Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5910
Review Article(ISSN: 2638-5910) 
The Effect of Glibenclamide Administration on Gastrin Release in Diabetic Patients Volume 3 - Issue 2
George C Nikou*, George Evangelou, Vera Nikou and Efrosyni Kitsiou
- Section of Neuroendocrine Tumors, 3rd Department of Internal Medicine, National and Kapodistrian University of Athens, Greece
Received:NFebruary 22, 2021; Published: March 4, 2021
Corresponding author: George C Nikou, Section of Neuroendocrine Tumors, 3rd Department of Internal Medicine, National and Kapodistrian University of Athens, “Sotiria” Hospital, Athens, Greece
DOI: 10.32474/ADO.2021.03.000159
Abstract
The effects of sulfonylureas on gastrointestinal function in man is not yet quite clear. The aim of this study was to investigate the effect of oral administration of glibenclamide on gastrin release in patients with non-insulin dependent diabetes mellitus. Twelve non-insulin dependent diabetic patients (six men, six women, median age 57 years, range 46-63 years) were studied. Glibenclamide or placebo were given on different days and in a random order 10 minutes before a standard meal (73.6 g corned beef + 5ml olive oil + 60g bread). Blood samples for the determination of gastrin, glucose and C-peptide in serum, before (-15 and 0 minutes) and 30, 60 , 90 , 120 and 180 minutes after the standard meal, were obtained. Initial mean values of gastrin in serum did not differ significantly between the two meals. As expected gastrin levels increased significantly after taking the meals. However, no significant differences concerning mean gastrin concentrations between the two meals were noted at all time intervals studied, although there was a trend for the glibenclamide-preceded meal to exert lower gastrin values, especially at 60 minutes (p: 0,06). Mean serum glucose levels were, as anticipated, significantly lower after the glibenclamide-meal. Similarly, serum C-peptide concentrations were higher after this meal. It is concluded that acute glibenclamide oral administration does not influence post-stimulatory 3 gastrin levels in non-insulin dependent diabetic patients. Thus, in the clinical situation, sulfonylurea administration does not seem to interfere with gastrin release.
Introduction
The effect of PPIs (Proton Pump Inhibitors) on serum gastrin levels has been well known since the early years of patient treatment with omeprazole [1]. On the contrary, the effects of sulfonylureas on gastrointestinal function in man is not yet quite clear. Sulfonylureas are known to have various extrapancreatic actions [2]. In addition to their stimulatory effect on insulin release from pancreatic islets. However, the effects of sulfonylureas on gastrointestinal function and gut hormones release remain unclear. More specifically, as it regards gastrin, there are only a few studies in which the effect of sulfonylureas is investigated [3,4]. furthermore, these studies show a discrepancy between results obtained in man and animal models [5,6] and, in addition they describe only the effect of sulfonylureas on fasting and not the postprandial serum gastrin concentrations [3,4]. The present study was undertaken, therefore, to investigate the effect of glibenclamide oral administration in patients with Non-Insulin Dependent Diabetes Mellitus (NIDDM) in combination with a test meal.
Results
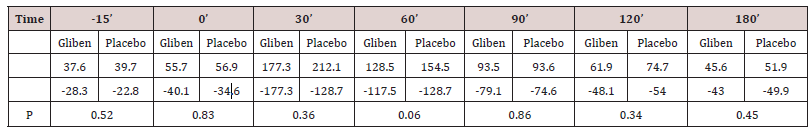
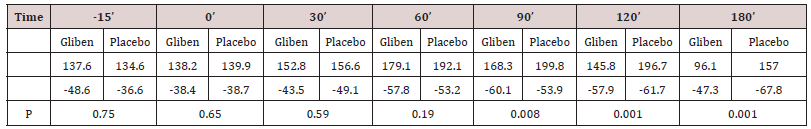
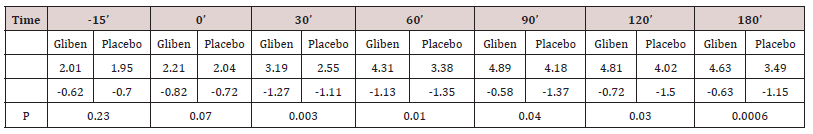
Initial mean values of gastrin in serum did not differ significantly between the two meals and were within the normal range (<90 pmol.1). As expected, gastrin levels increased significantly after taking the meals (Table 1). However, no significant gastrin concentrations between the two meals (meal + glibenclamide and meal + placebo) at all intervals studied, although there was a trend for the glibenclamide-preceed meal to exert lower gastrin values, especially at 60 minutes (p:0.063) (Table 1). Blood glucose variations are summarized in (Table 2). Mean serum glucose levels were, as anticipated significantly lower after the glibenclamide-meal as compared to those after placebo-meal. Similarly, postprandial C-peptide concentrations in serum were significantly higher after the glibenclamide-meal comparing to the placebo-meal (Table 3).
Discussion
In this study, gastrin concentrations in serum were found not to be altered significantly in NIDD patients after oral administration of glibenclamide, in combination with a meal, as compared to those who received the same meal with placebo. Although there was a trend for patients taking glibenclamide to present lower postprandial serum gastrin levels, this difference was not statistically significant. These findings are in agreement with the results of a previous study in which injectable solution of glibenclamide was administered intravenously or per os to healthy volunteers [3]. In the above study, gastrin levels were estimated in periphal and portal blood and were found to be essentially unchanged, under all conditions studies [3]. In another study, tolbutamide was reported to inhibit gastrin release in man [4]. In that case the drug was administrated intravenously or per so to normal subjects as well as in patients with atrophic gastritis, duodenal ulcer and IDDM [4].
Our study Differs from the previous reports:
a) Because it concerned exclusively patients with NIDDM, who are mainly treated with glibenclamide and
b) In the parallel administration of a test meal.
Therefore, it is obvious that the present study was planned accordingly to simulate the everyday conditions in diabetic patients taking a meal in combination with glibenclamide. These results, as well as the above-mentioned study [4], suggest a possible inhibition of gastrin release by Sulfonylureas in man. However, in animal models, gastrin release has been reported to be stimulated by sulfonylureas [5,6]. Indeed tolbutamide was found to stimulate both somatostatin and gastrin secretion from the isolated perfused rat stomach [5]. Also, glibenclamide was reported to stimulate gastrin release from the antral mucosa of cats [6]. It is possible that differences in animal species, as well as in the experimental design and drug dosage may account for this discrepancy. Basal gastrin values of diabetic patients in the present study were in normal range. Hupergastrinaemia has not been reported previously in diabetic humas, except in the diabetic pesuedo-Zolinger-Ellison syndrome [7] and a number of patients with clinical manifestations of autonomic neuropathy [8-10]. It has been further suggested that increased serum gastrin leels in those NIDD patients are not related to hypochlorhydria but, instead, are resulting from the autonomic dysfunction [8,9]. In the present study, patients did not show clinical manifestations of autonomic neuropathy. This, as well as the fact that they did not have impaired renal function, might well explain the normal initial mean gastrin values. Finally, it is noted that the present experiments deal with acute glibenclamide administration. Also, it would be interesting to investigate further the possible effect of sulfonylureas on gastrin levels in diabetic patients presenting hypergastrinaemia.
References
- Nikou GC, Vlavianos S, Kyriaki D, Vafiadou I, Alevizou V, et al. (1992) Variations of serum gastrin depending on the daily dose and the duration of treatment with omeprazole. Regulatory Peptides 40: 200-216.
- Blumenthal SA (1997) Potentiation of the hepatic action of insulin by chloropropamide. Diabetes 26(5): 485-489.
- Raptis S, Loeprecht H, Schelegel W (1977) Modulation of insulin, C-peptide, gastrin, secretin, pancreoenzymin release and blood glucose concentration in portal and peripheral blood following glibenclamide in man. Diabetologia 13: 420-426.
- Chiba T, Okimura Y, Kodama H, Kadowaki S, Chibara K, et al. (1988) Tolbutamide inhibits gastrin release in man. Horm Metab Res 20(10): 641-644.
- Chiba T, Kadowaki S, Taminato T, Chibara K, Fujita T (1983) Tolbutamide stimulates gastrin release in rats. Horm Metab Res 15: 510-516.
- Uvnas Wallensten K, Effendic S, Uvnas B, Lundberg JM (1979) Release of gastrin from the skeletal muscles and from the antral mucosa in cats induced by sulforunic drugs. Acta Physiol Scand 106(3): 267-270.
- Owyang C, Vinik AL (1982) Diabetic pseudo Zollinger-Ellison syndrome. Gastroenterology 82: 1140-1144.
- Sasaki H, Nagulesparam M, Dubois A, E Straus, I M Samloff, et al. (1983) Hypergastrinaemia in obese non-insulin dependent diabetes: A possible reflection of high prevalence of vagal dysfunction. J Clin Endocrinol Metab 56(4): 744-750.
- Guilford MC, Bicknell JE, Scarpello JHB (1988) Evaluation of gastrin secretion in diabetic subjects with normal or abnormal cardiovascular autonomic function tests. Acta Diabetol Lat 25(4): 275-282.
- Nikou GC, Pazaitou-Panagiotou K, Dimitroulopoulos D (2016) Results of a prospective multicenter neuroendocrine tumor registry reporting on clinicopathologic characteristics of Greek patients. BMC Endocr Disord 16: 1-8.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...