Lupine Publishers Group
Lupine Publishers
Menu
Research Article(ISSN: 2641-6875) 
Antibacterial Efficacy of Vernonia Amygdalina Against Bacteria Strains Recovered from Hospital Fomites, Nigeria Volume 2 - Issue 2
Thonda Oluwakemi Abike1*, Okorie Debbie1, Ogidi Clement Olusola2, Aladejana Oluwatoyin Modupe1, Olowookere Boyede D3 and Olawoye Abimbola A1
- 1Department of Biological Sciences, Microbiology Unit, Kings University, Nigeria
- 2Department of Biological Sciences, Biotechnology Unit, Kings University, Nigeria
- 3Department of Chemical Sciences, Kings University, Nigeria
Received: October 14, 2020; Published: November 02, 2020
*Corresponding author: Thonda Oluwakemi Abike, Department of Biological Sciences, Microbiology Unit, Kings University, Odeomu, Osun State, Nigeria
DOI: 10.32474/CTBM.2020.02.000131
Abstract
This study was carried out to evaluate the occurrence of bacteria from hospital fomites and the antibacterial activity extract from Vernonia amygdalina against bacteria isolates. The colonies obtained were subjected to colonial characteristics and conventional biochemical test with reference to Bergey’s Manual of Determinative Bacteriology. The antibiotic susceptibility of the isolates was performed using the Kirby-Bauer’s disc diffusion methods while the antimicrobial activity of the extract was performed by using well diffusion method. Proteus species (18%) were the most prevalent bacteria followed by Staphylococcus spp (16%) while Actinobacter spp and Photobacterium spp have the least of 1%. All the isolates showed high resistant (100%) to various antibiotics tested while they are sensitive to ofloxacin. The bioactive extract of Vernonia amygdalina revealed the presence of some active medicinal constituent. The antibacterial activity of the extract against the organisms produced a zone of inhibition which ranged between 4.5-15mm at 100mg/ml concentration while it ranged between 2.0-12.1mm at 50mg/ml. In conclusion, this study showed that hospital fomites harbour highly pathogenic bacteria which have the potentials of causing epidemics in the nearest future. Therefore, the efficacy of Vernonia amygdalina against clinical resistant isolates could be explored for further pharmaceutical use and should be encouraged in the formulation and production of new antibiotics.
Keywords: Fomites; Phytochemicals; Vernonia amygdalina; Hospital acquired infections; Resistance; Antibacterial
Introduction
Nosocomial (Hospital acquired infection) infections are important public health problems in developing countries and also in the developed countries [1]. Nosocomial infection is associated with a considerable increase in morbidity and mortality rate of patients at hospital and also significant increases in the costs. Nosocomial infections occur in 5% to 17% of hospitalized patients and the 5th causes of death in hospitals [2]. It was estimated that in year 2002, a total of 1.7 million hospital acquired infections occurred (4.5 per 100 admissions), and 99,000 deaths resulted from it or were associated with a hospital acquired infections [3]. Nosocomial infections has increased and are more alarming in the 21st century because of hospitals housing large numbers of people who are sick and whose immune system are weak, increase in number of outpatient treatment, wrong medical procedures, spread of pathogens via medical staff who moves from patient to patient, inadequate sanitation protocols regarding uniforms equipment sterilization, washing and other preventive measures that may either be unheeded by hospital personnel or too lax to sufficiently isolate patients from infectious agents, and the routine use of antimicrobial agents in hospitals creates selection pressure which leads to the emergence of resistant strains of microorganisms. Other infections such as healthcare-associated infections occur in both adult and pediatric patients [4]. Bloodstream infections, followed by pneumonia and urinary tract infections are the most infections in children, urinary tract infections are the most common healthcare associated infections in adults [5]. Hospital acquired infection is the sixth leading cause of death in the United States. One of the most common wards where HAIs occur is the Intensive Care Unit (ICU), where doctors treat serious diseases. About 1 in 10 of the people admitted to the hospital contract Hospital Acquired Infection (HAI). They are also associated with significant morbidity, mortality, and as well as hospital costs [6]. Fomite is any inanimate object which can transfer disease to a new host when contaminated with infectious agents, such as bacteria or viruses. Fomites are associated particularly with Hospital Acquired Infections (HAI), as they are possible routes to transfer pathogens between patients [7].
Scientist and researchers have discovered that smooth (nonporous) surfaces like door knobs transmit bacteria and viruses better than porous materials like paper and money because porous materials (especially fibrous) absorb and trap the contagion, thus making it harder to contract through simple touch. In the hospital, fomites include patient care items such as stethoscopes, thermometers and environmental surfaces such as carpets, beddings, chairs. It has been demonstrated that such items (eg, medical equipment, surfaces) are frequently contaminated with pathogens and can serve as a reservoir or source for multidrugresistant organisms [8]. In hospitals, fomites serve as a reservoir of pathogens being spread from the inanimate environment to an animate (patient) environment through the hands of Health Care Workers (HCW) [9-11]. To reduce morbidity and mortality in hospitals, it is important to identify common fomites associated pathogens in any hospital settings. Because prevention of a disease is to identify what has been transferring the diseases which is the most important factor. Medicinal plants and traditional medicine with antimicrobial activities have been used widely in the West African regions and it is recognized as the primary health care system in many communities due to reasons such as affordability low cost and accessibility. Plants of medicinal important have shown to be effective even where treatments with antibiotics has failed [12]. Vernonia amygdalina is a perennial shrub which belongs to the family Asteraceae [13]. Vernonia amygdalina is a shrub that grows to 10m tall with petiole leaf of about 6mm in diameter and elliptic in shape and grows throughout tropical Africa and has been domesticated in various parts of West Africa including Nigeria, where it is locally used as vegetable in soups [14]. It is used to treat many ailments including diabetes, malaria, helminth infections, fever [15], promote wound healing [16], and used to treat microbial infections [17].
Materials and Methods
Fomites samples were swabbed from hospitals and health centers in Odeomu regions. The fomites were swabbed with sterile cotton swabs stick moistened with sterile distilled water. All the samples were transported immediately to the laboratory for processing and analysis. The isolates were identified and characterized based on their cultural, morphological characteristics and biochemical reactions. Kirby-Bauer disc diffusion method was used for antibiotic susceptibility testing. Resistance profiles of the isolates were determined by measuring the diameter of zones of inhibition of each antibiotics and comparing these zones of inhibition with [18] tables of interpretative zones.
Collection of Vernonia amygdalina
Fresh leaves of bitter leaf were purchased at a local herbal market in Osun State, Nigeria. The leaves were sorted to remove any dead matter and other unwanted particles. The plants were identified and authenticated at Kings University with the voucher specimen number IFE/16885 and Ife herbarium.
Preparation of extract
Fresh bitter leaves were collected, air dried at room temperature and pulverized with pulse blender/mortar and pestle. 800g of bitter leaf (Veronica amygdalina) was weighed using analytical balance and mixed with 3200ml of n-hexane into a container to soak for 3 days for defatting while the temperature was maintained at room temperature. The mixture was filtered using muslin and the filtrate was removed from the solution. 3000ml of methanol was added to concentrate the extract. Defatting adds to the weight of the extract, so it is important to defat the extract to remove fat.
Sterility test of the plant extracts
The sterility of the extract was tested for by inoculating 1ml of the extract on sterile nutrient agar and incubated at 37°C for 24h. The plates were observed for growth. No growth in the extract after incubation indicated that sterility of extracts.
Phytochemical qualitative analysis
The plant extracts, methanolic aqueous solutions were assessed for the existence of the phytochemical analysis for major constituents including tannins, saponins, anthraquinones, steroids, terpenoids, flavonoids and glycosides was carried out using standard qualitative methods according to the methods of [19-22].
Test for anthraquinones
Ten millimeters (10ml) of benzene was added to 6g of Ephedra powder sample in a conical flask and soaked for 10 minutes, it was then filtered. Furthermore, 10ml of 10% ammonia solution was then added to the filtrate and shaken vigorously for 30 secs and pink, violet, or red color indicated the presence of anthraquinones in the ammonia phase.
Test for tannins
10ml of bromine water was added to 0.5g aqueous extract. Decolouration of bromine water showed the presence of tannins.
Test for saponins
5.0ml of distilled water was mixed with aqueous crude plant extract in a test tube and it was mixed. The frothing was mixed with few drops of olive oil and mixed vigorously, and the foam appearance showed the presence of saponins.
Tests for flavonoids
Shinoda Test: Pieces of magnesium ribbon and concentrated HCl were mixed with aqueous crude plant extract for few minutes, after few minutes, appearance of pink color showed the presence of flavonoid.
Alkaline Reagent Test: 2ml of 2.0% NaOH mixture was mixed together with aqueous plant crude extract; concentrated yellow colouration was produced, which became colorless after the addition of 2 drops of diluted acid to the mixture. This result indicated the presence of flavonoids.
Tests for glycosides
Liebermann’s Test: 0ml of acetic acid and 2ml of chloroform with whole aqueous plant crude extract was mixed. The mixture was then allowed to cool and H2SO4 concentrated was added. Green colour showed the entity of aglycone, steroidal part of glycosides.
Keller-Kiliani Test: A solution of glacial acetic acid (4.0ml) with 1 drop of 2.0% FeCl3 mixture was mixed with the 10ml aqueous plant extract and 1ml H2SO4 A brown ring formed between the layers which showed the entity of cardiac steroidal glycosides.
Salkowski’s Test: 2ml of H2SO4 concentrated was added to the whole aqueous plant crude extract. A reddish brown colour formed indicated the presence of steroidal aglycone part of the glycoside.
Test for terpenoids
2.0ml of chloroform was added with 5ml aqueous plant extract and evaporated on the water path and was then boiled with 3ml of H2SO4 concentrated. A grey colour formed showed the entity of terpenoids.
Test for steroids
2ml of chloroform and concentrated H2SO4 were added with 5ml aqueous plant crude extract. In the lower chloroform layer, red colour appeared indicated the presence of steroids.
Antibacterial Assay
Antibacterial activity of the methanolic extracts was carried out using agar well diffusion method. All isolated organisms were inoculated in normal saline, which was incubated for 18 hours and the concentration of the suspensions was adjusted to 0.5 McFarland standard by using a spectrophotometer. A solution of 100mg/ ml and 50mg/ml of the extract was prepared by dissolving the extract in sterile distilled water, The wells were filled with 0.1ml of the extract containing different concentration and antibiotics (streptomycin) used for control were introduced into the wells. Isolates were then seeded on Mueller Hinton agar plates by using sterilized cotton swabs on the surface. Agar surface was bored by using sterile gel cork borer to make wells (6mm). 100μl of the extract were introduced into separate wells. The standard antibiotic disc was placed on the agar surface as positive control. Plates were incubated at 37°C for 48 hours. The zones of inhibitions were recorded in mm using ruler.
Results
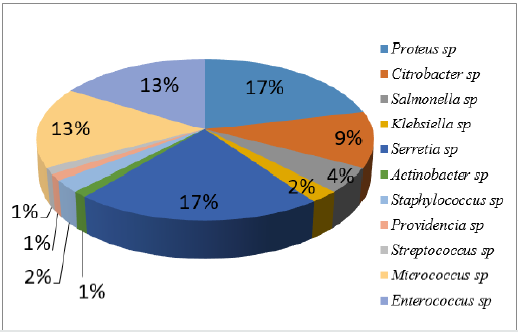
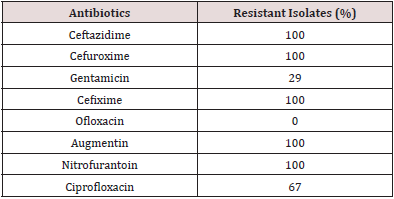
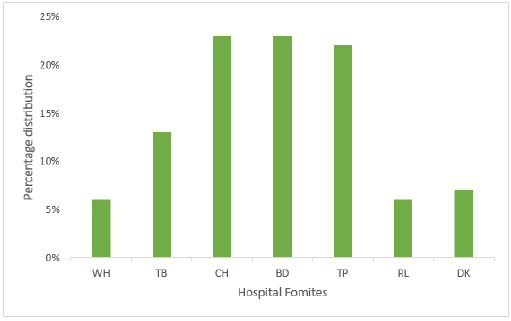
The bacteria identity includes Gram positive organisms (34%) which are Micrococcus sp, Staphylococcus sp, Streptococcus sp, and Enterococcus sp, while the organisms recovered for Gram negative (64%) were Actinobacter sp, Pseudomonas aeruginosa, Photobacterium sp and Vibrio sp. Other enterobacteria recovered from the samples are Klebsiella sp, Shigella sp, Proteus sp, Salmonella sp, Escherichia coli and Enterobacter sp. Among the isolated bacteria, Proteus sp and Staphylococcus sp had the highest frequency of 18% and 16% respectively, followed by Klebsiella sp (11%), while Actinobacter sp and Photobacterium sp were the least. (Figure 1) depicted the distribution of bacteria based on the hospital formites. The highest distribution of bacteria isolates was recovered on chairs (23%) followed by beddings at 23%, taps (22%), tables (13%), doorknob (7%), while wash hand basin and stair rail has 6% respectively. Proteus sp and Serratia sp was the most prevalence bacteria recovered from chairs with 17% each followed by Micrococcus sp and Enterococcus sp with 13% each. Proteus sp and Klebsiella sp has the highest percentage of 25% each and followed by Staphylococcus sp with 12% as showed in Figure 2. In this study, all isolates showed high resistance towards Augmentin, cefixime, cefuroxime, nitrofurantoin and ceftazidime (100%). However, they are less resistance to ciprofloxacin (67%) and gentamicin (29%), while they are susceptible to ofloxacin Table 1. Among the total isolates, Multiple Antibiotic Resistance (MAR) was recorded in all of the bacterial isolates. Fifty-one percent (51%) of the isolates showed MAR pattern for six antibiotics while 25% showed MAR pattern to seven antibiotics. The multi-resistance patterns displayed by the isolates were shown in (Table 2).
Table 1: Antibiotic resistance profile of bacteria isolated from fomites.
Interaction of blood grouping with clothes color is given in Table 1.
Table 2: Multiple Antibiotic Resistance (MAR) profile among bacteria isolates.
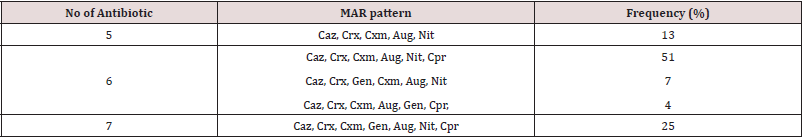
Keys: Caz- Ceftazidime, Crx- Cefuroxime, Cxm- Cefixime, Aug- Augmentin, Gen- Gentamicin, Nit- Nitrofurantoin, Cpr- Ciprofloxacin.
Figure 1: Distribution of isolates according to different hospital fomites.
Keys: WH-Wash Hand basins, TB: Tables, CH: Chairs, DK: Doorknobs, RL: Stair Rails TP: Tap, BD: Beddings.
The susceptibility of bacteria isolates to antibiotics is depicted in Figure 3. It was shown that ofloxacin was 100% susceptible. Bacteria isolates were most sensitive to ofloxacin while gentamicin and ciprofloxacin was 47% and 37% respectively, whereas isolate were totally resistant to ceftazidime, cefuroxime, Augmentin and nitrofurantoin. Table 3 represented the various phytochemical constituents present in the Vernonia amygdalina methanolic extract. The phytochemical screening of V. amygdalina revealed the presence of medicinally active constituent such as alkaloid, saponin, tannins, glycosides, flavonoid, and anthraquinone while phlobatannins and terpenoids are absent. The zone of inhibition formed by the extracts against the bacterial strains on Muller Hinton agar is depicted in Figure 4. Among the varying concentration of the extracts, higher concentration exhibited maximum antibacterial activity against the clinical isolates. The antibacterial activity of the extract against the organisms produced a zone of inhibition which ranged between 4.5-15mm at 100mg/ml concentration while it ranged between 2.0-12.1mm. Similarly, the extract showed no activity on both Salmonella sp and Pseudomonas sp at 100mg/ml. Thus, antibacterial activity was expressed at varying degrees with the increase in concentration. Higher concentration of the leaf extract showed highest antibacterial activity.
Figure 3: Susceptibility of bacteria isolates to antibiotics.
Keys: Caz- Ceftazidime, Crx- Cefuroxime, Cxm- Cefixime, Aug- Augmentin, Gen- Gentamicin, Nit- Nitrofurantoin, Cpr-
Ciprofloxacin.
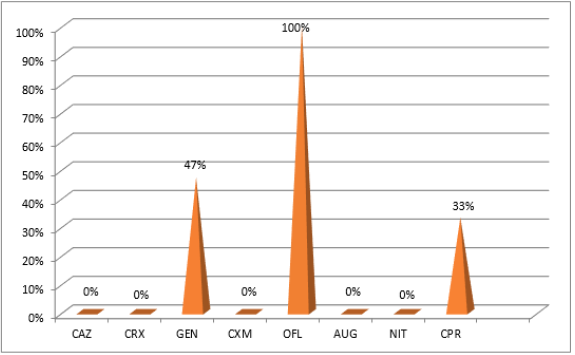
Figure 4: Antibacterial activity of Vernonia amygdalina methanolic extract against pathogenic organisms.
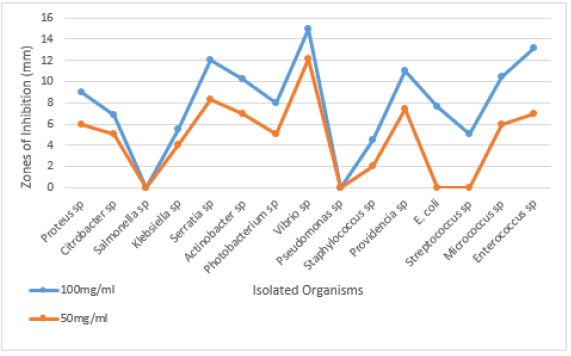
Table 3: Phytochemical analysis of Vernonia amygdalina methanolic extract
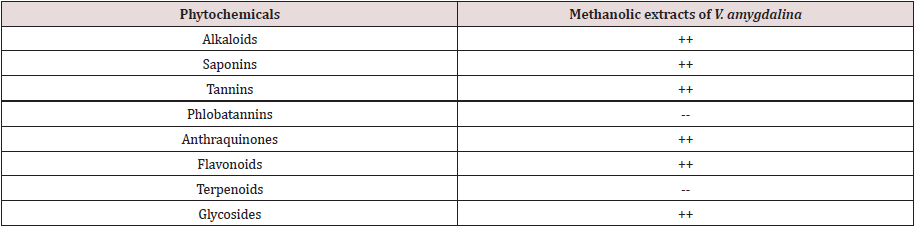
Keys: ++: present, -- : Absent
Discussion
Fomites should be regarded as a possible source of nosocomial infections or hospital acquired infections since they are bacteria from objects in the hospitals. The presence of Enterobacteriaceae in hospital fomites shows unhygienic practices of hospital management. In this study, Proteus sp and Staphylococcus sp were the most predominant isolates with 23% each. This study corroborated the study of [23] who investigated that hospital ward surfaces in Brazil and about 40% of tested surfaces were found to be contaminated with Staphylococcus aureus. An observation was also reported by [24] where about 23% of specimens were contaminated with S. aureus. Ulger F [25] showed that health care workers’ hands and mobile phones were contaminated with numerous types of microorganisms. Hospital fomites have been found to harbour disease causing microbes and are easily transmitted through hand contact or body contact with these fomites and from one person to another. Isolation of such enterobacteria is highly suggestive of faecal contamination, poor personal hygiene and most especially poor hand washing practices amongst health workers and patients [26]. Fomites are involved in the transmission of pathogens in health care environments [27]. In Nigeria, Staphylococcus sp have been reported to have the highest prevalence value of 30.2% and 28.4% in [28]. The results from this study established that bacteria were associated with fomites and this agrees with the findings [29]. It can be deduced from this study that various inanimate objects called fomites harbour various strains of bacteria which may be pathogenic even in the hospital environment. These pathogenic microorganism may cause hospital acquired infections in patients in the hospital or health care center. Kramer A [10] concluded in their study that the common nosocomial pathogens may survive well or persist on surfaces for months and can therefore be a continuous source of transmission from time to time if no regular prevention, surface disinfection is performed. Microorganisms found in fomites and surfaces in this study if transferred via any media and ingested or inhaled by humans could cause severe health problems.
All the isolates were highly resistant to cefixime, cefuroxime, ceftazidime, and Augmentin (100%). The antibiotic resistance of the isolated bacteria maybe due to indiscriminate use (overuse, misuse, prolonged use, self-medication) of antibiotics in humans who generally do not complete the dose of antibiotics prescribed by physician. The results of this study are in accordance with the findings of [30] who isolated Methicillin-resistant and Methicillin-sensitive S. aureus, E. coli, K. pneumoniae, Acinetobacter, E. faecalis and P. aeruginosa. Higher rate of antibacterial resistance in the isolates may have a major implication for human health. Mutations and other various factors may have aided these organisms to mount resistance towards commonly used antibiotics which has make and rendered treatment of infectious diseases inefficient. Ofloxacin were the most effective antibiotics in the study with 43.65%. On the contrary, all the microorganisms were resistance to ceftazidime, cefuroxime and amoxicillin. Medicinal plants are potent source of human health due to availability and the presence of active phytochemical compounds responsible for its various pharmacological activities. In this study V. amygdalina extract showed the presence of some compounds which are rich in those constituents. The phytochemical screening of the extracts showed variation in their phytochemical constituents with the presence and or absence of some components. The presence of glycosides, alkaloids, and flavonoids were believed to exhibit the antibiotic properties of V. amygdalina leaves and confirmed their antimicrobial efficacy against selected pathogens. This study suggested that plants phytochemical constituents that can either inhibit the growth of pathogens or kill them be considered as potential candidates for developing new antimicrobial drugs. The presence of flavonoids had been reported to be associated with the naturally occurring phenolic compounds that possess antioxidant properties in the human diet [31,32]. In various studies, the presence of terpenoids and steroids in V. amygdalina possess different biological activities which include anti-diabetes, anti-inflammatory, anticancer, antibacterial, analgesic, hepatoprotective, and antioxidant [33-35]. Tannins been useful for the prevention of cancer as well as treatment of inflamed or ulcerated tissues [36]. The presence of alkaloids could be well correlated with antimicrobial activities. Thus, this plant species has many medicinal uses. Most alkaloids have a strong bitter taste and are very toxic and because of the bitterness they are used by plant to defend themselves against herbivory and attacks by microbial pathogens and invertebrate pests [37]. Saponins as a class of natural products are involved in complexation with cholesterol to form pores in cell membrane bilayers [38,39] and as such may be used as anti-cholesterol agents or cholesterol lowering agent. The antibacterial activity of V. amygdalina was expressed at varying degrees with the increase in concentration. The high concentration of the leaf extract showed highest antibacterial activity.
Conclusion
This study investigated the occurrence of pathogens in hospital fomites and the efficacy of V. amygdalina against the multidrug resistant isolates. This study concluded that frequently used fomites harbored highly pathogenic bacteria, which have the potential of causing epidemics in the nearest future. Because of huge emergence of multi-drug resistant bacteria, it is an urgent need to discover new therapeutics that would be effective against this strains, therefore V. amygdalina can be considered as potential candidates for developing new antimicrobial drugs and as antibacterial agents that can be used to prevent enteric diseases. Finally, it could be concluded that the susceptibility of these pathogens to ofloxacin and ciprofloxacin will help the clinicians in the better management of hospital acquired infections.
References
- Celik N, Inci A, Denk E, Sevim D, Yasar M (2005) Prevalence of Hospital acquired infections in Anesthesiology intensive care unit. Fırat Tip Dergisi 10(3): 132-135.
- Qyli Z (2017) Prevalence of potential nosocomial pathogens isolated from environments of regional infections due to Gram negative bacteria. The New England Journal of Medicine (3): 171.
- Anton YP, David CH (2010) Hospital Acquired infections due to Gram negative bacteria. The New England Journal of Medicine 362(19): 1804-1813.
- Haidee TC (2016) Hospital acquired infection. Med scape.
- Grohskopf LA, Sinkowitz RL, Garrett DO, Sohn AH, Levine GL, et al. (2002) A national point-prevalence survey of pediatric intensive care unit acquired infections in the United States. Nigerian medical journal: Journal of Nigerian medical association 140(4): 432-438.
- Graham R (2016) What Are Nosocomial Infections?
- Abad FX, Pinto RM, Bosch A (1994) Survival of enteric viruses on environmental fomites. Applied and Environmental Microbiology 60(10): 3704-3710.
- Kanamori H, Rutala WA, Weber DJ (2017) The Role of Patient Care Items as a Fomite in Healthcare Associated Outbreaks and Infection Prevention. Clinical Infectious Diseases 65(8): 1412-1419.
- Bhalla A, Pultz NJ, Gries DM, Ray AJ, Eckstein EC, et al. (2004) Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol 25(2): 164-167.
- Kramer A, Schwebke I, Kampf G (2006) How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 6: 130.
- Ikeh EI, Isamade ES (2011) Bacterial flora of fomites in a Nigerian multi-disciplinary intensive care unit. Lab. Med 42(7): 411-413.
- Oshim IO, Desmond CO, Anyi R, Nwobu U, Ezugwu UM, et al. (2016) Kinetics of minimum inhibitory concentration, minimum bactericidal concentration and minimum fungicidal concentration of Vernonia amygdalina (Bitter leaf) on microorganisms isolated from wound infections. International Journal of Surgical Research 5: 8-14.
- Gashe F, Zeleke G (2017) Antimicrobial activities of Vernonia amygdalina Del and Prunus africana extracts against multidrug resistant clinical strains. Research Journal of Medicinal Plants 11: 142-147.
- Etim KH, Ofor SE, Mandor UA, Ogar M (2012) Evaluation of the antibacterial potential of Vernonia amygdalina on foodborne pathogens isolated from kunu sold in Calabar, Nigeria. Journal of Microbiology and Biotechnology Research 2: 778-782.
- Magadula JJ, Erasto P (2009) Bioactive natural products derived from the East African flora. Natural Product Reports 26: 1535-1554.
- Adetutu A, Morgan WA, Corcoran O (2011) Ethnopharmacological survey and in vitro evaluation of wound-healing plants used in South-western Nigeria. Journal of Ethnopharmacology 137(1): 50-56.
- Noumedem JAK, Mihasan M, Kuiate JR, Stefan M, Cojocaru D, et al. (2013) In Vitro antibacterial and antibiotic-potentiation activities of four edible plants against multidrug-resistant gram-negative species. BMC Complementary and Alternative Medicine 13: 190.
- CLSI Clinical and Laboratory Standards Institute (CLSI): Performance Standard for Antimicrobial Susceptibility Testing. 16th Informational supplement. 2018 CLSI document M100-S16. Wayne, PA.
- Debiyi OO, Sofowora FA (1978) Phytochemical screening of medicinal plants, Iloyidia. 3: 234-246.
- Trease GE, Evans WC (1989) Phenols and Phenolic glycosides. In: Textbook of Pharmacognosy Balliese, Tindall and Co Publishers, London, UK, 12: 343-383.
- Sofowora A (1993) Phytochemical Screening of Medicinal Plants and Traditional Medicine in Africa. Spectrum Books Ltd, Ibadan, Nigeria.
- Roopashree RD, Rani RHS, Narendra C (2008) Antibacterial activity of antipsoriatic herbs: Cassia tora, Mormodica charantia and Calendula officinalis. International Journal of Applied in Natural Products 3: 20-28.
- Carvalho KS, Melo MC, Melo GB, Gontijo Filho PP (2007) Hospital surface contamination in wards occupied by patients infected with MRSA or MSSA in a Brazilian university hospital. J Basic App Pharm Sci 28(2): 159-163.
- Narmeen S, Jaladet M (2009) Isolation and identification of Staphylococcus aureus using classical and molecular methods. The 2nd Kurdistan Conference on Biological Sciences 12(1): 10-16.
- Ulger F, Esen S, Dilek A, Yanik K, Gunaydin M, et al. (2009) Are we aware how contaminated our mobile phones with nosocomial pathogens? Annals Clin Micr Antimic 8: 7.
- Bellifa S, Hassaine H, Balestrino D, Charbonnel N, M Hamedi I, et al. (2013) Evaluation of biofilm formation of Klebsiella pneumoniae isolated from medical devices at the University Hospital of Tlemcen, Algeria. African Journal of Microbiology Research 7(49): 5558-5564.
- Prescott LM, Harley JP, Klein DN (1999) Microbiology. The McGraw-Hill Companies, Inc, 4th (edn.), New York.
- Kihla AJ, Ngunde PJ, Evelyn MS, Gerard N, Ndip RN (2014) Risk factors for wound infection in health care facilities in Buea, Cameroon: Aerobic bacterial pathogens and antibiogram of isolates. Pan African Medical Journal 18: 6.
- Orji MU, Mbata TI, Kalu OU (2005) Isolation of pathogenic bacteria from hospital staff apparel in Nigeria. Malawi Med J 17(4): 128-130.
- Bhatt MP, Bhalla GS, Tandel K, Jindamwar P, Chaudhari CN, et al. (2015) Antimicrobial Susceptibility Profile of Methicillin-resistant Staphylococcus aureus at a Tertiary Care Centre. Archives of Clinical Microbiology 6(3): 6.
- Atangwho IJ, Egbung GE, Ahmad M, Mun F Yam, Mohd Z Asmawi (2013) Antioxidant versus anti-diabetic properties of leaves from Vernonia amygdalina growing in Malaysia. Food Chem 141(4): 3428-3434.
- Ashok kumar R, Ramaswamy M (2014) Phytochemical screening by FTIR spectroscopic analysis of leaf extracts of selected Indian medicinal plants. Int J Curr Microbiol Appl Sci 3(1): 395-406.
- Erasto P, Grierson DS, Afolayan AJ (2007) Evaluation of antioxidant activity and the fatty acid profile of the leaves of Vernonia amygdalina growing in South Africa. Food Chem 104: 636-642.
- Nlandu Roger Ngatu, Maiko K Okajima, Maki Yokogawa, Ryoji Hirota, Mikiro Takaishi, et al. (2012) Anti-allergic effects of Vernonia amygdalina leaf extracts in hapten-induced atopic dermatitis-like disease in mice. Allergol Int 61(4): 597-607.
- Alara OR, Abdurahman NH, Ukaegbu CI, Kabbashi NA (2019) Extraction and characterization of bioactive compounds in Vernonia amygdalina leaf ethanolic extract comparing Soxhlet and microwave-assisted extraction techniques. Journal of Taibah University for Science 13(1): 414-422.
- Adegboye MF, Akinpelu DA, Okoh A (2008) The bioactive and phytochemical properties of Garcinia kola (Heckel) seed extract on some pathogens. African Journal of Biotechnology 7(21): 3934-3938.
- Harbone JB (1998) Methods of extraction and isolation. In: Phytochemical Methods. Chapman and Hall, London pp: 60-66.
- Francis C, George G, Zohar K, Harinder PS, Makhar LM, et al. (2002) The biological action of saponins in animal system: A review. British Journal of Nutrition 88(6): 587-605.
- Usunobun U, Okolie P, Ngozi (2016) Phytochemical analysis and proximate composition of Vernonia amygdalina. International Journal of Scientific World 4(1): 11-14.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...