad samples were carefully examined for the presence of parasites, segments of cestodes and larva of adult nematodes.
Microscopy: A weighed sample (10 g) was placed into a sterile beaker and washed with 100 ml physiological saline solution (0.9% NaCl). After removing fragments of the vegetable sample from the washed saline using clean forceps, it was kept for 24 hours to allow sedimentation to take place. Afterward, the top water was discarded, and the remaining wash water was centrifuged at 2000rpm for 15 min. The supernatant was discarded, the residue was carefully collected and examined in lugol stained slides using light microscopy. The preparation was examined under a light microscope using X10 and X40 objectives for detection of parasites. Cysts and eggs of parasites found under the microscope were identified Downes and Ito et al., 2001. The same experiment was carried out for each of the samples. The examination of these specimens was done according to the methods described by Cheesbrough et al., (2006) [13].
Sedimentation Technique: A portion of the wash water was filled into a clean test tube and taken to the centrifuge then balanced with another test tube with the same volume of water or another sample. It was then centrifuged for 5 minutes at 3000rpm after which the supernatant was discarded and the deposits were re-suspended. Two clean grease free microscope slides were taken, and a drop of the re-suspended deposit was placed on each of the slides. On one of the slide containing the deposit, a drop of Lugols iodine solution was added to stain the nucleus of cyst of parasites if present. They were then covered with grease free cover slips and were examined with X 10 objective X 40 objective lenses under the microscope and results recorded.
Floatation Technique: About 50g of the vegetables samples were washed in saturated salt solution (100ml) and filtered. The filtrate was then added into a test tube until it formed a convex meniscus after which a clean grease free slide was placed on top of the test tube containing the sample and left to stand upright for 1 hour. The slide was gently removed and covered with a cover slip and was examined under X10 and X40 objectives lenses of the microscope for detection of eggs of parasite.
Result
This study evaluates the bacteria load and parasitic contamination of salad vegetables (Cabbage and Lettuce) sold in Iruekpen market.
Bacteriological Analysis of Salad Vegetables (Lettuce and Cabbage)
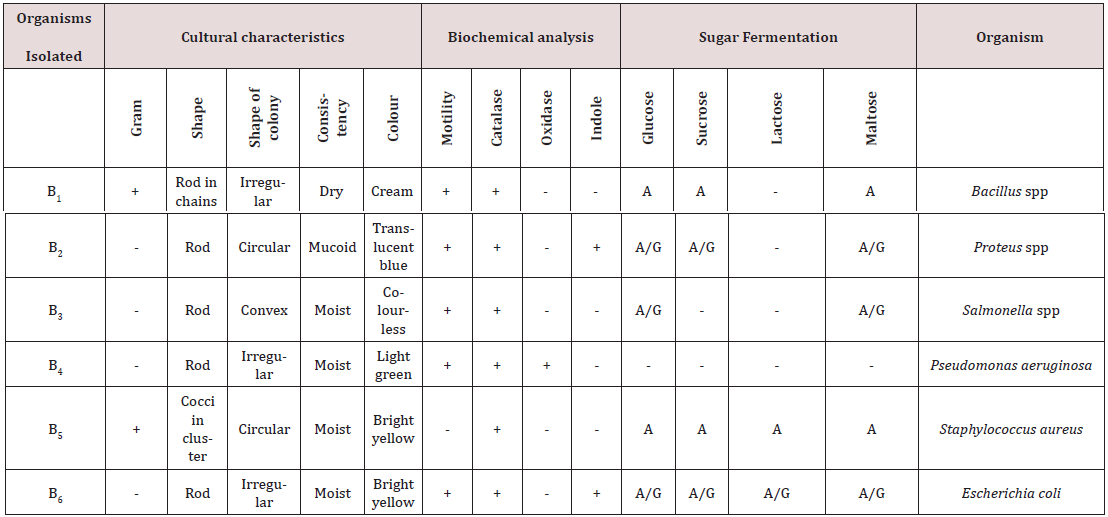
In this study the bacterial were randomly isolated from salad vegetables (lettuce and cabbage) were enumerated mainly to assess the level of bacterial contamination. In the present study high percentages of total bacterial counts were observed in cabbages. The six (6) bacteria species isolated and identified from the salad vegetable samples were Staphylococcus aureus, Proteus spp, Bacillus subtilis, Salmonella spp, Pseudomonas aeruginosa and Escherichia coli. The result of the Cultural Characteristics and Biochemical Analysis Bacterial Isolates conducted on the bacteria colonies during fermentation and storage period is shown in Table 1. The organisms isolated, some were gram positive and others were gram negative. The organisms on the agar plate showed circular to irregular shape, they were bright yellow, creamy, grey, translucent blue and translucent creamy in colour with surface appearances (consistency) which were moist, mucoid and dry.
Key: + = Positive; - = Negative; A = Acid; A/G = Acid/Gas; B = Bacteria.
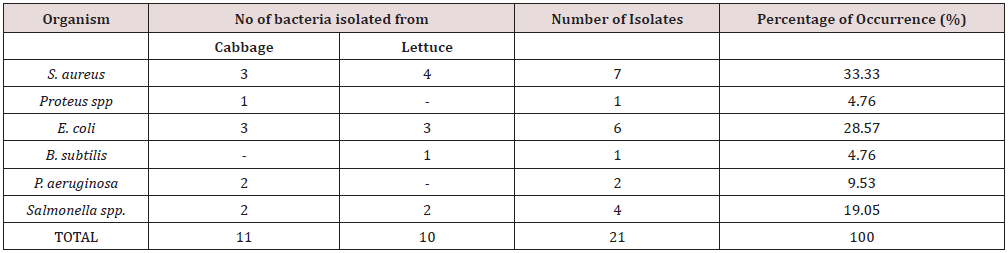
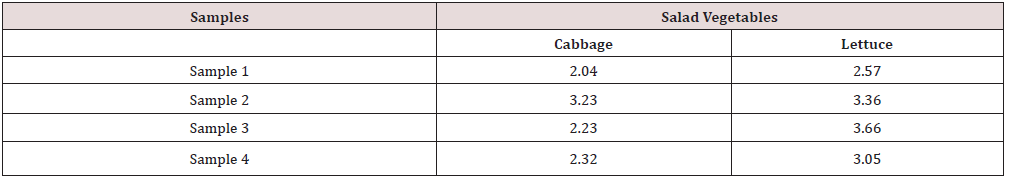
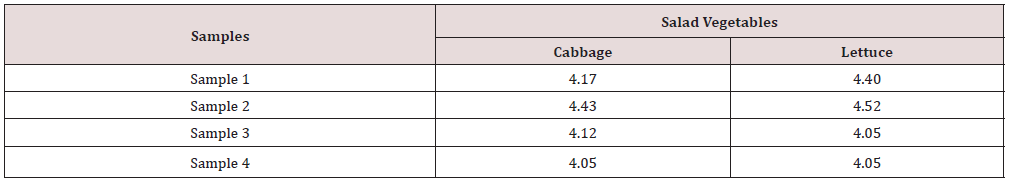
The distribution of bacteria contaminant in salad vegetables studied is shown in Table 2. Staphylococcus aureus has the highest percentage of occurrence (33.33%), followed by Escherichia coli (28.57%), Salmonella spp (19.05%), Pseudomonas aeruginosa (9.53%) while Proteus spp and Bacillus subtilis has same percentage of occurrence of 4.76%. Table 3 presents the mean Total Aerobic Mesophilic Count (cfu/g) of salad vegetables (Cabbage and Lettuce) studied. Total aerobic mesophilic count was highest in lettuce sample 2 (4.52 x 106cfu/g). Table 4 presents the mean Total coliform Counts (cfu/g) of salad vegetables (Cabbage and Lettuce) studied. Total coliform count was highest in lettuce sample 3 (3.66 x 106cfu/g) and lowest in cabbage sample 1 (2.04 x 106cfu/g).
Table 3: Mean Total Aerobic Mesophilic Count (x 106cfu/g) of salad vegetables (Cabbage and Lettuce).
Prevalence of Parasites on Vegetables
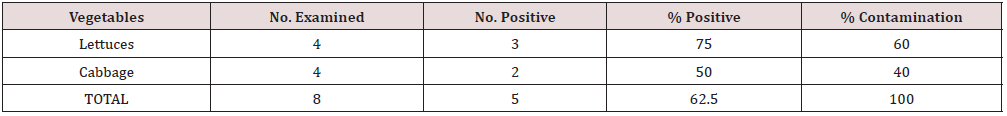
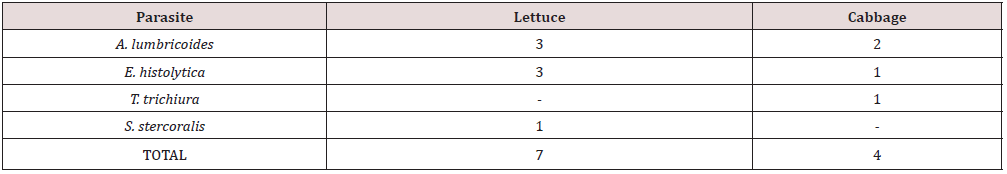
A total number of 8 samples of two different types of salad vegetables (Cabbage and Lettuce) were examined for parasites (protozoa and geohelminths) of which 5 (62.5%) were found positive for different parasite forms. Among the salad vegetables (Cabbage and Lettuce) examined in the study area, lettuce had the highest parasite prevalence of 60%, followed by cabbage (40%) (Table 5). The prevalence of each parasite on each salad vegetable showed that A. lumbricoides and E. histolytica had the highest infection rate in Lettuce, followed by S. stercoralis while on the cabbage examined, A. lumbricoides had the highest prevalence, followed by E. histolytica and T. trichiura with same number of prevalence (Table 6).
Discussion
The microorganisms present in salad vegetables are a direct reflection of the sanitary quality of the cultivation water, harvesting, transportation, storage and processing of the produce [14]. All the parasite and bacterial isolates were present on the outer surfaces of the samples. In developing countries like Nigeria, intestinal parasites are very common in the environment. Fresh vegetables are an important route of their transmission. The results of this study showed that locally consumed vegetables are often contaminated with human intestinal parasites especially in areas where night soil or wastewater reuse is practiced. Fresh salad vegetables have been found to be a salient mode for the transmission of parasitic infections [15,16]. Vegetable consumers and agricultural workers are a major target of these infections. Four species of parasites recorded in this study have been reported as frequent gastro-intestinal parasites in many parts of Nigeria [17,18]. The persistent occurrence of these parasites suggests a high level of contamination and longtime transmission; however the occurrence of these parasitic species in various salad vegetables varied.
The variation of contamination among the salad vegetables might be due to uneven surfaces which make the parasitic stages attach more easily to the surface of these vegetables. The occurrence of pathogenic microorganisms in vegetables most especially salad vegetables is an indication of the quality of the overall process of cultivation, irrigation and post-harvest handling [19]. The present study has attempted to assess the bacteria and Parasitic Load of Salad Vegetables Sold in Iruekpen Market. The overall parasitic contamination rate was found to be 62.5%. Out of 8 samples of salad vegetables (lettuce and cabbage) examined, 5 (62.5%) (Cabbage and lettuce) (Table 5) were positive to parasite. Although the population in each is not high, it is however a greater indication that much care should be taken in handling salad vegetables before consumption. It is not also the population that matters but the deleterious harmful effects to human health. The parasites encountered include Ascaris lumbricoides, Entamoeba histolytica, Trichuris trichiura and Strongyloides stercoralis. These percentages suggest a high risk of human infection, since parasites which exist in association with these vegetables are capable of infecting human; especially Ascaris lumbricoides, Entamoeba histolytica and Trichuris trichuria are highly prevalent from both farms and markets.
[15] examined different types of vegetables for parasitic contamination and reported that cabbage had the highest contamination rate of (64%), followed by lettuce (40%) which were higher than the present study which is in contrast with this study. The observed differences in prevalence rates of the different intestinal parasites from fresh vegetables reported in the present work and those reported by others is expected. Several factors may contribute to such differences. These may include, among other factors, geographical location, type and number of samples examined, methods used for detection of the intestinal parasites, type of water used for irrigation, and pre-harvest handling methods of such vegetables. Parasites’ eggs and trophozoites attach to themselves surface of salad vegetables more easily. The presence of these parasites may be due to lack of modern toilet facilities, inadequate public health enlightenment and illiteracy that make people defecate indiscriminately resulting in pollution of water and farmland [15].
The risk of infection with intestinal parasites to the population is increased because these contaminated salad vegetables are sometimes eaten raw, under parboiled to retain the natural taste and preserve heat labile nutrients [12]. Contamination of soil and water sources with human faeces and poor sewage disposal such as use of the soil for fertilizers Mustafa et al., (2001), eggs in the soil can be transferred on to salad vegetables. These findings are comparable to various studies carried out on vegetables and fruits in some markets in other parts of Nigeria; In Ibadan, only Ascaris lumbricoides, hookworm and Strongyloides stercoralis were isolated, Ascaris lumbricoides was the most prevalent similar to our findings Alli et al., (2011). In Ebonyi, only Ascaris lumbricoides, hookworm and Trichuris trichuria were isolated with Ascaris lumbricoides also as the most prevalent [20]; this is also similar to our findings although hookworm and Trichuris trichuria was not found in ours and all these three parasites were also isolated in Ilorin markets. In Jos, Trichomonas hominis was found to be the most prevalent [15] which is not the case in our findings and in the other two parts of Nigeria aforementioned. In all these findings Ascaris lumbricoides is common in all the markets.
Recently, [21] reported the detection of intestinal parasites in 29% (13/45) of native garden vegetables consumed in Ardabil city, Iran. Similarly some previous studies have reported vegetable contamination with intestinal parasites ranged from 1.94% to 68.3% in different parts of Iran [22]. In line with this finding, Oliveira and Germano (1992) [23] reported that from the parasites studied, Ascaris eggs were the highest in number which is in close agreement with this result. [24] in Turkey detected soil-transmitted helminths (mainly A. lumbricoides) in 14% (14/100) of fresh vegetables, in 84% of soil samples where vegetables are cultivated and in 61% of irrigation water. Another study from Iran, reported prevalence of 25% and 29% for intestinal parasites in vegetables of markets and gardens, respectively with A. lumbricoides eggs being detected in 2% of samples examined which is very low compared to the present result [21]. Previous studies from different countries where STH infection is endemic have shown that vegetables were highly contaminated with Ascaris lumbricoides eggs and had similar findings with the present study, e.g. Malaysia, Thailand, and Philippines [25].
Data showing the presence of Ascaris lumbricoides in this study are similar to those identified by other investigators [26, 27] in Korea reported that ascaris eggs were found to be the highest (49.0%) among lettuce (Lactuca sativa), young radish (Raphanussapivus) and Chinese cabbage (Brassica pekinensis) where Chinese cabbage showed the highest degree of contamination (91.1%) and lettuce being next (49.0%) in positivity of ascarid eggs. A study from Saudi Arabia also reported the detection of Ascaris lumbricoides in 16% of leafy vegetables examined which was lower than the present study [28]. Another study from Iran, reported prevalence of 25% and 29% for pathogenic parasites in vegetables of markets and gardens, respectively with A. lumbricoides eggs being detected in 2% of samples examined [25]. A. lumbricoides is the causative agent of ascariosis. The reservoir host of the parasite is man. The eggs contaminates the soil or vegetable though promiscuous defecation or through the use of manure as organic fertilizer on farmland. The eggs are ingested by humans with contaminated food, soil and less frequently drinking water. The occurrence of A. lumbricoides in some stream, river and other sources of water has been reported by the work of Nwele et al., (2013), Solomon et al., (2013) [29,30] which is also a predisposing factor to the contamination of vegetables when such water is used for irrigation. One sixth of the human population is estimated to be infected by Ascaris lumbricoides or another roundworm (Harhay et al., 2010).
Ascariasis is prevalent worldwide and more so in tropical and subtropical countries. Infections with these parasites are more common where sanitation is poor (Harhay et al., 2010) and raw human faeces are used as fertilizer. Often, there are no symptoms with an A. lumbricoides infection. However, in the case of a particularly bad infection, symptoms may include bloody sputum, cough, fever, abdominal discomfort and passing worms (Harhay et al., 2010). Infection with A. lumbricoides was associated with increased risk of asthma (p < 0.001) and atopy in China rural children Lyle et al., (2002). In previous study [31] reported that E. histolytica was detected in 100% (27/27) of fresh lettuce samples which was higher than the present study. As shown from the results the degree of contamination for vegetables was different from each other depending on the kind of vegetable. This could be due to the difference in distance between the soil and the plant leaves.
The presence of Strongyloides stercoralis is of serious concern due to its ability to exist in a free living state and it usually does not require a host for its proliferation.
The detection however is an indication that fecal contamination of salad with the parasite and other extrinsic bacterial and viral agents that can cause serious infection is present. Farmers need to be sensitized on the potential health risk associated with the use of sewage contaminated water for irrigation. Food vendors in particular and the general public must be informed on the need to properly wash raw vegetables with salts before consumption. According to Umoh et al., (2001) [17] the rate of contamination of food is dependent on the sanitation in a particular environment and sanitary habits of people living in such environment. This is also in line with the result of this study which also showed that the parasite occurrence varied with market location. Dalomo et al., (2003) reported that the degree of pollution is attributed to poor sanitary habits of the people where human wastes are indiscriminately disposed around houses and vegetable gardens.
This study also showed that Entamoeba histolytica and Ascaris lumbricoides were the most common parasite species found in the food products. This confirmed the findings of Leon et al. (1992) [19,33]. The water used for washing the vegetables might have introduced these parasites. The presence of soil transmissible helminthes is an indication of poor Socio-Economic condition, as well as poor environmental and sanitation practices. The high level of these parasites can also be attributed to favorable weather and climatic condition, such as high temperature, high humidity and rainy season. [33], in Sanliurfa, Turkey, detected soil transmitted helmin thes (mainly A. lumbricoides) in 14% of fresh vegetables, in 84% of soil samples where the vegetables were cultivated, and in 61% of irrigation water. The findings strongly indicate that the vegetables were irrigated with sewage water.
Farmers should, therefore, ensure that salad vegetables are grown hygienically, the use of sewage or waste water with potential risks of transmitting infectious pathogens should be minimized or discouraged. Salad vegetable sellers and consumers should endeavour to wash all products thoroughly before selling and consumption. This can be achieved through proper washing in clean chlorinated water. The discrepancy between the present study and previous studies might be as a result of the variations in geographical locations, climatic and environmental conditions, the kind of sample and sample size examined, the sampling techniques, methods used for detection of the intestinal parasites, and socioeconomic status. So long as these factors differ, consequently the discrepancy of the results would be expected.
The high microbial load obtained from the isolation of microorganism from the vegetable samples could probably be directly linked to the recorded use of waste water in irrigation for watering the field, or the manure used for fertilization and the unhygienic condition of the area where the vegetables were being grown and sold. The result correspond to the findings of Buck et al., (2003) [34] who reported that the presence of many pathogens in the soil was thought to be from historical application or environmental presence of feaces or untreated sewage and pathogens existing in the soil or water can be the source of both pre- and post-harvest contamination respectively. The slight variation in the microbial load from other sources can be traced to prewashing of vegetables with portable water.
Among the organisms encountered during this study Staphylococcus aureus with the highest occurrence is in agreement with the studies of Akinyele et al., (2013) [35]. [36] also showed presence of Salmonella, Serratia, Enterobacter, Staphylococcus aureus, E. coli and P. aeruginosa in vegetables and fruits. S. aureus is a normal microbial flora of the mucus membrane and on the human skin. It is an opportunistic pathogen and enterotoxigenic strains which are known to cause serious food borne disease and has been reported that ingestion of the thermostable enterotoxins, rather than the bacterium itself is responsible for food borne illness [35]. They cause boils, abscesses, post-operative infections, toxic shock syndrome and food poisoning in man [37]. Pre-harvest conditions can come from irrigation water, improperly composted manure used as fertilizer, fecal contamination from human and domestic animals [38].
Other organisms encountered during the study and their respective health implications include Bacillus spp causing diarrhoeal. The occurrence of Bacillus spp in vegetables agrees with the findings of Mead et al. [39] who reported that estimated 27,000 cases of food borne illness are due to Bacillus species. Bacillus spp. are part of the natural flora and normally lives in the soil. They are among the most common vegetable spoilage bacteria [37], though some Bacillus species are capable of causing food borne illness. The occurrence of E. coli may be linked to animal dung and manure used during the cultivation of vegetables as fertilizers. Other organisms encountered include Salmonella spp which has been reported to be responsible for typhoid fever [40] and several cases of an outbreak of typhoid fever have been associated with consumption of contaminated vegetable grown in or fertilized with contaminated soil or sewage [41].
Pseudomonas aeruginosa is a prominent inhabitant of soil and organism is responsible for diseases of vegetables like angular leaf spot of many vegetables it has become an important cause of infection and it is a frequent cause of nosocomial infections such as pneumonia, urinary tract infections (UTIs), and bacteremia [42]. It invades burns area, causes septic shock and responsible for cystic fibrosis in human. They are also associated with spoilage of vegetable. [37] described Proteus spp as an organism that occur in the intestine of human and in a wide variety of animals, in polluted water, soil and they are opportunistic pathogens. In the present study, coliform counts were lower in all samples. This agreed with reports of earlier workers of Cornish et al. [26,38]. This result was closer with the findings of Thunberg et al. (2004). They have reported total viable count as 5.0×108, 4.0×108, 3.1×107, 2.5×107, and 2.0×106 CFU g-1 for spinach samples collected from various farm sites. Bacterial numbers recorded in this study exceed the International Commission on Microbiological Specifications for Food limits of 103 to 105 coliforms 100 g-1 wet weight of vegetables usually eaten raw ICMSF (1998).
Aerobic organisms reflect the exposure of the sample to contamination and the existence of favorable conditions for multiplication of microorganisms. For export purposes, it is important that fresh vegetables should not have a total aerobic count exceeding 4.9×106 CFU g-1 which is the acceptable limit by some countries [43]. Therefore, reducing the total count on the products is a priority to ease the economic impact of such contamination. However, some studies showed low levels of contamination in Egypt, Turkey and Taiwan food Products reported by Lund et al., (1993). In contrast Fang et al., (2003) reported high aerobic plate count on vegetable samples in Taiwan ranging from 2.0×103 to 4.4×108 CFU g-1. Furthermore, Vural and Erkan et al., (2008) in Turkey had a range of aerobic count of microorganisms from 2.7×106 to 4.3×107cfu g-1. Similarly for salad vegetables collected from Johannesburg in South Africa, Christison et al., (2008) reported an average aerobic plate count of 1.0×107cfu g-1. In other studies a range from 1.0×102 to 1.0×106cfu g-1 was obtained by different scholars [44,45]. In close agreement with the present result, a study in India Viswanatha and Kaur et al., (2001) showed that the total aerobic plate count for cabbage and lettuce was found to be 2.8 ×106 and 1.2 ×108 CFU g-1 respectively. Whereas in the same study the total coliform count for these two vegetables was from 2.0×102 - 2.5×103 for cabbage and 1.2×105 - 7.0×106 for lettuce.
In this study, the high prevalence of bacteria and parasites was further enhanced by unhygienic mode of transportation of these consumable products. Also local practice of using organic manure, such as human, animal and poultry dropping as fertilizer contributed immensely to the occurrence of the pathogen research. The contamination of vegetables by pathogenic bacteria and parasites could also be as a result of poor handling practices in food supply chain, storage conditions, distribution, marketing practices and transportation [46,47]. Lettuces and carrot are produced and transported from the Northern parts of Nigeria where human and animal wastes are used as manure to supplement fertilizers and contaminated waters are used for cleaning the farm products before they are offered for sale. The use of feacal contaminated water and hands to wet the vegetable in the bid to make it fresh and attractive are also important contaminating agents. The habit of eating raw salad vegetables like cabbage and lettuce is commonly practiced in the study area. Hence, the findings of the present study are of public health importance, requiring an appropriate intervention to prevent transmission of bacteria and parasitic diseases that can be acquired through consumption of contaminated fruits and vegetables. However, this study did not address the effect of seasonal variation on the contamination of the salad vegetables. The findings of this study could not underscore the infectivity of the parasitic stages detected as viability study was not conducted.
In conclusion, contamination of fresh salad vegetables sold in Iruekpen market with pathogenic bacteria and intestinal parasites may pose a health risk to consumers of such products. The local health and environmental authorities should educate the public on the health hazards of fresh salad vegetables and the importance of washing and disinfecting them before consumption. This study highlighted the importance of salad vegetables as the potential source of transmission for intestinal parasites to humans. The salad vegetables contamination with the pathogenic parasites poses health risk to the consumers if consumed without proper cleaning and or cooking. Prevention of contamination remains the most effective way of reducing food borne parasitic infection. Although salad vegetables are commonly associated with food poisoning, they harbour disease causing organisms. The growth of these in the environment such oil which is a rich source of microbes. Poor agricultural practices such as irrigation with contaminated water also may introduce microorganisms. Poor storage and transportation practices can result in contamination as well as poor handling by dealers, processing and consumer in the home. Side by side is the huge nutritional benefit derivable from consumption of these vegetable especially that are therapeutic, curative and preventive. Just as with other foods consumer have some responsibilities to carry when handling these vegetables, wash hand with warm water and soap before and after handling the vegetables. Rinse raw product in clean water, brush off debris. For cutting, plastic board should be used instead of wooden ones.
Conflict of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or not-for- profit sectors.
Acknowledgements
The authors would like to thank all the Laboratory and technical staffs of St Kenny Research Consult, Edo State for their excellent assistance and for providing medical writing support/ editorial support in accordance with Good Publication Practice (GPP3) guidelines.
References
- Rajvanshi A (2010) Bacterial Load on Street Vended Salads in Jaipur City, India. Internet J Food Safety 12: 136-139.
- Taura DW and Habibu AU (2009) Bacterial Contamination of Lactuca sativa, Spinacia olerencea, and Brassica olerencea in Kano Metropolis. Int J Biomed Hlth Sci 5 (1): 55-57.
- WHO/FAO (2016) WHO and FAO announce global initiative to promote consumption of fruit and vegetables.
- Whigham LD, Valentine AR, Johnson LK, Zhang Z, Atkinson RL, et al. (2012) Increased fruit and vegetable consumption during weight and fat loss. Nutr Diabetes 2(10): 48.
- WHO (2003) Diet, nutrition and the prevention of chronic diseases. Report of a joint FAO/WHO expert consultation. Geneva, World Health Organization, WHO Technical Report Series.
- Amoah P, Drechsel P, Henseler M and Abaidoo R (2007) Irrigated Urban Vegetable Production in Ghana: Microbiological Contamination in Farms and Markets and Associated Consumer Risk Groups. Journal of Water and Health 5(3): 455-466.
- Khan MR, Saha ML, Kibria AM (1992) A bacteriological profile of salad vegetables in Bangladesh with special reference to coliforms. Applied Microbiology 14: 88-90.
- Tambekar D and Mundhada R (2006) Bacteriological Quality of Salad Vegetables Sold in Amravati City (India). Journal of Biological Sciences 6(1): 28-30.
- Elhariry HM (2011) Biofilm formation by Aeromonas hydrophila on green-leafy vegetables: Cabbage and lettuce. Foodborne Patho Dis 8(1): 30-35.
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, et al. (2008) Global trends in emerging infectious diseases. Nature 451(7181): 990-993.
- Graziani BL, Battisti C, Franco A, Ricci A, Vio D (2004) Antibiotic resistance in Salmonella enterica serotypes Typhimurium, Enteritidis and Infantis from human infections, foodstuffs and farm animals in Italy. Epidemiol Infect 132(2): 245-225.
- Slifko TR, Smith HV and Rose JB (2009) Emerging parasites Zoonoses associated with water and food. International Journal of Parasitology 30(12-13): 1379-1393.
- Cheesbrough M (2006) Microbiological tests: biochemical tests to identify bacteria. District laboratory practice in tropical countries. 2nd Cambridge University Press, Cambridge, USA p. 62-70.
- Ray B, Bhunia AK (2007) Fundamental Food Microbiology. 4th, CRC Press, USA, pp. 492.
- Damen JG, Banwat EB, Egah DZ, Allanana IA (2007) Parasitic contamination of vegetables in Jos, Nigeria. Annals of African Medicine 6(3): 115-118.
- Adamu BN, Adamu YJ and Dauda M (2012) Prevalence of helminth parasites found on vegetables sold in Maiduguri, Northeastern Nigeria. Food Control 25 (1):23-26.
- Umoh VI, Okafor C, Galadima M (2001) Contamination by helminthes of vegetable cultivated on land irrigated with urban wastewater in Zaria and Kaduna, Nigeria. The Nigeria journal of parasitology 22(2): 95-104.
- Uneke CJ, Uneke BI (2007) Occurrence of Cryptosporidium species in surface water in Southeastern Nigeria: The public health implication. eHealth J 7(2).
- Ogunleye VF, Babatunde SA and Ogbolu DO (2010) Parasitic contamination of vegetables from some market in southwest Nigeria. The Tropical Journal of Health Sciences 17(2): 23-26.
- Eni A, Oluwawemitan I, Solomon O (2010) Microbial Quality of Fruits and Vegetables Sold in Sango Ota, Nigeria. African Journal of Food Science 4(5): 291-296.
- Daryani A, Ettehad GH, Sharif M, Ghorbani L, Ziaei H (2008) Prevalence of intestinal parasites in vegetables consumed in Ardabil. Iran Food Control 19(8): 790-794.
- Sahebani N, Foladvand MA, Dalimi AH (2001) Survey of parasitic contamination in vegetables in Boosher. Proceedings of the 3rd National Congress of Medical Parasitology, pp. 204.
- Oliveira CA and Germano PM (1992) Presence of intestinal parasites in vegetables sold in the metropolitan region of Sao Paulo, SP, Brazil. Search of helminths. Rev Saude Publica 26(4): 283-289.
- Ulukanligil M, Seyrek A, Aslan G, Ozbilge H, Atay S (2001) Environmental pollution with soil-transmitted helminthes in Sanliurfa, Turkey. Memorias do Instituto Oswaldo Cruz 96(7): 903-909.
- de Leon WU, Monzaon RB, Aganon AA, Arceo RE, Ignacio EJ, et al. (1992) Parasitic contamination of selected vegetables sold in metropolitan Manila, Philippines. Southeast Asian J Trop Med Public Health 23(1): 162-164.
- Amoah P, Drechsel P, Abaidoo C (2005) Irrigated urban vegetables production in Ghana: Sources of pathogen contamination and health risk elimination. Irrig Drainage 54(s1): 49-61.
- Choi DW, Lee S (1992) Incidence of parasites found on vegetables collected from markets and vegetables gardens in Taegu area. Korean J Parasitol 10(1): 44-51.
- Al-Binali AM, Bello CS, El-Shewy K, Abdulla SE (2006) The prevalence of parasites in commonly used leafy vegetables in South Western Saudi Arabia. Saudi Medical Journal 27(5): 613-616.
- Nwele DE, Uhuo AC, Okonkwo EC, Ibiam GA, Onwe CS, et al. (2013) Parasitological examination of Ava stream used in irrigation in Enugu State, South- Eastern Nigeria: An implication for helminth transmission. Journal of Parasitology and Vector Biology 5(8): 112-115.
- Solomon C, Michael U, Bitrus J, Micheal A, Aloysius U, et al. (2013) Parasitological Evaluation of Domestic Water Sources in a Rural Community in Nigeria. British Microbiology Research Journal 3(3): 393-399.
- Abougrain AK, Mohammad HN, Nuri SM, Mohammad MS, Khalifa SG (2009) Parasitological contamination in salad vegetables in Tripoli-Libya. Food Control 21(5): 760-762.
- Leon W, Monzoon RB, Agnon AA, Arco RE, Ignaua EJ, et al. (1992) Parasitic contamination of selected vegetables sold in metropolitan Manila, Philipines Southeast Asia. Journal of public health and hygiene 23:162-164.
- Atay S, Ulukanligil M, Seyrek A, Aslan G, Ozbilge H (2001) Environmental pollution with soil-transmitted helminthes in Sanliurfa, Turkey. Memorias do Instituto Oswaldo Cruz 96(7):903-909.
- Buck JW, Walcott RR, Beuchat LR (2003) Recent trend in microbiological safety of fruits and Vegetables. Online. Plant Health Progress 10(1): 9-14.
- Akinyele BJ, Oladejo BO, Bankefa EO and Ayanyemi SA (2013) Microbiological analysis and antimicrobial sensitivity pattern of microorganism isolated from vegetables sold in Akure, Nigeria. International Journal of Current Microbiology and Applied Sciences 2(10): 306-313.
- Poorna V (2001) Prevalence and growth of pathogens on salad vegetables, fruits and sprouts. International Journal of Hygiene and environmental health 203(3): 205-213.
- Michael JP, Chan ECS and Noel RK (2005) Microbiology, New Delhi, Tata McGraw Hill. 5th Pp. 793-811.
- Cornish G, Mensah E, Ghesquire P (1999) Water Quality and Periurban Irrigation. An Assessment of Surface Water Quality for Irrigation and its Implications for Human Health in the Peri-urban Zone of Kumasi, Ghana. Report OD/TN 95. HR Wallingford Ltd, Wallingford, UK, pp. 44.
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, et al. (1999) Food related illness and death in the United States. Emerging Infectious Disease Journal 5(5): 607-625.
- Nester EU, Roberts CE, Nester MT (2004) Microbiology a Human Perspective. WC Brown Oxford. 6th Edn, pp. 524- 540.
- Beuchat LR (1998) Surface Decontamination of Fruits and Vegetables Eaten Raw: A Review. Food Safety Unit, World Health Organization. WHO/FSF/FOS/Publication 98.2. Geneva. pp. 31-42.
- Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y (2006) Multidrug-Resistant Pseudomonas aeruginosa: Risk Factors and Clinical Impact. Journal of Antimicrobial Chemotheraphy 50(1): 43-48.
- Nguyen the C, Carlin F (1994) The microbiology of minimally processed fresh fruits and vegetables. Crit Rev Food Sci Nutr 34(4): 371- 401.
- Angelidis AS, Chronis EN, Papageorgiou DK, Kazakis II, Arsenoglou KC, Stathopoulos GA (2006) Non-lactic acid contaminating flora in ready-to-eat foods: A potential food quality index. Food Microbiol 23(1): 95-100.
- Aycicek H, Oguz U, Karci K (2006) Determination of Total Aerobic and Indicator Bacteria on Some Raw Eaten Vegetables from Wholesalers in Ankara, Turkey. International Journal of Hygiene and Environmental Health 209(2): 197-201.
- Effiuvwevwere BJO (2000) Microbial Spoilage Agents of Tropical and Assorted fruits and Vegetables (An Illustrated References Book). Paragraphics publishing company, Port Harcourt. 1st Pp. 1-39.
- Ochei J, Kolhatker A (2000) Medical microbiology [parasitology] medical laboratory science theory and practice. Tatamc Graw-Hill. Pp. 909-912.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...