Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4706
Research Article(ISSN: 2637-4706) 
Effect of Combination Superdisintegrants on the Drug Delivery of Tolvaptan. Hcl Volume 3 - Issue 5
Raghavendra Kumar Gunda*1, Prasada Rao Manchineni2 and Rajini Sudireddy3
- 1Department of Pharmaceutics, M.A.M College of Pharmacy, Andhra Pradesh, India
- 2Department of Pharmaceutical Analysis, M.A.M College of Pharmacy, Andhra Pradesh, India
- 3Department of Pharmacy Practise, M.A.M College of Pharmacy, Andhra Pradesh, India
Received: February 16, 2021; Published: March 03, 2021
Corresponding author: Raghavendra Kumar Gunda, Department of Pharmaceutics, M.A.M college of Pharmacy, India
DOI: 10.32474/DDIPIJ.2021.03.000176
Abstract
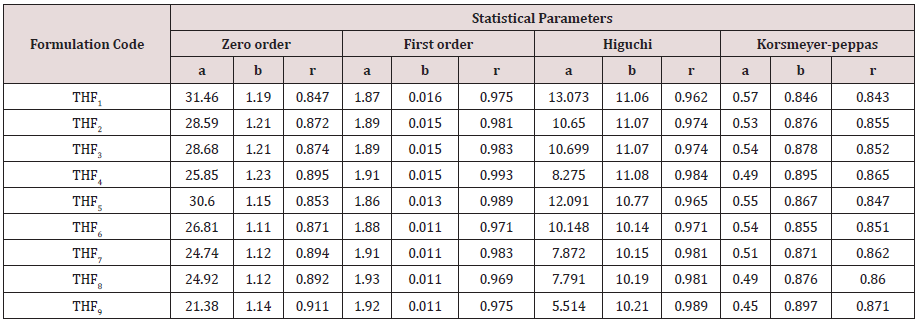
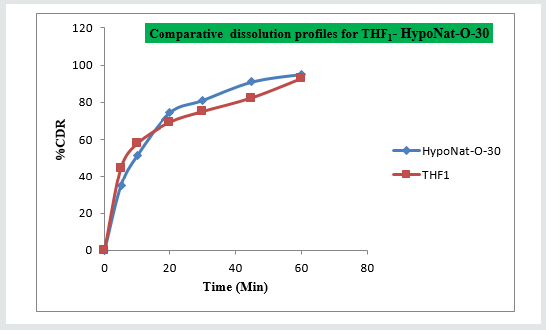
The main objective of the current work is to study the effect of super disintegrants in combination for the drug release of Tolvaptan. HCl by developing Fast Disintegrating Tablets (FDT). Tolvaptan. HCl, a newer aquaretic sorvaptan. It mainly used for the effective management of Hyponatremia associated with Syndrome of Inappropriate Anti Diuretic Hormone (SIDAH). Mouth dissolving formulations of Tolvaptan. HCl were prepared using different quantities of Kollidon & Primellose employed as Superdisintegrants by Direct Compression technique. Amount of super disintegrants need to get desired drug release was labeled as factors. Disintegration time, wetting time, times taken for dissolution were labeled as responses. Nine trials were formulated as per 32 factorial design and characterized for quality control parameters. Data obtained from the results reveals that formulations show satisfactory compendial limits. Data obtained from the dissolution study fitted well to kinetic modeling. THF1 composed of 50 milligrams of Kollidon & 50 milligrams of Primellose considered as best compared with all formulations. It shows similarity with HYPONAT-O-30 (f2= 71.32, f1= 4.84). THF1 follow higuchi kinetics with nonfickian diffusion of first order model (n= 0.846).
Keywords: Tolvaptan HCl; Super disintegrants; Kollidon; Primellose; Non-Fickian Diffusion
Introduction
Oral Dissolving Tablets (ODT) occupied special place in the pharmaceutical market. Fast/oral/Rapid or quick dissolving, Rapimelts, oro dispersible tablets were the synonyms for melt in mouth tablets [1]. They disintegrate/dissolve quickly in mouth within (<) 60 seconds. ODTs exhibit variations in terms of Mouth feel, Taste due to variety of organoleptic additives used in their formulation. Based on the method of manufacturing employed, they exhibit modulations in crushing index, drug delivery, stability and clinical outcome. Most widely used manufacturing methods for Rapimelts as follows Cotton candy process, Lyophilization, granulation techniques, named technolofies (Orosolv, Durasolv), Direct Compression and Spray drying molding [2]. Tolvaptan. HCl is a selective, competitive receptor blocker for vasopressin V2 (antagonist) located in renal tubule. Due to this effect induces the diuresis by lowering the osmality known as aqueresis ultimately enhances the serum sodium level. It mainly useful for treating hypervolemic, euvolemic hyponatremia and also associated with cardiac cirrhosis, Syndrome of Inappropriate Anti Diuretic Hormone (SIDAH) and congestive cardiac failure. It is available as uncoated tablets in the market with various strengths like 15 mg, 30 mg [3-5].
An attempt was made to achieve enhanced drug release from the dosage form by employing various concentrations of combination super disintegrants (Primellose, Kollidon) by formulating the Fast-dissolving tablets for Tolvaptan. HCl as per 32 factorial design [6]. Application of polynomial based response surface morphology occupies major volume in case of pharmaceutical product development. Most widely used methods in the abovementioned category as follows Factorial Design (23,32,33), CCD, BBD. Manufacture of tablets processed by Direct Compression technique is frequent method, observed in many of Pharmaceutical Industries. A two factor, 3-levels study utilized for observe the combination effect of both moieties on the dissolution of formulation (to see the effect of factors on the responses, thereby improving the clinical efficacy of the active ingredient).
Materials and Methods
Materials
Tolvaptan HCl was a gift sample procured from Konis Pharma Limited, Baddi, India. Lactose, Avicel, Kollidon, Primellose were procured from Aman Scientifics, Hyderabad. Remaining all materials obtained from sathi chemicals Guntur.
Design & Preparation of Fast Disintegrating Tablets for Tolvaptan.Hcl
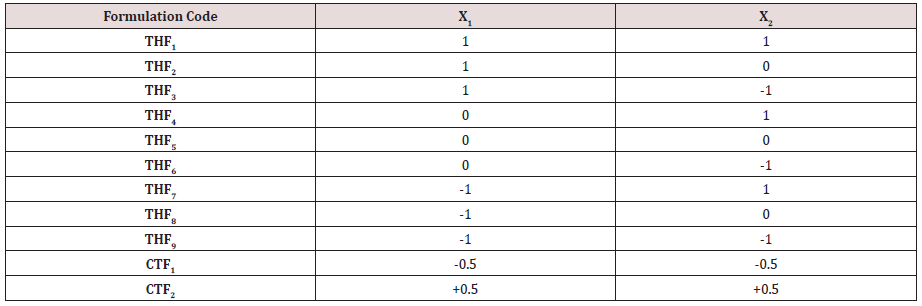
Quantities required for the Kollidon, Primellose for the development of Tolvaptan. HCl fast dissolving tablet formulations were taken as factors. t10%, t50%, t90%, Wetting Time & Disintegration Time was chosen as Responses. RSM prediction equations (polynomial) were derived for responses. 15%, 20%, 25% of Kollidon were taken as 3 levels for X1. 3 levels of X2 (Primellose) were 15, 20%, 25% (% with respect to total weight of Tablet). Nine Tolvaptan. HCl fast dissolving tablets were developed with 2 factors of X1& X2 and their relative effects were studied to optimize the amounts of super disintegrants for obtaining desired release profile of Tolvaptan. HCl from the formulation.
Preparation of Tolvaptan. Hcl Fast Dissolving Tablets
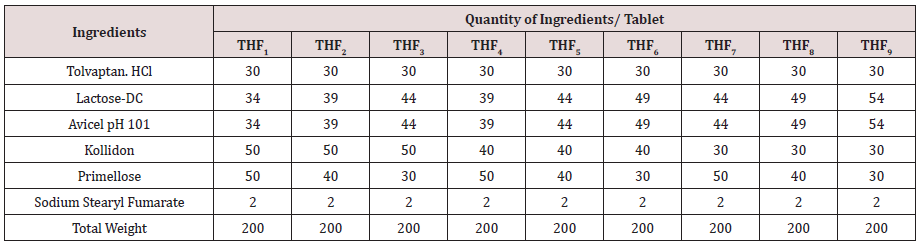
A 3 level, 2- factor design was utilized for the present research work. Amount of HPMCK100M chosen as X1 & Amount of LCG chosen as X2 shown in (Table 1). 3 levels of both factors chosen indicated as -1= 15%; 0= 20%;+1= 25% (% as per average weight of tablet). Fast dissolving tablets were prepared by using direct compression method; each formulation contains 30 mg Tolvaptan. HCl. Formulae for the preparation of tablets were presented in (Table 2). Accurately weighed ingredients were screened for obtaining uniform size to ensure proper mixing, to obtain polymer mixture. The drug was then mixed with the polymer mixture for 10 minutes for uniform mixing of powder blend. Blend was lubricated with sodium stearyl fumarate. Powder blend was subjected to compression with the help of rotary tablet compression machine (Tablet Minipress). Compressed tablets were processed for Quality Control measures as per Pharmacopoeia. Final formulations were transferred to airtight and light resistance packaging bottles [7].
Characterization 0f Tolvaptan. Hcl Fast Dissolving Tablets
Hardness
The breaking/ crushing strength for the dosage forms were obtained by the diametric break of tablets using Pfizer apparatus [8].
Friability
Friability test was performed by using Friability test apparatus (Roche). Selected number of tablets (20) were weighed accurately weight was noted (W0), tablets were subjected to rotations (25 rpm for 4 minutes) again weight was noted (W). % weight loss was determined using formula [9-12].
Weight loss (%) = [W0- W / W0] x 100
Drug Content
Assay was performed by triturating stated number of tablets in Indian pharmacopoeia (20) converted to powder; powder equivalent to 100mg Tolvaptan. HCl was added to 100 mL volumetric flask containing simulated gastric fluid, resultant mixture was subjected to sonication followed by straining. Suitable aliquots were prepared and checked for absorbance using UVVisible spectrophotometer at 268 nm [3-6].
Wetting Time
Tablets to be tested for Determination of wetting time were placed on a Petri dish containing paper soaked in 5mL of distilled water (2.5inch internal diameter). Time taken by the tablet to wet was recorded in seconds [9-12].
In-vitro Dissolution Study
Tolvaptan. HCl oral disintegrating tablets subjected to dissolution test with the help of USP XXIII type-II tablet dissolution test apparatus using 900 ml of pH 1.2 buffer as per official method specified in monograph. Absorbance for samples was noted at 268 nm using UV Visible spectrophotometer (after suitable dilutions if necessary) [3-5]. Data obtained from the dissolution study was fitted to kinetic modeling [12-14].
Disintegration Test
This test was performed as per the provisions of modified disintegration test for tablets. A cylindrical vessel with 10# was placed in such way that only 2 ml of medium would be placed below the sieve. Disintegration time was recorded [1].
Results and Discussion
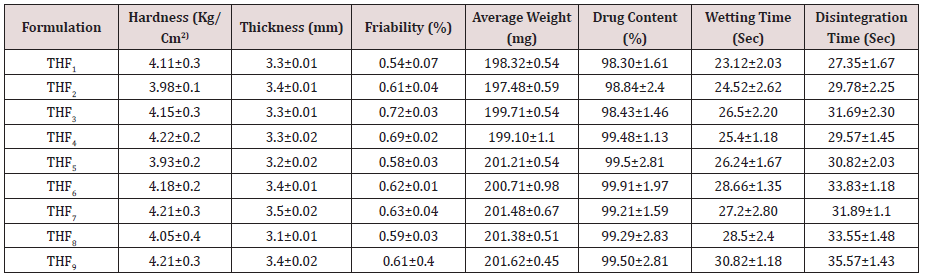
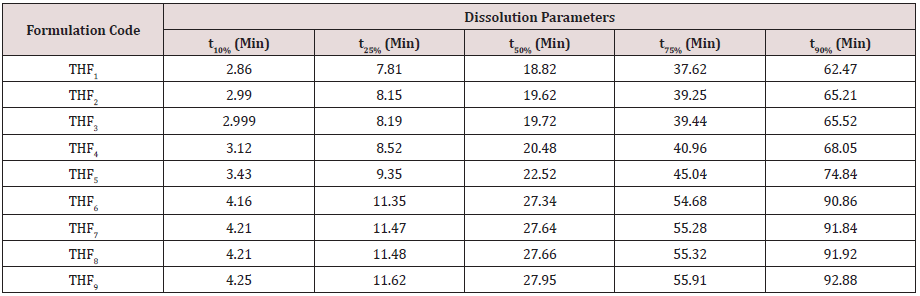
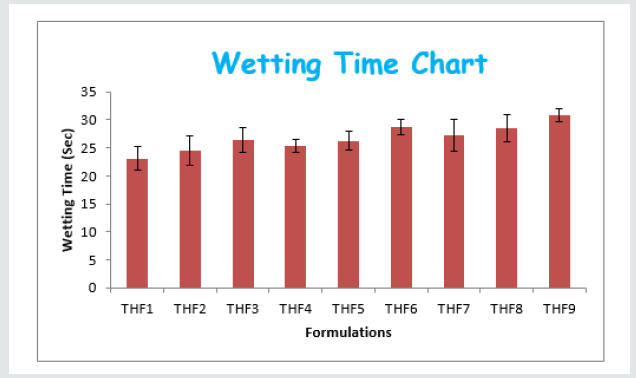
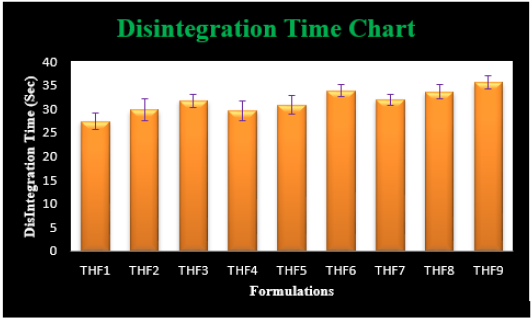
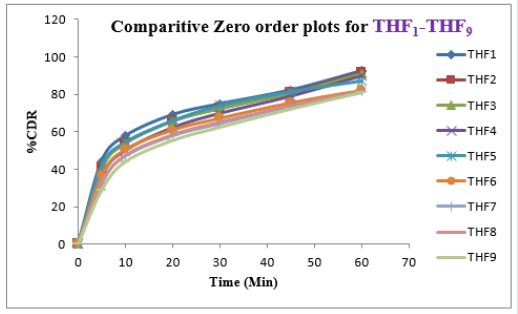
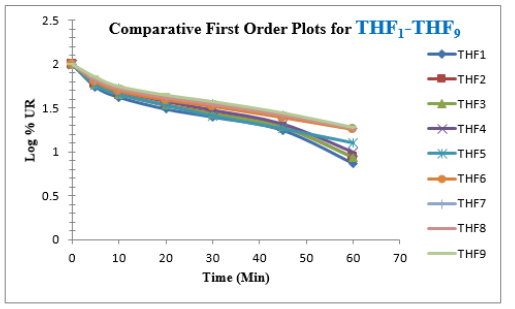
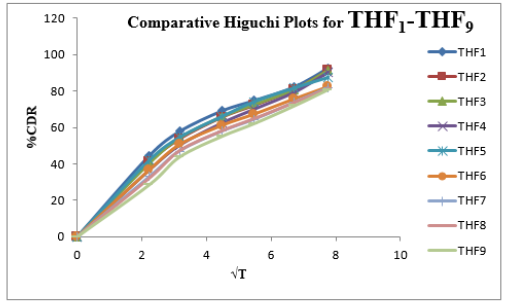
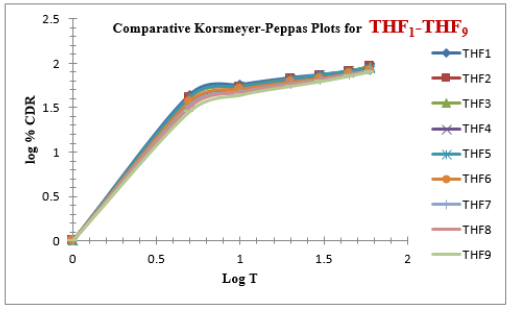
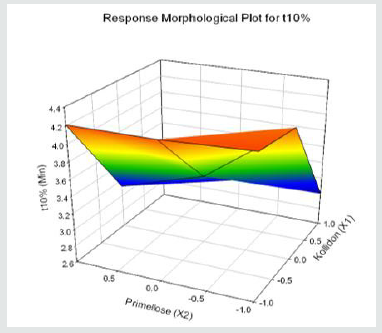
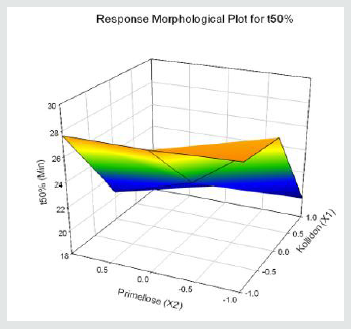
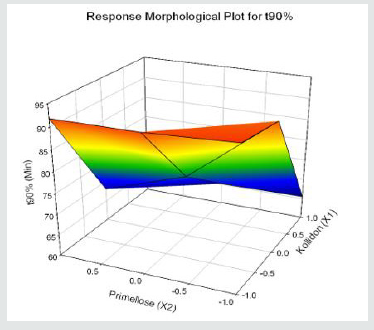
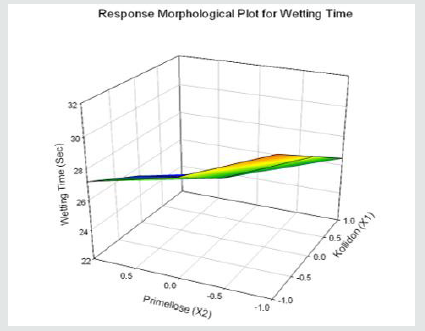
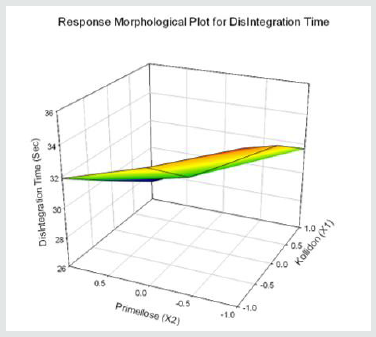
Tolvaptan. HCl Fast dissolving tablets were developed as per 3-level; 2- factor design for optimizing the combination of super disintegrants (Kollidon and Primellose) along with diluents. Formulation design was presented (Table 1). Amount of Kollidon (X1) and Primellose (X2) were chosen as factors and time for obtaining wetting, disintegration and dissolution chosen as responses. Nine batches were formulated as per 32 factorial model; formulae was presented in (Table 2). All trials have Tolvaptan. HCl (30 mg) as a fast-dissolving formulation, obtained as tablet by direct compression. Developed formulations were evaluated for pharmaceutical product performance tests. Data was presented in (Table 3). All tablets possess adequate mechanical strength, less friable and values were within the limits. All batches pass the drug content uniformity test. All formulation batches passed the Weight variation test. Results for wetting time were lie between 23 to 31 seconds. The values for Disintegration time present between 27 to 36 seconds. And the same was presented as Wetting Time Chart Disintegration Time Chart in (Figures 1-2). Dissolution rate test was carried as per standard procedures, the specifications such as 900 mL of Simulated Gastric Fluid (SGF); paddle rotated at a speed of 50 rpm; temperature maintained as 37±0.5oC throughout the test period. Dissolution profile well fit drug release kinetic models, data for kinetic analysis summarized in (Table 4). and the same was presented as plots from (Figure 3-6). From the results, observed that there was a clear relationship existed between amount (or) concentration of super disintegrants to the rate of dissolution and mouth feel. Predicted quick release of drug was obtained by appropriate composition of both X1, X2. Based on the desirability factor, THF1 was considered to be ideal which meets the objective of current work. THF1 composed of Polypladone and Primellose in same proportion i.e 50 mg each. RSM polynomial equations were derived for all responses using PCP-Disso. RSM plots were obtained with the help of Design-Expert 7.0. RSM plots were presented as (Figure 7-11). Dissolution parameters for THF1-THF9 were summarized in (Table 5).
RSM equations for the determination of predicted kinetic parameters as follows:
Y1= 3.58-0.64X1-0.21X2-0.03X1X2+0.018 X12+0.06X22 ( t10%)
Y2= 23.53-4.19X1-1.35X2-0.16 X1X2+0.12 X12+0.4 X22 (t50%)
Y3= 78.17-13.91X1-4.48X2-0.51 X1X2+0.24 X12+0.78 X22 (t90%)
Y4= 26.78-2.07X1-1.70X2+0.06 X1X2+0.01 X12+0.5 X22 ( Wetting Time, WT)
Y5 = 31.56-2.03X1-2.05 X2-0.17 X1X2+0.24 X12+0.27 X22 ( DisIntegration time, DT)
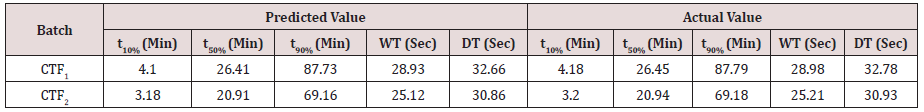
Results for the Predicted responses vs actual responses presented in (Table 6). No much deviation was observed in the predicted vs actual responses. It indicates validity of developed equation. THF1 was considered to be ideal, it shows similarity factor (f2) 71.32, difference factor (f1) 4.84, tcal<0.05 when compared with HypoNat-O-30. Comparative dissolution plots for best formulation (THF1) and marketed product shown in (Figure 12).
Conclusion
The current research investigation focuses about influence of utilization of superdisinterants such as Kollidon and Primellose in the formulation development Tolvaptan. HCl ODT. The optimized formulation (THF1) follows first order release, Non-Fickian Diffusion. THF1 utilized for the treatment of hypervolemic, euvolemic Hyponatremia. It improves the therapeutic outcome in case of hyponatremia associated with SIDAH, Congestive cardiac failure and Cardiac cirrhosis with better compliance for end user.
Acknowledgement
Authors acknowledge sincere thanks to the Management and Staff of M.A.M College of pharmacy, India for the facilities granted & constant encouragement for the completion of current research investigation.
References
- RK Gunda, JNS Kumar (2018) Formulation Development and Evaluation of Amisulpride Fast Dissolving Tablets. FABAD J Pharm Sci 43(2): 105-115.
- Raghav K Gunda, S Jayakumari (2016) Formulation Development and Evaluation of Risperidone Fast Dissolving Tablets. J Pharm Res 10(9): 579-588.
- Ramesh, B. Chandra Shekar (2015) Design and evaluation of tolvaptan solid dispersions using hot-melt extrusion and spray drying technique-A comparative study. Der Pharm Let 7: 218-231.
- Sree Giri Prasad B, Gupta VRM, Tamilselvan A (2015) Formulation and Evaluation of Fast Dissolving Tablet of Tolvaptan. J Glob Tre Pharm Sci 6(1): 2403-2410.
- K Ramesh, Chandra Shekar B (2015) Development characterization and in vivo evaluation of Tolvaptan solid dispersions via evaporation technique. Int J Drug Del 7(1): 32-43.
- Gunda RK, Swarooparani T (2016) Formulation Development and Evaluation of Clopidogrel Fast Dissolving Tablets. Iran J Pharm Sci 12(2): 61-74.
- Raghavendra Kumar Gunda, Jujjuru Naga Suresh Kumar (2017) Formulation Development and Evaluation of Moxifloxacin. Hcl Fast Dissolving Tablets. Pharm Meth 8(2): 160-167.
- Raghavendra Kumar Gunda, Masilamani K (2020) Development In-vitro and In-vivo evaluation of gastro retentive formulations for Moxifloxacin. Res J Pharm Tech 13(10): 4668-4674.
- Raghavendra Kumar Gunda, JNS Kumar (2016) Formulation Development and Evaluation of Carbamazepine Fast Dissolving Tablets. J Pharm Res 10(5): 216-225.
- Gunda RK, Vijayalakshmi A (2019) Formulation Development and Evaluation of Gastro retentive Bio adhesive drug delivery system for Moxifloxacin. HCl. Ind J Pharm Edu Res 53(4): 724-732.
- RK Gunda A, Vijayalakshmi (2020) Formulation and evaluation of gastro retentive floating drug delivery system for novel fluoro quinolone using natural and semi synthetic polymers. Iran J Pharm Sci 16(1): 49-60.
- Gunda RK, Manchineni PR (2020) Statistical Design and Optimization of Sustained Release Formulations of Pravastatin. Turk J Pharm Sci 17(2): 221-227.
- Higuchi T (1963) Mechanism of sustained-action medication Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 52: 1145-1149.
- Peppas NA (1985) Analysis of fickian and non-fickian drug release from polymers. Pharm Acta Helv 60(4): 110-111.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...