Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4692
Research Article(ISSN: 2637-4692) 
Expression of Metalloproteinase-8 in Gingival Crevicular Fluid, Peri-Implant Sulcular Fluid and Saliva in Healthy and Diseased Periodontal and Peri-Implant Tissue. Volume 4 - Issue 5
Theodosia Lazaridou1, Aikaterini Elisavet Doufexi*2, Giorgos Menexes3 and Lazaros Tsalikis4
- 1DDS, Graduate student, Department of Preventive Dentistry, Periodontology and Implant Biology, Aristotle University of Thessaloniki, Greece
- 2DDS, Ph D, Department of Preventive Dentistry, Periodontology and Implant Biology, Aristotle University of Thessaloniki, Greece
- 3PhD Associate Professor, School of Agriculture, Laboratory of Agronomy, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 4DDS, Ph D, Professor at the Department of Preventive Dentistry, Periodontology and Implant Biology, Aristotle University
Received: April 01, 2021 Published:April 29, 2021
Corresponding author: Aikaterini-Elisavet Doufexi, Department of Preventive Dentistry, Periodontology and Implant Biology, Aristotle University of Thessaloniki, Greece
DOI: 10.32474/MADOHC.2021.04.000197
Abstract
The study aimed to compare the levels of Metalloproteinase- 8 (MMP-8) in saliva, Gingival Crevicular Fluid (GCF), and Peri Implant Sulcular Fluid (PISF) in health and disease before and after treatment of periodontal and peri implant diseases. 42 patients treated with dental implants were divided into 3 groups: 15 periodontally healthy patients with healthy implants (Group A), 14 patients with gingivitis and peri implant mucositis (Group B), and 13 patients with periodontitis and peri implantitis (Group C). Clinical recordings, sulcular fluid sampling, and saliva collection were performed before and 3 months after treatment. Immunological characteristics were compared using metalloproteinase-8 (MMP-8) protein levels results using Enzyme-Linked Immunosorbent Assay (ELISA). Therapy lowered the PPD, PPDmax, and PI values of teeth and PI values of implants only in group C. Levels of MMP- 8 in GCF and PISF were similar for all groups; MMP-8 levels in saliva of group B were statistically higher compared to the other groups. Saliva showed higher MMP-8 levels than GCF and PISF. In all groups and fluids, MMP-8 levels were statistically unchanged after treatment. The analysis of MMP-8 in saliva could potentially offer insight into the molecular pathways involved in periodontal and peri-implant tissue inflammation
Keywords: Collagenase; Oral fluids; Periodontal inflammation Peri-Implant inflammation Expression
Introduction
Periodontal diseases are highly prevalent oral diseases caused by accumulation of bacterial biofilm adherent to tooth surfaces. Untreated gingivitis may progress to periodontitis, a destructive form of periodontal disease affecting connective tissue attachment and supporting bone around teeth. Apart from the presence of bacteria, the most critical factor for tissue destruction is the host response [1-3]. The bacterial plaque causing inflammation in periodontal tissues can also affect peri implant tissues. Nowadays, the predictability of osseointegrated dental implants for replacing missing teeth has resulted in their widespread use [4-7]. However, complications involving pathological changes in peri implant tissues may sometimes occur after osseointegration and dental implant occlusal loading. Peri mucositis is defined as a reversible mucosal inflammatory condition, similar to gingivitis, whereas peri-implantitis is the advanced stage of the disease characterized by destruction of bone and soft tissues around the implant [8,9]. Current research suggests that soft tissues surrounding an implant, despite differences, closely resembles the periodontium in natural dentition and that similar bacterial flora is associated with both chronic periodontitis and peri -implantitis [10]. Diagnosis of these inflammatory diseases is based upon clinical periodontal parameters including Bleeding on Probing (BOP), Probing Pocket Depth (PPD), Clinical Attachment Level (CAL), and also radiographic bone resorption [11]. Additionally, in peri implant mucositis, redness and swelling of soft tissues are symptoms aiding diagnosis, but bleeding on probing is currently recognized as the primary feature [12]. Peri-implantitis lesions are often associated with suppuration and increasing pocket depths, but always accompanied by loss of supporting marginal bone. However, these diagnostic indicators have limited value, because they depend on subjective examination and relate only to the disease progression, not its current status. This highlights the need for different diagnostic methods to supplement clinical ones [13].
New diagnostic methods should detect the presence of active disease, predict future disease progression, and evaluate responses to therapy, thereby improving clinical management of periodontal patients [14]. Extensive research suggests that periodontal risk can be identified by objective measures such as biomarkers indicating inflammation or tissue destruction [15]. These could include inflammatory mediators such as Matrix Metalloproteinases (MMPs) detectable in Gingival Crevicular Fluid (GCF), Peri-Implant Sulcular Fluid (PISF), and saliva. MMPs constitute a multigene family of over 25 structurally related, but genetically distinct, secreted or cell surface-associated proteolytic enzymes that can degrade many extracellular, pericellular, and non-matrix substrates [16-18]. The main tissue destructive MMP is considered to be MMP- 8 (collagenase 8s). High levels of MMP-8 have been demonstrated in chronic periodontitis and in peri- implantitis [19-21]. Tissue response similarities between periodontitis and peri-implantitis have led to expanding the therapeutic modalities of chronic periodontitis to also include treatment of peri-implantitis [22,23]. Unfortunately, insufficient knowledge exists concerning the effects of therapy on the clinical and immunological parameters specifically characterizing peri implant diseases. The purpose of this study was to compare protein levels of MMP-8 in GCF, PISF, and saliva in
a) Gingival health,
b) Gingivitis,
c) Periodontitis,
d) Peri implant health
e) Peri implant mucositis, and
f) Peri-implantitis before and after treatment of periodontal or peri implant disease.
Materials and Methods
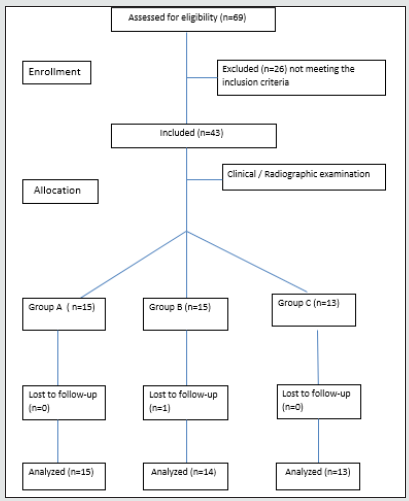
The present study was prospective, comparing clinical and immunological characteristics of periodontal and peri implant tissues at inflammation and after treatment of periodontal or peri implant disease. The study was conducted between December 2012 and June 2013 at the Department of Preventive Dentistry, Periodontology, and Implant Biology, School of Dentistry, Aristotle University of Thessaloniki in Greece. The outline of the study is described in a flowchart Figure 1.
Study Population
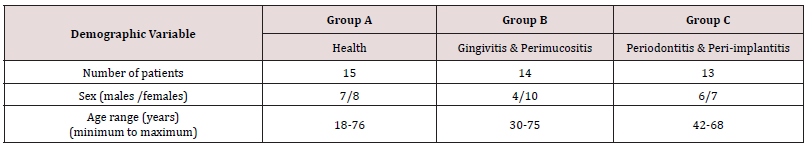
43 patients previously treated with dental implants in the Postgraduate Clinic of Periodontology and Implant Biology, Aristotle University of Thessaloniki, Greece, were enrolled in this study. The sample population comprised 18 men and 25 women: ages ranging from 18 to 76 years. Detailed demographic data for the study participants are presented in Table 1. The study protocol was approved by the Ethics Committee of the Aristotle University of Thessaloniki, Greece and the study were conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000. All patients red and signed an appropriate consent document prior to participation and agreed to attend all scheduled follow-up appointments.
Inclusion-Exclusion Criteria
For inclusion in the study, subjects had to be over 18, systemically healthy, partially edentulous (having>18 erupted teeth), with one or more missing teeth restored with fixed implant supported restorations loaded for at least to 12 months. Subjects with systemic diseases affecting the healing process (e.g., uncontrolled diabetes mellitus) were excluded from this study. Patients receiving periodontal therapy in the previous 6 months, smokers (>10 cigarettes/day), patients with liver/kidney or salivary gland dysfunction, under cancer therapy or organ transplant, pregnant or lactating women, individuals using antibiotics or immunosupressive medication within the last 3 months, needing antibiotics for infective endocarditis prophylaxis during dental procedures, having orthodontic appliances, presenting oral mucosal inflammatory conditions, HIV positive and or with history of hepatitis were also excluded. Furthermore, patients whose implants had mobility or needed Guided Bone Regeneration (GBR) or sinus elevation before their placement were excluded as well.
Group classification
All patients fulfilling inclusion criteria were first clinically examined. After clinical recordings, patients were divided into 3 groups: A, B and C. The patients not meeting the specific criteria for inclusion in each group were excluded. Group A comprised periodontally healthy patients exhibiting PPD ≤2mm in teeth and dental implants, and absence of Bleeding on Probing (BOP). These patients had implants without signs of peri-implant disease or radiographic change of the peri-implant bone level. Group B included patients with gingivitis and peri-implant mucositis. They exhibited bleeding on probing and PPD was >2 mm and ≤4mm. Implants in this group showed no radiographic change of the peri-implant bone level. Finally, group C comprised patients with periodontitis and periimplantitis, presenting PPD ≥5mm, and bleeding on probing. Dental implants in this group showed radiographic bone loss compared with the peri-implant bone level on the day of installation [24, 25].
Sample size and experimental design
MMP-8 was considered the primary outcome. A minimum of 11 patients per group was required to detect a mean difference in MMP-8 of 10ng/ml (±9ng/ml Standard Deviation-SD within each group) between any two groups (either before or after treatment), at a significance level 0.05 with power 0.80, using one-tailed t-test for independent samples. This difference was considered clinically significant. The anticipated SD is a conservative estimate according to clinical study (SD≈80% of the mean difference) [26]. Hence, the inclusion of at least 11 patients in the study would yield adequate statistical power for group comparisons. The power analysis was performed using the G*Power v.3 software [27,28]. Patients receiving dental implant therapy within the previous 3 years were examined and if meeting the inclusion criteria, were divided into the 3 groups of the study. Complete medical and dental histories were obtained using both patients’ records and interviews to ascertain any changes in their history. All study participants were followed up for a period of 3 months. Finally, 15 patients were included in group A, 15 patients in group B, and 13 patients in group C, allowing for possible dropouts. Clinical data were recorded at the beginning of the study (baseline) and after 3 months. According to periodontal and peri implant condition of the patients in each group, an appropriate prophylaxis treatment approach was administered. Radiographic evaluation of the implant sites took place at baseline to confirm initial diagnosis. Finally, samples of GCF, PISF, and saliva were collected at baseline and after 3 months.
Clinical recordings
Clinical recordings included: a) PPD, b) PPDmax, c) CAL, d) CALmax, e) BOP, f) PI, were recorded for each patient. Each recording included 6 surfaces for each tooth and implant (mesiofacial, midfacial, distofacial, mesiolingual, midlingual, and distolingual) using a 15mm North Carolina periodontal probe (PCP-UNC 15, Hu- Friedy Manufacturing Co., Chicago, IL, USA). Initially, PI and BOP were recorded (full mouth) using the plaque and calculus sensor. The presence or absence of plaque or bleeding was also recorded.
Other parameters were recorded using a periodontal probe. PPD for teeth is the distance from the gingival margin to the bottom of the pocket, whereas CAL is defined as the distance from the cementoenamel junction to the bottom of the pocket. For implants, PPD is the distance from the gingival margin to the bottom of the implant pocket when applying a light probing force. CAL is defined as the distance from the lower side of the implant platform to the bottom of the pocket.
GCF, PISF sampling, and saliva collection
GCF and PISF were collected the day after clinical recordings. PISF samples were collected from the mesiobuccal surface of the implants and GCF samples from teeth presenting on the contralateral side of the implant, so 1 sample of GCF and PISF were collected from each patient. Following site isolation with cotton rolls to prevent contamination with saliva, supragingival plaque was removed with sterile gauze, and teeth and implants were air dried for 5-10 sec. GCF and PISF were collected using filter paper strips (PerioPaper, Oraflow, Smithtown, NY, USA), gently inserted for 30 seconds into the orifice of the periodontal or peri-implant pocket, 1-2mm subgingivally, until slight resistance was felt. Samples visibly contaminated with blood were discarded. The collected samples were immediately transferred into sterile 1ml microcentrifuge Eppendorf tubes containing 120μl Phosphate Buffered Saline (PBS) centrifuged for 10 minutes, and stored at -80°C. The same day, saliva samples were collected between 09:00 and 11:00 hours using a specific protocol [29]. Patients should not have eaten, drunk (except water), chewed gum, and used any antiseptic mouthwash prior to collection. After rinsing their mouth with tap water, they expectorated whole saliva into sterile tubes, while seated in an upright position, for 5 minutes. The collected samples were placed immediately on ice and aliquoted before freezing at -80°C.
MMP-8 analysis
Enzyme-Linked Immunosorbent Assay kits (ELISA) were utilized to identify and quantify MMP-8 protein levels in all samples (Quantikine-Human Total MMP-8 Immunoassay, R&D Systems, Inc, Minneapolis, MN, USA) according to the manufacturer’s directions.
Therapeutic Procedure
After completing clinical recordings and collecting samples of GCF PISF, and saliva, therapeutic procedures for each group of patients were implemented. Group A patients were extensively instructed in oral hygiene according to the Bass method, and had their teeth and implants polished professionally with rubber cups and paste. Group B patients also received oral hygiene instruction and encouragement to use interdental brushes or dental floss. Subsequently they underwent supra- subgingival debridement in all 4 quadrants; local anaesthesia with 2% lidocaine (epinephrine 1:80,000) being used as needed. For periodontal treatment, the clinician used tips A and P (Swiss Instruments PM, EMS, Nyon, Switzerland) of ultrasound (EMS Piezon®, EMS, Nyon, Switzerland) and curettes (Hu-Friedy Gracey Standard SG 3/4, 11/12, 13/14, Hu-Friedy, Chicago, IL, USA). For implant surfaces special plastic curettes were used. Mechanical non-surgical therapy is recognized as effective in treating peri-implant mucositis lesion [30]. No adjunctive antimicrobial toothpaste or mouthrinses were used. Finally, patients had their implants and teeth polished. Group C patients received supra and subgingival debridement and root planning without any use of adjunctive chlorhexidine, antimicrobials or methods of access flap surgery. The quadrants containing teeth or implants were treated simultaneously. The subgingival debridement in tooth and implant surfaces were performed under local anesthesia and with identical tools to group B. Oral hygiene instructions and polishing were also performed.
Statistical analysis
Data are presented as mean ± Standard Error (SE). Data for PPD, PPD max, CAL, CAL max, BOP, PI, and MMP-8 were analyzed using the ANOVA (Analysis of Variance) method, in the frame of the Mixed Linear Models in order to accommodate the hierarchical structure of the data [31]. Specifically, the model involved three fixed effect factors; one factor between patients, the “group”, and two factors within patients: factor “site” and factor “time” with two repeated measures. In addition, there was one random effect factor: “patients” nested within groups. ANOVA was mainly used for estimating the appropriate error mean square for statistical comparisons of means. Mean values of the parameters were compared with the protected Least Significant Difference (LSD) criterion at p≤0.05. Additionally, the association between MMP-8 and other parameters measured in the current study was tested using Spearman’s rho rank correlation coefficient either at p≤0.05 for the whole sample or at p≤0.10 within groups. All the statistical analyses were performed with the SPSS v.15.0 statistical package (SPSS Inc, Chicago, IL, USA).
Results
All participants except one received the intended treatment and completed the study. Data from 42 patients were analyzed for primary and secondary outcomes. No adverse events following the appropriate treatment protocol were observed.
Before treatment
A. Clinical periodontal measurements
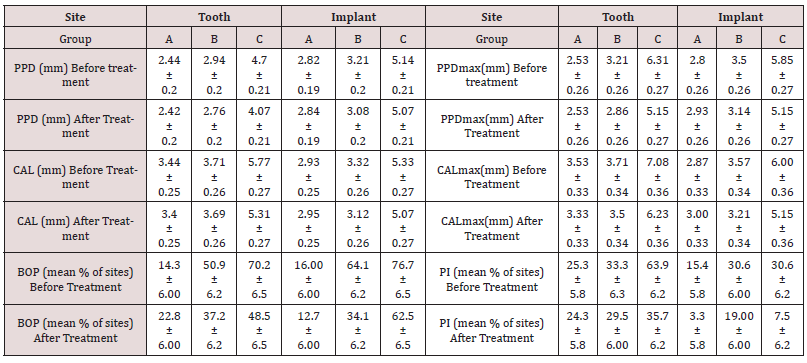
The mean PPD, PPD max, CAL, and CALmax values of implants and teeth in group C were significantly higher compared with groups A and B Table 2. The mean BOP values for implants and teeth in group C were significantly higher than group A and the mean PI values for implants did not differ among the groups; however, teeth in group C presented higher PI levels than groups A and B Table 2. Comparison of mean PPD, PPD max, CAL, and BOP values for implants and teeth, showed no significant differences among all 3 groups. Only CAL max and PI values of teeth in group C were significantly higher than the implants of the same group.
Table 2: Clinical parameters-PPD, PPD max, CAL, CALmax, BOP, and PI of tooth and implant sites for the different groups, before and after treatment (mean±SE)
B. Biochemical data in GCF, PISF and saliva samples
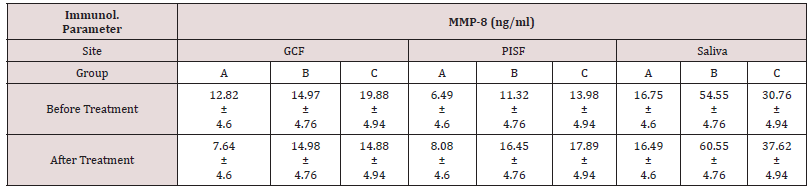
MMP-8 levels in GCF and PISF did not present statistically significant differences among the 3 groups, even though the groups with inflammation exhibited higher levels. MMP-8 levels in saliva showed a significant difference between group A and B, and between group B and C with levels in group B being statistically significantly higher. In group A there was no significant difference between the levels of MMP-8 in GCF, PISF, and saliva. In group B the levels in saliva were significantly higher than in GCF or PISF and in group C the levels in saliva were significantly higher than in PISF Table 3.
C. Correlations
In groups A and B no correlation between MMP-8 levels in GCF and PISF, and clinical parameters was evident, neither for teeth or implants, whereas for teeth in group C there was a correlation between MMP-8 and PPD (rho=0.480, p=0.097). For teeth in whole sample there was a correlation between MMP-8 and PPD (rho=0.386, p=0.012), and between MMP-8 and CAL (rho=0.298, p=0.05) whereas for implants in whole sample no correlation was found between MMP-8 and clinical parameters. Furthermore, saliva MMP-8 levels correlated with BOP in all implants (rho=0.527, p<0.001) and with PPDmax (rho=0.305, p=0.049), and BOP (rho=0. 355, p=0.021) in all teeth. Saliva MMP-8 levels also correlated with CAL in implants (rho=0.456, p=0.088) and PI in teeth (rho=0.454, p=0.089) of group A, with PPDmax in teeth of group B (rho=0.467, p=0.092), and with PPD in teeth of group C (rho=0.530, p=0.063). These correlations occurred for levels of MMP-8 <40ng/ml. Finally, GCF MMP-8 levels in the whole sample were correlated with MMP- 8 saliva levels (rho=0.329, p=0.033) and with PISF MMP-8 levels (rho=0.380, p=0.013). Especially in group B, PISF levels were correlated with GCF MMP-8 levels (rho=0.534, p=0.049) and with saliva levels (rho=0.497, p=0.070). In group C, GCF levels were correlated with saliva levels (rho=0.736, p=0.004).
<After treatment
A. Clinical periodontal measurements
The mean PPD, PPDmax, CAL, and CAlmax values of implants and teeth in group C were significantly higher than groups A and B. There was no significant difference among the groups for the BOP and PI values Table 2.
Comparison of mean PPDmax, CAL, and BOP values for implants and teeth, showed no significant differences among all 3 groups. For PPD value, only group C showed a statistically significant difference, because teeth exhibited a higher decrease of PPD after treatment; CAL max values of teeth in group C were also significantly higher than the implants of the same group. PI values also differed between implants and teeth in group C, where teeth exhibited higher levels.
B. Biochemical data in GCF, PISF and saliva samples
The mean MMP-8 in GCF and PISF did not present differences among the 3 groups Table 3. MMP-8 levels in saliva showed a significant difference between group A and B, group B and C, and between group A and C as in group B the MMP-8 levels were higher. The levels in saliva were higher compared to GCF and PISF levels in groups B and C, and these differences were found statistically significant.
C. Correlations
Implants in group C showed a correlation between MMP- 8 and PPD (rho=0.690, p=0.009), and between MMP-8 and CAL (rho=0.690, p=0.009). Teeth in group C exhibited a correlation between MMP-8 and BOP (rho=0.702 and p=0.007). For teeth in whole sample there was a correlation only between MMP-8 and BOP (rho=0.334, p=0.030), and for implants in the whole sample a correlation between MMP-8 and PPD existed (rho=0.289, p=0.064). Salivary MMP-8 levels were not correlated with the clinical parameters in teeth and implants of any group or in the whole sample. Salivary MMP-8 levels were correlated with PISF levels (rho=0.343, p=0.026) and with GCF levels in the whole sample (rho=0.375, p=0.014). Especially in group A and B, saliva levels were correlated with PISF levels for group A (rho=0.481, p=0.069) and PISF levels for group B (rho=0.459, p=0.098).
The Effect of Treatment
A. Clinical periodontal measurements
After appropriate treatment, PPD and PPD max values were found statistically significantly lower only for teeth in group C. The levels of CAL and CALmax were not found to be statistically significantly lower, neither for teeth nor implants in all groups. For BOP values, implants and teeth of all groups did not exhibit any statistically significant difference. PI values for implants and teeth decreased significantly only in group C Table 2.
B. Biochemical data in GCF, PISF and saliva samples
The levels of MMP-8 showed no statistically significant difference after treatment, although they seemed reduced for GCF and appeared higher for PISF and saliva.
Discussion
This study examined the levels of MMP-8 in GCF, PISF, and saliva in periodontal and peri implant health and disease. Clinical parameters are very important in diagnosis and treatment but not exact indicators of current disease status, so new methods, that analyze levels of certain biomarkers in oral fluids could be potentially useful [15]. Findings showed that PPD and PPDmax for teeth and dental implants increase in gingival inflammation. Implants differed from teeth after treatment only in group C, showing higher parameters for implants, such as PPD and CALmax, maybe because of greater accumulations of plaque and calculus. Teeth with severe inflammation seem to benefit more as their levels of PPD and PPDmax are lower than in implants after treatment. Both GCF and PISF are osmotically mediated inflammatory exudates arising from vessels of the mucosal plexus around teeth and implants. They are considered useful in diagnosis as they require non-invasive methods and contain molecules whose levels may reflect local or systemic inflammation [32]. Additionally, circulating molecules in these biological phases have been detected at high levels in the whole saliva of patients with inflammation [33]. The most important immunological results of the study were the high levels of MMP-8 in GCF and PISF in sites with severe inflammation, in contrast to healthy sites or ones with initial inflammation. However, [34] have found that higher levels of MMP-8 were found in GCF of periodontal patients compared with controls, and [35] similarly concluded that significantly higher MMP-8 levels were observed in periodontitis cases. Similarly with our results a previous study found higher levels of MMP-8 in sites with ongoing vertical bone loss around dental implants [36]. This is explained by MMP-8 having a unique role in tissue destruction by degrading type I and III collagen. Secondly, it was found that the levels of MMP-8 in GCF, PISF and saliva were significantly higher in presence of inflammation. Similarly, [37] found no difference in health and diseased between the levels of MMP-8 in GCF and PISF, although in peri-implantitis sites the rate of tissue destruction was elevated. Similarly, [38] concluded that the levels in PISF were higher than those in GCF. Particularly in cases of experimental gingivitis and peri-implant mucositis, [39] found that levels of MMP-8 were statistically significantly higher at implants sties compared with teeth sites. It is interesting that in our study the highest levels of MMP-8 in saliva were detected in sites of mucositis and gingivitis compared to either healthy sites or sites with bone loss. The therapeutic procedure did not affect the levels of MMP-8 in teeth and implants in all groups. [34] found that the levels of MMP-8 in GCF decreased 3 months after periodontal therapy and [40] also recorded that therapy resulted in significant decrease of MMP-8 GCF levels. It is possible that the levels of only the active MMP-8 and not the levels of total-latent MMP-8 [41]. Currently however, no studies exist examining how levels of MMP- 8 in PISF change after non-surgical therapy. In our study, before therapy, the levels of MMP-8 in teeth were correlated in the whole sample with PPD and CAL and the higher the levels of MMP-8, the larger the periodontal destruction. [42] came to the same conclusion examining the correlation of MMP-8 with PPD in female periodontal-patients. In our study, after therapy, MMP-8 in GCF was correlated positively with BOP. This is very important since BOP is a strong indicator of active inflammation. Furthermore, the levels of MMP-8 in PISF after therapy were correlated positively in all groups with PPD. This finding agrees with [43] who showed that MMP-8 levels demonstrated positive correlations with gingival index and probing depth in implant sites. Salivary samples in our study showed that in patients with inflammation, the levels of MMP-8 were higher than in healthy ones, especially in cases of gingivitis and peri-implant mucositis, which showed the highest levels of MMP- 8. Studies exist in the literature examining healthy and patients with periodontitis, agreeing with this result. [44] and [45] found that patients with severe periodontitis present elevated salivary concentrations of MMP-8. There are also studies disagreeing with this finding as they found that in severe inflammation the levels of MMP-8 are higher than in gingivitis and peri-implant mucositis sites [46,47]. In our study, MMP-8 levels in initial inflammation are higher maybe because MMP-8 is associated with the active phases of disease present in these cases. The main finding was that saliva was the only statistically significant indicator of the different stages of inflammation; as such it constitutes a very sensitive diagnostic and prognostic tool that can improve treatment planning in periodontics and implant dentistry.
In our study, we found that MMP-8 levels in saliva were significantly higher than in other fluids in all groups, and these levels do not conform to the levels in GCF, PISF since saliva is a more general non-specific reflection of oral status [48]. In contrast to our study [49] found that MMP-8 protein levels were higher in GCF than in saliva in different groups of periodontal health status and smoking status indicating that GCF might be a better sample to detect differences in MMP-8 levels. Saliva did not show the benefit of treatment as the salivary MMP-8 levels after therapy were unchanged [50] with this finding, finding that salivary levels of MMP-8 were decreased after therapy in patients with periodontitis. As saliva mirrors the inflammation in teeth and implants, the salivary levels of MMP-8 remained unchanged after therapy, due to unchanged levels of MMP-8 in PISF. Also, MMP-8 in saliva was highly correlated with clinical parameters such as PPDmax, CAL, PI, and BOP in teeth and implants before therapy [46] also reached the same conclusion. This result supports the fact that saliva mirrors oral status. Finally, as it was found that saliva MMP-8 levels were correlated positively with GCF and PISF levels, confirming that inflammation in teeth and implants is reflected in saliva as well. In conclusion, biomarkers ‘analysis in saliva offers certain advantages, in comparison with GCF and PISF analysis, because this method is quick, easy, non-invasive, and more sensitive, although non-specific among healthy sites, and those with early or severe stages of inflammation. Further studies are needed to develop new methods of oral fluid MMP tests to diagnose, follow, monitor, and predict periodontal and peri-implant disease or health.
Acknowledgment
The authors would like to thank Mrs. Konstantina Topouzidou for aiding in the MMP-8 analysis of the samples using the ELISA method. The authors report no conflict of interest with regard to this study.
References
- Socransky SS, Haffajee AD (1992) The bacterial etiology of destructive periodontal disease: Current concepts. J Periodontol 63(4 Suppl): 322-331.
- Genco RJ (1992) Host responses in periodontal diseases: Current concepts. J Periodontol 63(4 Suppl): 338-355.
- Kirkwood KL, Taba M Jr, Rossa C, Newman M, Takei H (2012) Molecular biology of the host-microbe interaction in periodontal diseases. Selected topics: Molecular signaling aspects of pathogen-mediated bine destruction in periodontal diseases. Carranza’s periodontology Elvesier St. Louis pp: 285-293.
- Adell R, Lekholm U, Rockler B, Branemark Pi (1981) A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg 10(6): 387-416.
- Quirynen M, Naert I, Van Steenberghe D, Nys L (1992) A study of 589 consecutive implants supporting complete fixed prostheses. Part 1: Periodontal aspects. J Prosthet Dent 68(4): 655-663.
- Lanq Hua BH, Lang NP, Lo EC, McGrath CP (2013) Attitudes of general dental practitioners towards implant dentistry in an environment with widespread provision of implant therapy Clin Oral Implants Res 24(3): 278-284.
- Lang NP, Zitsmann NU (2012) Working Group 3 of the VIII European Workshop on Periodontology. J Clin Periodontol 39(12): 133-138.
- Tonetti MS, Schmid J (1994) Pathogenesis of implant failures. Periodontol 2000 4: 127-138.
- Klokkevold PR, Newman MG (2000) Current status of dental implants: A periodontal perspective. Int J Oral Maxillofac Implants 15(1): 56-65.
- Listgarten MA, Lang NP, Schroeder HE, Schroeder A (1991) Periodontal tissues and their counterparts around endosseous implants. Clin Oral Implants Res 2(3): 1-19.
- Armitage GC (2004) Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000 34: 9-21.
- Lindhe J, Meyle J, Group D of European Workshop on Periodontology (2008) Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol 35(8 Suppl):282-285.
- MacClanahan SF, Bartizek RD, Biesbrock AR (2001) Identification and consequences of distinct Löe Silness gingival index examiner styles for the clinical assessment of gingivitis. J Periodontol 72(3): 383-392.
- Souza SL, Taba M Jr (2004) Cross-sectional evaluation of clinical parameters to select high prevalence populations for periodontal disease: the site comparative severity methodology. Braz Dent J 15(1): 46-53.
- Taba M Jr, Kinney J, Kim AS, Giannobile WV (2005) Diagnostic biomarkers for oral and periodontal diseases. Dent Clin North Am 49(3): 551-571.
- Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ Res 92(8): 827-839.
- McCawley LJ, Matrisian LM (2001) Matrix metalloproteinases: They're not just for matrix anymore! Curr Opin Cell Biol 13(5): 534-540.
- Golub LM, Wolff M, Roberts S, Lee HM, Leung M, et al. (1994) Treating periodontal diseases by blocking tissue-destructive enzymes. J Am Dent Assoc 125(2): 163-169.
- Mäntylä P, Stenman M, Kinane D (2006) Monitoring periodontal disease status in smokers and nonsmokers using a gingival crevicular fluid matrix metalloproteinase-8-specific chair-side test. J Periodontal Res 41(6): 503-512.
- Teronen O, Konttinen YT, Lindqvist C (1997) Human neutrophil collagenase MMP-8 in peri-implant sulcus fluid and its inhibition by clodronate. J Dent Res 76(9): 1529-1537.
- Kivelä Rajamäki M, Maisi P, Srinivas R (2003) Levels and molecular forms of MMP-7 (matrilysin-1) and MMP-8 (collagenase-2) in diseased human peri-implant sulcular fluid. J Periodontal Res 38(6): 583-590.
- Klinge B, Gustafsson A, Berglundh T (2002) A systematic review of the effect of anti-infective therapy in the treatment of peri-implantitis. J Clin Periodontol 29 (Suppl 3): 213-225.
- Ross Jansaker AM, Renvert S, Egelberg J (2003) Treatment of peri-implant infections: A literature review. J Clin Periodontol 30(6): 467-485.
- Lindhe J, Meyle J, Group D of European Workshop on Periodontology (2008) Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol 35(8 Suppl): 282-285.
- Zitsmann NU, Berglundh T (2008) Definition and prevalence of peri-implant diseases. J Cl Periodontol 35(Suppl. 8): 286-291.
- Han DH, Shin HS, Paek D, Kim HD (2012) Gingival crevicular fluid levels of matrix metalloproteinases cross-sectionally related to periodontitis and metabolic syndrome in community Koreans. J Clin Periodontol 39(12): 1125-1131.
- Faul F, Erdfelder E, Lang AG, Buchner A (2007) G Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods 39(2): 175-191.
- Faul F, Erdfelder E, Buchner A, Lang AG (2009) Statistical power analyses using G Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods 41: 1149-1160.
- Navazesh M (1993) Methods for collecting saliva. Ann N Y Acad Sci 694: 72-77.
- Renvert S, Roos Jansåker AM, Claffey N (2008) Non-surgical treatment of peri-implant mucositis and peri-implantitis: A literature review. J Clin Periodontol 35(8 Suppl): 305-315.
- West BT, Welch KB, Galecki AT (2007) Linear mixed models: A practical guide to using statistical software.
- Erica N Recker, Gustavo Avila Ortiz, Carol L Fischer, Keyla Pagan Rivera, Kim A Brogden, et al. (2015) A Cross-Sectional Assessment of Biomarker Levels Around Implants Versus Natural Teeth in Periodontal Maintenance Patients. J Periodontol 86(2): 264-272.
- Lorena Da Rós Gonçalves, Márcia Regina Soares, Fábio CS Nogueira, Carlos Garcia, Danielle Resende Camisasca, et al. (2010) Comparative proteomic analysis of whole saliva from chronic periodontitis patients. J Proteomics 73(7): 1334-1341.
- Andrea M Marcaccini, Cesar A Meschiari, Leonardo R Zuardi, Tiago Sampaio de Sousa, Mario Taba Jr, et al. (2010) Gingival crevicular fluid levels of MMP-8, MMP-9, TIMP-2, and MPO decrease after periodontal therapy. J Clin Periodontol 37(2): 180-190.
- Rai B, Kaur J, Jain R, Anand SC (2010) Levels of gingival crevicular metalloproteinases-8 and -9 in periodontitis. Saudi Dent J 22(3): 129-131.
- Arakawa H, Uehara J, Hara ES, Wataru Sonoyama (2012) Matrix metalloproteinase-8 is the major potential collagenase in active peri-implantitis. J Prosthodont Res 56(4): 249-255.
- Nomura T, Ishii A, Shimizu H (2000) Tissue inhibitor of metalloproteinases-1, matrix metalloproteinases-1 and -8, and collagenase activity levels in peri-implant crevicular fluid after implantation. Clin Oral Implants Res 11(5): 430-440.
- Ling Xu, Zhao Yu, His Ming Lee, Mark S Wolff, Lorne M Golub, et al. (2008) Characteristics of collagenase-2 from gingival crevicular fluid and peri-implant sulcular fluid in periodontitis and peri-implantitis patients: pilot study. Acta Odontol Scand 66(4): 219-224.
- Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, et al. (2012) Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res 23(2): 182-190.
- Konopka L, Pietrzak A, Brzezińska Błaszczyk E (2012) Effect of scaling and root planning on interleukin-1β, interleukin-8 and MMP-8 levels in gingival crevicular fluid from chronic periodontitis patients. J Periodontal Res 47(6): 681-688.
- Al-Majid A, Alassiri S, Rathnayake N, Tervahartiala T, Gieselmann DR, et al. (2018) Matrix Metalloproteinase-8 as an Inflammatory and Prevention Biomarker in Periodontal and Peri-Implant Diseases. Int J Dent.
- M Kraft Neumärker, K Lorenz, R Koch, T Hoffmann, P Mäntylä et al. (2012) Full-mouth profile of active MMP-8 in periodontitis patients. J Periodontal Res 47(1): 121-128.
- Basegmez C, Yalcin S, Yalcin F, Ersanli S, Mijiritsky E (2012) Evaluation of periimplant crevicular fluid prostaglandin E2 and matrix metalloproteinase-8 levels from health to periimplant disease status: A prospective study. Implant Dent 21(4): 306-310.
- Nilminie Rathnayake, Sigvard Akerman, Bjorn Klinge, Nina Lundegren, Henrik Janssonet al. (2013) Gustafsson Salivary biomarkers of oral health: A cross-sectional study. J Clin Periodontol 40(2): 140-147.
- Jeffrey L Ebersole, Julie L Schuster, Jason Stevens, Dolph Dawson, Richard J Kryscio, et al. (2013) Patterns of salivary analytes provide diagnostic capacity for distinguishing chronic adult periodontitis from health. J Clin Immunol 33(1): 271-279.
- Rai B, Kharb S, Jain R, Anand SC (2008) Biomarkers of periodontitis in oral fluids. J Oral Sci 50(1): 53-56.
- Ulvi K Gursoy, Eija Könönen, Sisko Huumonen, Taina Tervahartiala, Pirkko J Pussinen, et al. (2013) Salivary type I collagen degradation end-products and related matrix metalloproteinases in periodontitis. J Clin Periodontol 40(1): 18-25.
- JM Leppilahti, MM Ahonen, M Hernández, S Munjal, L Netuschil, et al. (2011) Oral rinse MMP-8 point-of-care immuno test identifies patients with strong periodontal inflammatory burden. Oral Dis 17(1): 115-122.
- Kasuma N, Oenzil F, Darwin E, Sofyan Y (2018) The analysis of matrix metalloproteinase-8 in gingival crevicular fluid and periodontal diseases. Indian J Dent Res 29(4): 450-454.
- Sexton WM, Lin Y, Kryscio RJ, Dawson DR, Ebersole JL, et al. (2011) Salivary biomarkers of periodontal disease in response to treatment. J Clin Periodontol 38(5): 434-441.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...