Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4579
Review Article(ISSN: 2637-4579) 
Unique Anatomy of the Spleen of One Humped Camel (Camelus Dromedarius): A Review Volume 3 - Issue 3
A. Bello1*, J.E Onu1, B.I Onyeanusi2, M. L Sonfada1, M.A Umaru3, A. Danmaigoro1 and S.A. Hena1
- 1Department of Veterinary Anatomy, Usmanu Danfodiyo University, Sokoto
- 2Department of Veterinary Anatomy, Ahmadu Bello University Zaria
- 3Department of Theriogenology and Animal production, Usmanu Danfodiyo University, Sokoto, Nigeria
Received: March 15, 2019; Published: March 22, 2019
*Corresponding author: Dr. A. Bello, Department of Veterinary Anatomy, Usmanu Danfodiyo University, Sokoto.
DOI: 10.32474/OAJBEB.2019.03.000161
abstract
Over the past years, defensive immunity has been recognized as having an important role as a front-line mechanism and as an integral part of the adaptive immune response. Immunity in One Humped Camel (Camelus dromedarius) exposed too many diseases are spleen-dependent. In this review, we discuss the general aspects of the gross anatomy of the spleen (both internal and external), based on location, position, relation, blood supply and innervations of the organ. The micro-architecture of the camel anatomy was discussed using eosin and hematoxylene stain and the cells visible in the aspect of normal tissue histology. The aim of the write-up is to examine the anatomy of the different sections in the spleen of the one-humped camel (Camelus dromedaries).
Keywords: Defensive Immunity; Camelus Dromedarius; Spleen; Gross Anatomy; Histology
Introduction
Camelids originated in North America, where they are now extinct, and did not reach the Old World until about two million years ago [1]. Camels belong to the suborder Tylopoda of the Artiodactyls (even-toed ungulates, along with pigs). Within their current native range of North Africa and central and western Asia the one-humped camel or dromedary (Camelus dromedarius) is a highly revered species, used for food, fibre, and racing [2]. The species reportedly has no known natural predators throughout this range. Australia is the only country in which camels have established a feral population, and is now the only location where the species lives in natural populations [3]. Spleen, located between the stomach, left kidney and diaphragm, the spleen is the largest lymphoid organ in the body, performing functions for the blood similar to those performed by the lymphnodes for the lymph [4]. It is a soft organ, conforming to the contours of the organs and structures surrounding it. At the hilus on the visceral surface, the splenic artery brings blood into the spleen, the splenic vein takes blood from the spleen to the hepatic portal system, and lymphatic drain lymph from the spleen.[5]. In some domestic species such as the horse and dog, the spleen functions as a reservoir from which blood can be mobilized when needed and in these species, smooth muscle is a prominent feature of the capsule and traberculae of the spleen [6].
Structure
The spleen represents the largest reticulo-endothelial accumulation in the body [6]. It has a thin fibrous capsule, to which the peritoneum adheres intimately. The fibrous tissue of the capsule extends into the spleen to form a series of traberculae between which lies the splenic pulp [5]. The spleen has an extensive blood supply consisting of traberculae arteries, central arteries, penicillar arteries, sinusoids, red pulp veins, and traberculae veins [7]. It is surrounded by a capsule, has traberculae, and is divided into red and white pulp. The spleen is very vascular and has red and white pulp [3].
Embryologically
The spleen develops in the dorsal mesentery of the stomach (dorsal mesogastrium). The spleen arises from cells of the mesentery, which migrate into the plane between the layers of the mesentery [8]. The mesentery covering the spleen becomes the visceral peritoneum of the spleen. The mesentery between the spleen and the gut tube becomes the gastrosplenic ligament. The mesentery between the spleen and the dorsal body wall becomes the splenorenal ligament (most of which subsequently fuses to become parietal peritoneum) [9].
Grossly
The spleen is about the size of the cupped hand. If forms the left lateral extremity of the lesser sac [10]. The spleen is a peritoneal organ in the upper left quadrant that is related to the left 9th, 10th, and II the ribs. Clinically, fracture of these ribs may injured or lacerate the spleen. The Spleen is located in the upper left abdominal quadrant. Unlike the lymph node, the spleen is inserted in the blood stream. The spleen clears the blood of aged blood cells and foreign material. It is the site of an immune response to bloodborne antigens, especially in children. In adults, the spleen is not essential to life.
In general, white pulp is the site of antibody synthesis and lymphocyte production. Red pulp is chiefly concerned with the removal and destruction of worn out erythrocytes. In as much as the spleen lies above the costal margin, a normal-sized spleen is not palpable. Enlarged spleen may be palpated below the left costal margin. The splenic artery and vein reach the hilus of the spleen by traversing the splenorenal ligament. Passing from it are the gastrosplenic ligament to the greater curvature of stomach (carrying the short gastric and left gastroepiploic vessels) and the lienorenal ligament to the posterior abdominal wall (carrying the splenic vessels and tail of the pancreas) [5]. The spleen is a peritoneal organ in the upper left quadrant that is related to the left 9th, 10th, and II the ribs. Fracture of these ribs may lacerate the spleen [5]. In as much as the spleen lies above the costal margin, a normal-sized spleen is not palpable. The enlarged spleen may be palpated below the left costal margin. The splenic artery and vein reach the hilus of the spleen by traversing the spleno-renal ligament.
Relations:
a) Ventrally: The left diaphragm, separating it from the pleura, left lung and the 9th, 10th and 11th ribs.
b) Cranially: The stomach.
c) Dorsally: The splenic flexure of the colon.
d) Medially: The left kidney.
The tail of the pancreas abuts against the hilum of the spleen through which vessels and nerves enter and leave this organ Figure 1.
Histologically
The structure resembles the lymph node except in some anatomical aspect, which includes; only efferent lymphatic drain the spleen. The sinusoids are blood vascular not lymphatic. As a result, all cells entering the spleen do so via the blood. Scattered primary nodules (germinal centers) are found throughout the spleen. These contain abundant lymphocytes and make up the “white pulp” (also known as Malpighian corpuscles). Plasma cells and macrophages are found here as well. Surrounding the white pulp nodules are venous sinuses containing abundant erythrocytes - the “red pulp”. These sinuses are lined with macrophages (RE cells) which are highly phagocytic. Between the sinuses are cords of cells - lymphocytes, monocytes and macrophages - referred to as the Cords of Billroth. The immune function of the spleen is especially important in infancy and early childhood. At these times, the rest of the lymphoid system is somewhat underdeveloped Figure 2.
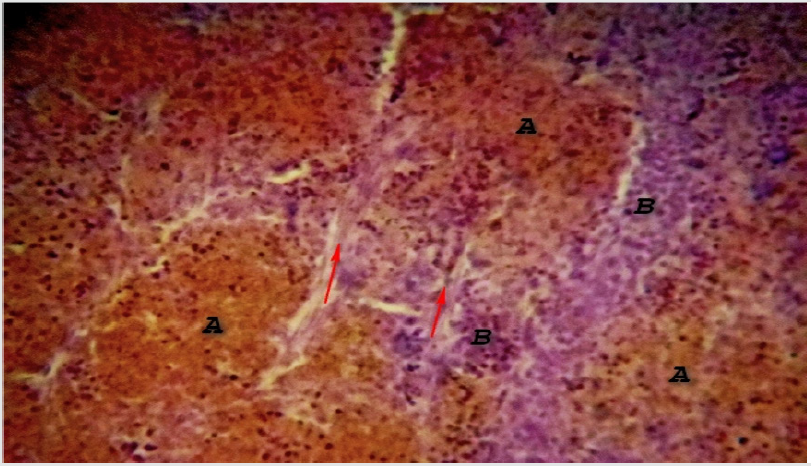
Figure 2: Photomicrograph of Camel Spleen showing clearly differentiated zones of white pulp (A) and Red pulp (B) cells with inter parenchymal traberculae collagen connective tissue fibers (Red arrow) H&E x200.
Microscopically, the spleen appears to consist of discrete 0.5-1 mm white nodules, called the white pulp, embedded in a red matrix called the red pulp. Microscopically, as shown here, the white pulp WP consists of lymphoid aggregations and the red pulp RP, making up the bulk of the organ, is a highly vascular tissue [10]. The spleen has a thin fibroelastic outer capsule C from which short traberculae T extend into the parenchyma Figure 3. The capsule is thickened at the hilum and is continuous with supporting tissues that sheath the larger blood vessels entering and leaving the organ. In dogs and horses the spleen is also a reservoir of blood and these supporting tissues contain smooth muscle to pump blood out, but in humans only a few smooth muscle cells persist Figure 4. The splenic artery divides into several major branches, which enter the hilum and branch to form numerous arterioles. [11].
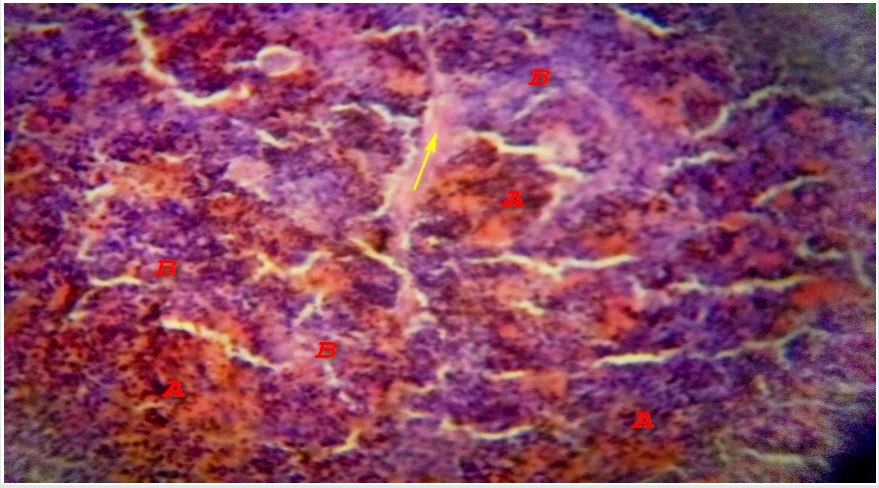
Figure 3: Photomicrograph of Camel Spleen showing clearly differentiated zones of white pulp (A) and Red pulp (B) cells with communication of paranchymal traberculae connective tissue fibers connective tissue (Red arrow) and thick connective tissue fibers capsule (Yellow arrow) H&E x200.

Figure 4: Photomicrograph of Camel Spleen showing clearly zones of white pulp (A) and Red pulp (B) cells with developed inter-paranchymal traberculae collagen connective tissue fibers (Yellow arrow) H&E x200
An overview of the splenic ultra-structure and circulation is shown in Figure 1 above; blood enters the spleen in the splenic artery which branches repeatedly within the parenchyma [5]. The larger arteries are surrounded by a fibrocollagenous sheath that disappears in the smaller branches. These central arteries A, are so named because they have a cylindrical cuff of lymphoid tissue around them, the periarteriolar lymphoid sheath PALS, consisting mainly of TH cells. The central artery gives off a number of short branches at right angles, which are called penicilliary arteries PALS and these terminate in two to three sheathed capillaries with only one is shown for each penicilliary artery [7]. These unique vessels are small blind-ending capillaries with no endothelial lining but surrounded instead by an aggregate of macrophages. Thus the blood arriving in a sheathed capillary must traverse this wall of macrophages before entering the red pulp RP. The sheathed capillaries therefore form the first part of the filtering mechanism of the spleen [6]. The splenic white pulp is of two types, T cell and B cell, together making up 5-20% of the total mass of the spleen. The functions of these areas appear to be similar to those of the paracortex and superficial cortex of lymph nodes respectively. The non-filtering areas of red pulp parenchyma as probably be considered part of the splenic lymphoid tissue mass also, but its immunological function remains to be elucidated [12].
White Pulp
White pulp consists of lymphoid tissue that unsheathes the central arteries (periarterial sheath) along with the associated nodules and germinal centers. The periarterial sheath is populated mainly by T lymphocytes [9]. The peripheral white pulp and germinal centers are populated mainly by B lymphocytes. In the white pulp, the T cell areas surround the central arteries, forming the periarteriolar lymphoid sheath. In humans, this lymphoid tissue is less well organized than in other animals, but the term PALS persists [12].
Red Pulp
Red pulp consists of splenic cords of Billroth and venous sinusoids [8]. Defective red blood cells resulting from aging or disease (as in sickle cell anemia, hereditary spherocytosis, or thalassemia syndromes) are delayed in their passage from Billroth cords into the venous sinusoids and phagocytosed by macrophages lining the cords. The splenic parenchyma is permeated by an interconnected network of sinuses S that drain in turn into larger sinuses, tributaries of the splenic vein and finally the hepatic portal vein [3]. The sinuses are lined by endothelial cells resting upon a basement membrane with numerous narrow slits. The reticulin fibers of the sinusoidal basement membrane are arranged in a circular fashion and are continuous with the reticulin meshwork of the parenchyma [1].
Blood Supply
The splenic artery is one of the three main branches of the coeliac axis. The splenic vein is joined by the superior mesenteric to form the portal vein [6]. Blood cells entering the parenchyma from the sheathed capillaries squeeze through the walls of the sinuses to drain out of the organ via the splenic vein, an arrangement known as the open circulation [4]. Note that the splenic vessels also provide the principal blood supply of the pancreas. [6].
References
- Hildebrandt PK (1981) The organ and vascular pathology of babesiosis. In: Ristic M, Kreier JP (Eds.), Babesiosis. Academic Press, New York, pp. 459-473.
- Hein WR, Dudler L (1993) Divergent evolution of T-cell repertoires: extensive diversity and developmentally regulated expression of the sheep gamma delta T-cell receptor. Basel Institute For Immunology, Switzerland, 12(2): 715-724.
- Brown W, Estes DM, Chantler SE, Kegerreis KA, Suarez CE, et al. (1998) DNA and a CpG oligonucleotide derived from Babesia bovis are mutagenic for B cells. Infect. Immun. 66(11): 5423-5432.
- (2006) Clinical Anatomy A revision and applied anatomy for clinical students by HAROLD ELLIS Published by Blackwell Publishing Ltd Blackwell Publishing Asia Pty Ltd, 550 Swanston Street, Carlton, Victoria 3053, Australia.
- Barbara young, john W Heath, (2004) wheaters functional histology, (5th edn), pp. 229-236.
- H Dieter Dellman (1993) Textbook of veterinary Histology, (4th edn).
- Hirose K, Nishimura H, Matsuguchi T, Yoshikai Y (1999) Endogenous IL-15 might be responsible for early protection by natural killer cells against infection with an avirulent strain of Salmonella choleraesuis in mice. J. Leukoc. Biol, Nagoya University School Of Medicine, Japan, 66(3): 382-390.
- Evans E , Alexander Delahunta (2004) Guide to the dissection of dog, (6th edn).
- Brown WC, Davis WC, Tuo W (1996) Human IL-12 up-regulates proliferation and IFN- production by parasite antigen-stimulated the cell clones and T-cells of cattle, Washington State University, USA, 31(795): 321-324.
- htp://www.cvm.okstate.edu/instruction/mm_curr/histology/ Histology Reference/index.htm
- Goff WL, Johnson WC, Horn RH, Barrington GM, Knowles DP, et al. (2003) The innate immune response in calves to Boophilus microplus tick transmitted Babesia bovis involves type-1 cytokine induction and NKlike cells in the spleen. Parasite Immunol. 25(4): 185-188.
- Goff WL, Johnson WC, Parish SM, Barrington GM, Elsasser TH, et al. (2002) IL-4 and IL-10 inhibition of IFN-- and TNF--dependent nitric oxide production from bovine mononuclear phagocytes exposed to Babesia bovis merozoites. Vet Immunol Immunopathol. USA, 84(3-4): 237-251.
Editorial Manager:
Email:
biomedicalengineering@lupinepublishers.com

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...