
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4676
Research Article(ISSN: 2637-4676) 
Mechanisms of Resistance to Organophosphorus and Pyrethroid Insecticides in Asian Citrus Psyllid, Diaphorina Citri Populations in Florida Volume 1 - Issue 3
Julius Eason1, Lambert HB Kanga1*, Muhammad Haseeb1, Jawwad A Quershi2 and Jesusa Legaspi3
- 1Center for Biological Control, Florida A&M University, USA
- 2Entomoogy and Nematology, Univeristy of Florida, USA
- 3United States Department of Agriculture, Agricultural Research Service, USA
Received: February 14,2018; Published: February 21,2018
Corresponding author: Lambert HB Kanga, Center for Biological Control, Florida A&M University, USA
DOI: 10.32474/CIACR.2018.01.000111
Abstract
Background: The Asian citrus psyllid (ACP) is the most devastating pest on citrus in Florida as it vectors causal pathogens of citrus greening a disease also known as huanglongbing. Florida is the largest citrus producer in the.com and the development of insecticide resistance in ACP populations is a serious threat to the citrus industry. Mechanisms of resistance in ACP are not fully understood. Thus, in order to develop a resistance management program, the primary type of resistance must be determined. We conducted several studies to investigate the mechanisms of resistance to two commonly used classes of insecticides in ACP populations.
Results: Enhanced metabolism by esterase (185.0-fold) and carboxyl esterase (30.33-fold) were major factors of resistance to organophosphorus insecticides. In addition, glutathione-s-transferees (2.5-44 fold), mixed function oxidise (2.17-fold), and altered target site (3.67-fold) were also significant factor of resistance to pyrethroid insecticides.
Conclusion: Mechanisms of resistance in ACP populations to insecticides were both metabolic and altered target site; therefore synergist probes such as piperonyl butoxide, S, S, S, -tri-n- butyl phosphorotrithionate, triphenyl phosphate and formamidine could be used successfully to design a resistance management strategy for the Asian citrus psyllid.
Keywords: Asian citrus psyllid; Citrus greening; Insecticide resistance; Mechanisms of resistance
Abbrevations: ACP: Asian Citrus Psyllid; PBO: Piperonyl Butoxide; SL: Susceptible Strain; CL: Confidence Limit; TEPP: Triphenyl Phosphate; SR: Synergism Ratio
Introduction
Florida is the largest citrus producer in the.com, and the citrus industry contributes to about $8.91 billion annually to the state [1]. The Asian citrus psyllid (ACP), Diaphorina citri Kuwayama, is the most devastating pest on citrus in Florida. First reported in Taiwan in 1907, it is spread throughout the citrus industries of Asia and was found in Florida in 1998 [2]. The ACP vectors the pathogens that cause the disease known as citrus greening. Since 2006 the combination of pest and disease has resulted in an approximate loss of $4.5 billion to Florida's citrus industry. The disease continues to spread and the entire U.S. citrus industry is threatened. Applications of insecticides have led to the development of insecticide resistance in ACP populations [3,4]. Therefore, in order to develop a resistance-monitoring program, the primary type of resistance must be determined. Mechanisms of ACP resistance are not fully understood. Insects have developed resistance to insecticides primarily as a result of three mechanisms: decreased penetration, reduced target site sensitivity, and enhanced metabolism [5,6]. There are three major enzymes associated with insecticide resistance: mixed-function oxidases, glutathione-s- transferees, and esterases [7]. Mixed-function oxidases are known to have a wide range of substrates, thus has a role in the metabolism and detoxification of various compounds [8]. Esterase-mediated mechanism of resistance has been detected against all classes of insecticides containing ester moiety [9]. Glutathione-s- transferees and Carboxyl esterase are enzymes that have been demonstrated to cause insect resistance to conventional insecticides [10,11]. Here we present results of several studies designed to determine the mechanisms of resistance to organophosphorus and pyrethroid insecticides in ACP populations from southern Florida. Such information is critical in the development of a successful resistance management strategy for the citrus industry
Materials and Methods
Insecticides and insect collections
All insecticides were technical grade samples (>98% purity). These include pyrethroids (cypermethrin) and organophosphates (malathion), they were purchased from Sigma-Aldrich (St. Louis, MO). The other Chemical tested included the piperonyl butoxide (PBO), diethyl maleate and formamidine were also purchased from Sigma-Aldrich (St. Louis, MO) and S, S, S, -tri-n-butyl phosphorotrithionate (DEF) was obtained from Chem Services (West Chester, PA).
Insects
The susceptible strain (SL) of adult ACP came from a population continuously reared at the Southwest Florida Research and Education Center, University of Florida. The susceptible strain was established in 2006 using field populations which had not been subjected to insecticides. The strain was maintained on orange jasmine, Murraya paniculata (L) Jack (Sapindales: Rutaceae) without exposure to insecticides in an air conditioned glasshouse maintained at 27±4°C; 70±10% RH under natural light. The field populations (RM) of ACP were collected using an aspirator from commercial citrus groves in Immokalee, FL (26 41'04 N 81 26' 20W). They were fed on young seedlings of Citrus macrophylla Wester until used for bioassays.
Synergism bioassays
The mechanisms of resistance were determined by using known synergists as diagnostic probes. The glass vial bioassay technique developed by Kanga and Plapp [12] was used for the synergism bioassays. The synergists used in these experiments were piperonyl butoxide (PBO) a mixed-function microsomal oxidase inhibitor; S, S, S, -tri-n- butyl phosphorotrithionate (DEF) a putative inhibitor of esterases; triphenyl phosphate (TEPP) an inhibitor of carboxylesterase; diethyl maleate (DEM) a glutathione s- transferee's inhibitor [13] and formamidine as a target site synergist. The concentrations of synergists in the bioassay were 50μg/vial of PBO, 25μg/vial of DEF, 50μg/vial of DEM, 25μg/vial of TEPP, and 50μg/vial of formamidine. The amounts of synergists used in these experiments were the highest concentrations that are not toxic to the psyllids during the pre-experimental runs. Two sets of bioassays were conducted at each time. In one set, the psyllids were treated with a synergistic mixture of the selected insecticide, and second sets of psyllids were tested with the selected insecticide only. Each insecticide was tested using eight concentration levels (plus an ethanol control) with 10 replicates of 5 psyllids per vial. All treated vials were held at room temperature (27±1°C, 65% RH) and the psyllid mortality was recorded 24 hours after exposure. Adult ACP that were unable to walk for a short distance (>5mm) after gentle probing with a fine brush were considered dead.
Statistical analysis
Concentration-mortality data with or without synergists were subjected to Probity analysis using the POLO program [14]. Percentage mortality in the treatments was corrected for control mortality using Abbotts formula [15]. The effects of synergists were calculated by dividing the LC50 for the insecticide alone by the LC50 for the selected insecticide and synergist mixture. The responses to synergists were considered not significant if the 95% confidence limit (CL) of the synergist ratio at the LC50 bracketed 1.016. A likelihood ratio test of equality was conducted to determine whether the regression lines of the two treatments were equal (the slopes and intercepts of the two lines were the same). A similar ratio test of parallelism was run to determine whether the regression lines were parallel (the slopes of the two lines were the same) [16].
Results and Discussion
Esterase-mediated resistance factor
The levels of toxicity (the LC50) of malathion alone and malathion with DEF to ACP were significantly different as the 95% confidence limit of the synergist ratio at the LC50 did not bracket 1.0 (Table 1). In addition, dose mortality regression lines for both treatments were not parallel (χ2=93.32; df=1; P=0.0001) and not equal (χ2=221.81; df=2; P=0.0001). The synergism ratio (SR) was 185.0-fold. These results suggested that esterase- mediated metabolism was a major resistance factor role in ACP populations.
Carboxylesterase resistance factor
The toxicity (LC50) of insecticide malathion alone and malathion with TEPP to ACP were significantly different (Table 1). There was a significant increase of 30.33-fold in the toxicity to ACP of malathion with the synergist of TEPP compared to malathion alone. These results suggested that carboxylesterase was also a major factor of resistance in ACP populations to malathion.
Table 1: Toxicity of Malathion with and without DEF, PBO and TEPP to Asian citrus psyllid Diaphorina citri
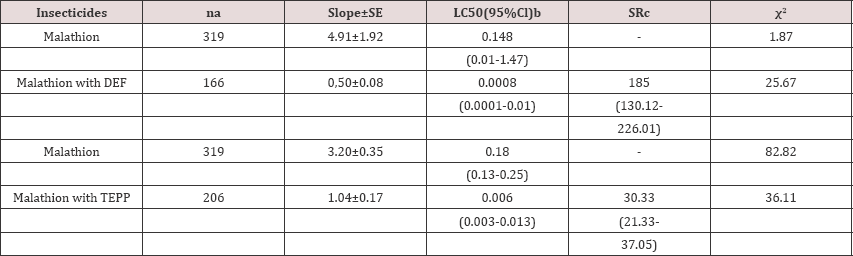
a-Number of adult Asian citrus psyllids tested.
b-Concentrations are expressed in ^g insecticide per vial.
c-Synergist ratio (SR) calculated by dividing the LC50 for insecticide alone by the LC50 for insecticide with the synergist.
Glutathion-S-transferase resistance factor
The pyrethroid, cypermethrin alone was significantly less toxic to ACP compared to cypermethrin with the synergist DEM (Table 2). In addition, the dose mortality regression lines for both treatments were parallel (χ2=0.023; df=1; P=0.88) but not equal (χ2=8.52; df=2; P = 0.014 ). Data indicated a significant increase of 2.5-fold in synergism ratio with DEM and suggested that glutathione-s-transferees were involved in insecticide resistance in ACP population
Mixed-function oxidase
Table 2: Toxicity of cypermethrin with and without DEM, PBO and Formamidine to Asian citrus psyllid Diaphorina citri.
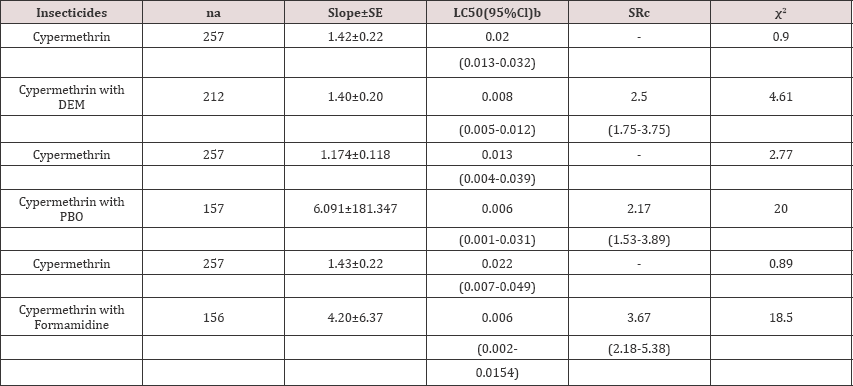
a-Number of adult Asian citrus psyllids tested.
b-Concentrations are expressed in ^g insecticide per vial.
c-Synergist ratio (SR) calculated by dividing the LC50 for insecticide alone by the LC50 for insecticide with the synergist.
Data indicated a significant increase of cypermethrin with the synergist PBO compared to cypermethrin alone to ACP (Table 2). In addition, the dose mortality regression lines for cypermethrin alone and cypermethrin with PBO were not parallel (χ2=14.24; df=2; P=0.001) and not equal (χ2=10.56; df=1; P=0.001).The synergism ratio (SR) was 2.17- fold indicating that mixed-function microsomal oxidases were one of the major factors of resistance in ACP populations to cypermethrin.
Target site resistance factor
The levels of toxicity (LC50) of the pyrethroid, cypermethrin alone and cypermethrin with the synergist formamidine to ACP were significantly different (Table 2). The dose mortality regression lines for both treatments were not parallel (x2=17.81; df=2; P=0.0001) and not equal (x2=3.91; df=1; P=0.0048). Data indicated a significant increase of 3.67-fold in synergism ratio with formamidine. The results suggested that altered target site was a major factor of resistance in ACP populations. From the overall data, DEF and TEPP significantly synergized the toxicity of malathion and suggested that enhanced metabolism by esterase and carboxylesterase was a major mechanism of resistance to organophosphorus insecticides in ACP populations. Similarly, significant increase in synergism activities with DEM, PBO and formamidine indicated that mixed-function oxidases, glutathione-s- transferases, and altered target site insensitivity are also major mechanisms of resistance to pyrethroid insecticides in populations of Asian citrus psyllid. Mechanisms of resistance in ACP populations to these classes of insecticides appear to be both metabolic and altered target site, therefore synergist probes such as piperonyl butoxide, S, S, S,-tri-n-butyl phosphorotrithionate, triphenyl phosphate and formamidine could be used successfully to design a resistance management strategy for the ACP population.
Acknowledgment
We are grateful to Janice Peters and Manuel Pescador (Florida A&M University) for providing useful discussions and reviews of the manuscript. We also thank Sabrina Hayes (Florida A&M University) for her technical assistance with this study.
References
- Hodges AW, Spreen T (2012) Economic impacts of citrus greening (HLB) in Florida, 2006/07 2010/11. Electronic Data Information (EDIS) FE903, University of Florida, USA.
- Halbert S, Manjunath K (2004) Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: A Literature Review and Assessment of Risk in Florida. Florida Entomol 87(3): 330-353.
- Tiwari SS, Mann RS, Rogers ME, Stelinski L (2011) Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manag Sci 67(10): 1258-1268.
- Wells R, Stelinski L (2011) UF: Insecticide resistance developing in psyllid that carries citrus disease. University of Florida, USA.
- Plapp FW (1976) Biochemical genetics of insecticide resistance. Annu Rev Entomol 21: 179 -197.
- Oppenoorth FJ (1984) Biochemistry of insecticide resistance. Pestic Biochem Physiol 22(2): 187-193.
- Bull DL (1981) Factors that influence tobacco budworm, resistance to organophosphorus insecticides. Bull Entomol Soc Am 27(3): 193-197.
- Scott JG (1999) Cytochromes P450 and insecticide resistance. Insect Biochem Mol Biol 29(9): 757-777.
- Alizadeh M, Bandani AR, Amiri A (2010) Evaluation of insecticide resistance and biochemical mechanism in two populations of Eurygaster integriceps Puton (Heteroptera: Scutelleridae). Munis Entomol Zool 5(2): 734-744.
- Che Mendoza A, Penilla RP, Rodriguez DA (2009) Insecticide resistance and glutathione s- transferases in mosquitoes a review. Afr J Biotech 8(8): 1386-1397.
- Devonshire AL, Moores GD (1982) A carboxylesterase with broad substrate specificity causes organophosphorus, carbamate and pyrethroid resistance in peach potato aphids (Myzus persicae). Pestic Biochem Physiol 18(2): 235-246.
- Kanga LHB, Plapp FW (1995) Development of a technique to monitor resistance to biodegradable insecticides in field populations of tobacco budworm (Lepidoptera: Noctuidae). J Econ Entomol 88(1): 487-494.
- Grant D, Bender D, Hammock B (1989) Quantiative kinetic assays for glutathione s-transferase and general esterase in individual mosquitoes using EIA reader. Insect Biochem 19(8): 741-751.
- Russel RM, Robertson JL, Savin NE (1977) POLO: A new computer program for Probit analysis. Bull Entomol Soc Am 23(3): 209-213.
- Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18(2): 265-267.
- Robertson JL, Preisler HK (1992) Pesticide bioassays with arthropods. CRC Press, USA.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...














