Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4676
Research Article(ISSN: 2637-4676) 
Effect of the Regrowth Age on the Chemical Composition, Digestibility and Polyphenol Content of Jatropha Curcas Volume 8 - Issue 1
Verdecia DM1*, Torres E2, Sánchez AR2, Álvarez-PerdomoGR2, Herrera RS3, Ramirez JL1, Paumier M1, Bodas R4, Giraldez FJ5, Grizelj J6 and Lopez S7
- 1Animal Production Study Center, University of Granma, Cuba
- 2Technical State University of Quevedo, Extension La María, Mocacha-Los Ríos, Ecuador
- 3Institute of Animal Science, Cuba
- 4ITACYL Agricultural Technological Institute, Spain
- 5Mountain Livestock Institute (CSIC-ULE), Spain
- 6University of Zagreb, Faculty of Veterinary Medicine, Croatia
- 7Animal Production Department, University of León, Spain
Received: November 11, 2019; Published: November 26, 2019
Corresponding author: Verdecia DM, Animal Production Study Center, University of Granma, PO Box 21, Bayamo, C.P. 85 100, Granma, Cuba
DOI: 10.32474/CIACR.2019.08.000276
Abstract
The present work was developed with the objective of evaluating the effect of the regrowth age on the chemical composition, digestibility and polyphenol content of the Jatropha curcas in an area of Cauto Valley. Was used piece of ground of Jatropha curcas with two years of establishment. A randomized block design with six replicas was used. The treatments were regrowth ages of 60, 120 and 180 days. At the beginning of each seasonal period a homogeneity cut was made at 1 m above the ground level. From there the sampling was carried out in 10 random plants eliminating the edge effect in an area of 0.5 ha, according to the treatments. The chemical composition of Jatropha curcas the dry matter content, DNF, ADF, ADL, increase with age with its best results at 180 days with 28.82; 51.53; 33.44 and 20.22%; while the CP and cellular content decrease with its highest results at 60 days 27.78 and 58.79%. The minerals showed a very variable behavior. The highest energy contribution and digestibility percentage at 60 days of age with a decrease of 11.04; 7.40; 8.90%; 1.58 and 1.11 MJ / kg. Polyphenolic compounds increase with age with the best results at 180 days of age with 11.26, 21.10, 113.93, 111.49 and 2.46 g / Kg, respectively; for total tannins, total phenols, TCT, TBCT and FTC. The results of the present investigation evidenced the marked effect of climatic factors and age on the quality of the Jatropha curcas, as the maturity of the forage progressed, its nutritional contribution decreased with a decrease in the CP, the nutrient content, digestibility and energy, as well as an increase in the fibrous fraction and the content of polyphenolic compounds.
Introduction
Due to the food deficit and the global economic crisis, Latin American countries have had to venture into other feeding strategies to increase animal production in tropical conditions, by supplying livestock with more protein and minerals, which in general are found deficit in grasses Diaz . In this sense, the biomass of trees, shrubs have a leading role for their considerable protein levels and acceptable nutritional value. Due to the perspectives and benefits of this plant for tropical livestock, it is necessary to know the essential characteristics of its chemical composition, nutritional value and its impact on the acceptability of the animal Boufennara et al. [1]. The presence of foliage of trees, shrubs and legumes in animal diets, whether in fresh or flour form, can improve production yields, which represents a practical and economical alternative to increase productivity in developing countries Boufennara et al. [1]. Most of these food sources have a well-balanced amino acid pattern in relation to the established requirements, so the total protein required in the diet is much lower than when cereals are the basis of it, and the waste is less of nitrogen during metabolism Murgueitio et al. [2]. Among these tree species is Jatropha curcas with outstanding nutritional qualities, rich in protein, vitamins and minerals, it is a multipurpose species, of tropical origin, belonging to the Euphorbiaceae family and their common names vary according to the different regions where it is grown. In Cuba it is recognized as: pinija pinion, fence pinion, purgative pinion; It is called piñoncillo in Mexico, piñol in Peru and tempate in Costa Rica. It can grow both in areas of high and low annual rainfall. It is fast growing and seed production can begin even in the first year of planting. Highly resistant to drought, it can be grown in marginal areas, without competing with the production of species for human consumption Toral et al. [3]. According to the above mentioned, the aim of this work was to evaluate the effect of the regrowth age on the chemical composition, digestibility and polyphenol content of Jatropha curcas in an area of the Cauto Valley.
Material and Methods
Geographic LOCATION
The research was carried out by the Productive Teaching Department of Granma University (Granma, Cuba). An area of 4410 m2 was used for the purposes of this experiment from 2007 to 2009. The type of soil was calcic haptustept Soil Survey Staff [4], with pH 6.2. The P2O5, K2O and N total content was 2.4, 33.4 y 3.0 mg/100g of soil, respectively, with 3.6% of organic matter.
Treatments and experimental design
According to weather conditions, the period of study was divided in two seasons: rainy season (May-October) and dry season (November-April). The rainfall during the rainy season was 894 mm, the mean, minimum and maximum temperatures registered were 26.7, 22.3 and 33.9 °C, respectively and the mean, minimum and maximum relative humidity were 80.8, 51.0 and 99.2 %, respectively. The rainfall during dry season was 364 mm; the mean, minimum and maximum temperatures were 24.5, 18.3 and 31.6 °C, respectively, and the mean, minimum and maximum relative humidity were 76.2, 44.2 and 97.0%, respectively. Within each season, three regrowth ages were considered: 60, 120 and 180 days; thus, the development state varied from a vegetative growing state at 60 days up to flowering at 180 days. A random block design with three replicates per block was used (9 plots of 396 m2).
Experimental procedure
At the beginning of each season, the plants were trimmed 1 m above ground level. Later on, at each regrowth age, 10 plants were randomly selected from each plot at every regrowth age, discarding the lowest and highest plants. Stalk and petioles with diameters inferior to 2 cm were chosen (considered, as a whole, the useful biomass to be eaten by animals). All select materials were mixed and a homogenous sample representative of useful biomass was obtained. This sample was dried at 65 °C in a forced-air circulation oven for 72 hours, in order to determine the dry matter content (DM). Once dried, samples were stored in a dry cool place until further analyses.
Chemical analysis
Neutral detergent fiber (NDF), acid detergent fiber (ADF) and acid detergent lignin (ADL) contents were determined according to Goering and Van Soest [5]. AOAC [6] procedures were used to determine crude protein (CP), ash, organic matter (OM) and minerals (calcium, phosphorus, magnesium and silica) contents. Phenolic compounds were extracted and analyzed following the procedures described by Makkar [7]. Total extractable phenols (TP) were determined using the Folin- Ciolateau reagent and tannic acid as standard. Total extractable tannins(TT)were estimated after adsorption of TP to insoluble polyvinylpyrrolidone, and measuring the remaining total phenols in the supernatant (or nonprecipitable phenols). Concentration of total extractable tannins (TT) was calculated through subtraction as follows TT = TP − nonprecipitable phenols. Free condensed tannins (FCT) were measured in the extract using the butanol–HCl assay Porter et al. with the modifications of Makkar [7]. Total condensed tannins attach (TCTA) were measured in the solid residue remaining after extraction of phenolic compounds. Concentration of total condensed tannins (TT) was calculated as follows: TT = FCT + CTA. Concentration of phenols and tannins were expressed in g tannic acid equivalent kg−1 DM.
In vitro digestibility
In vitro dry matter digestibility (IVDMD) was determined by the ANKOM procedure, using a Daisy II® incubator (ANKOM Technology, Fairport, NY-USA), as described by Robinson et al. [8]. Rumen fluid obtained from four canulated sheep was diluted (1:4 v/v) into the medium as reported by Menke and Steingass [9]. Four Merino sheep were provided permanent ruminale cannula, with weight lives off 53,8 ± 4,08 kg, housed in cubicles singular, consumed during the experiment medic hay (Medicago sativa) and Erica arboreal. Rumen fluid was obtained before the morning feed and filtered through four gauze layers. Samples of (250 ± 10 mg) diets were weighed into F57 Ankom bags with a pore size of 25 μm, heat-sealed and then placed into an incubation jar. In each digestion jar they were incubated a replica at random of each one of those ages of these species and a bag like white, with the purpose of generating the correction factor for the possible entrance of particles or loss of weight of the bags. The procedure was carried out on four replicate. Nine bags per treatment were used. Samples were incubated at 39 °C at constant levels of agitation and rotation. After 48 h of incubation the jars were emptied and the bags were rinsed with cold water and dried in an oven at 105 °C. Thereafter, was determined to estimate true digestibility (TD).
In situ digestibility
In situ dry matter digestibility (ISDMD) was determined using the nylon bag technique Ørskov [10]. Samples were ground using a 2 mm screen and about 4 g of sample was weighed into nylon bags (12 × 10 cm; pore size of 40 μm), which were introduced before the morning feeding and incubated in duplicate inside the rumen of each sheep for 72 h. Upon removal, bags were soaked in cold water for 15 minutes to stop the microbial activity, and then frozen at -30º C for 24 h to remove any microbial cells adhering to the particles. The bags were defrosted in a fridge at 4ºC, washed with cold water, oven dried at 60º C for 48 h and weighed to estimate DM disappearance. Afterwards, the residues were analyzed to calculate NDF, ADF and CP digestibility. Organic matter digestibility (OMD) was estimated according to Aumont et al. [11] and metabolizable energy (ME) and net energy for lactation (NEL) was calculated according to Caceres and Gonzalez [12].
Statistical Analysis and Calculations
An analysis of variance of double classification and comparison of submultiple ranges was used. For the normal distribution of the data, Kolmogorov-Smirnov tests Massey [13] and the homogeneity of the variances Bartlett [14] was performed.
Results
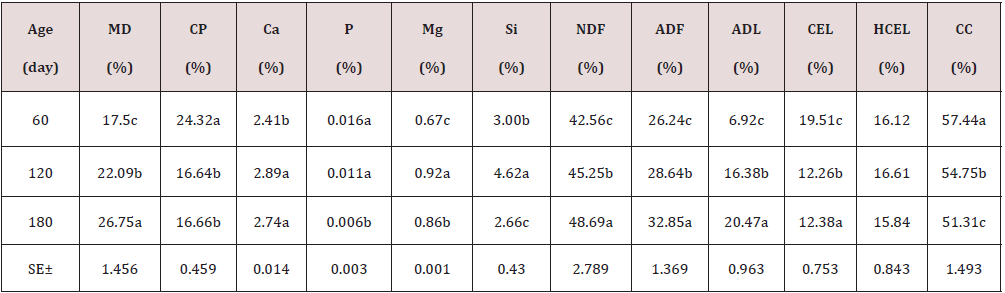
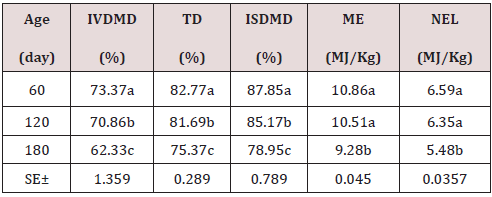
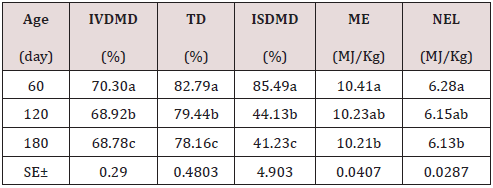
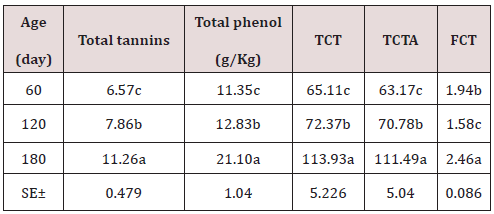
As can be seen in Table 1, the chemical composition of the Jatropha curcas, the dry matter content, NDF, ADF, ADL, increase with age with its best results at 180 days with 26.75; 48.69; 32.85 and 20.47%; while the CP and cellular content decrease with their highest results at 60 days 24.32 and 57.44% respectively. On the other hand, minerals showed a very variable behavior. During the dry season, the chemical composition of the Jatropha curcas Table 2 maintained a similar behavior to the rainy season, but with different values, the dry matter content, NDF, ADF, ADL, increased with age with its best results. 180 days with 28.82; 51.53; 33.44 and 20.22%; while the CP and cellular content decrease with their highest results at 60 days 27.78 and 58.79% respectively. On the other hand, minerals showed a very variable behavior. The highest energy contribution and digestibility percentage at 60 days of age (Table 3) with a decrement for IVDMD, TD, ISDMD, ME and NLE of 11.04; 7.40; 8.90%; 1.58 and 1.11 MJ / kg up to 180 days, respectively, is due to differences in tissue types and characteristics independently of each plant, since depending on the constitution of the cell wall, its digestibility varies; From 100% in mesophilic cells to 0% in the xylem, this variation occurs in different tissues within a part of the plant and between similar tissues in different forage species. During the dry season, the contribution of energy and digestibility (Table 4) maintained a similar behavior to the rainy season, with a decrease of 1.52, 4.63, 44.26 percentage units for IVDMD, TD, ISDMD and 0.2, 0.15 MJ / Kg of dry matter for ME and NLE. Regarding the behavior of polyphenolic compounds during the rain (Table 5), these increase with age with the best results at 180 days with 11.26, 21.10, 113.93, 111.49 and 2.46 g / Kg, respectively; for total tannin, total phenol, TCT, TCTA and FCT. While during the low rainfall these indicators also showed an increase of 10.55, 0.56, 32.14, 31.03 and 1.12 g / Kg, respectively from 60 to 180 days for total tannins, total phenols, total condensed tannins, total bound condensed tannins and free condensed tannins (Table 6).
Table 2: Chemical composition of the Jatropha curcas during dry season.
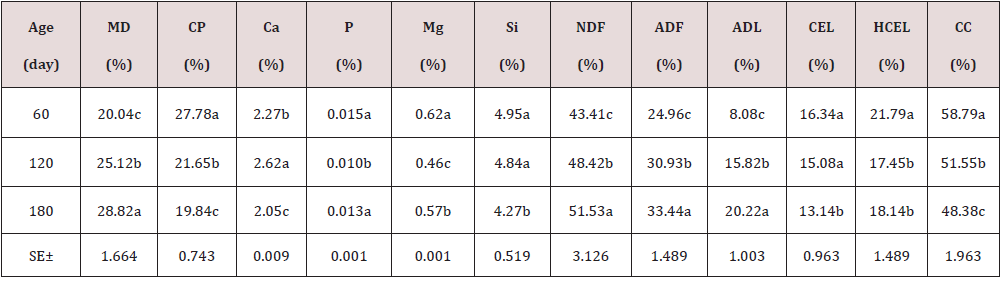
a.b.c Means with different superscript in file differ at (p<0.05)
Table 6: Polyphenol content of the Jatropha curcas during the dry season.
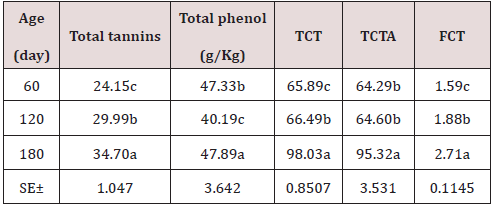
a.b.c Means with different superscript in file differ at (p<0.05).
Discussion
This behavior was given Tables 1 & 2 among other aspects
by the aging of the plant and with it the increase of the structural
components of this or that was related reduction of the synthesis
of protein compounds, to the decrease of the amount of leaves,
to the increase of the fraction stem and increase in the synthesis
of structural carbohydrates (cellulose, hemicellulose and lignin)
Verdecia et al. [15]. The decrease in protein levels with age may
be related to the reduction of the synthesis of protein compounds,
due to the decrease in the number of leaves and increase in the
proportion of stems Verdecia et al. [16]. The levels reached in this
investigation are comparable to the concentrations in most of the
trees used in the tropics Pedraza et al. [17], specifically, are higher
than the values obtained Garcia et al. [18,19], when evaluating
different cultivars of this species and similar to those obtained by
Verdecia et al. [20] in the Cauto Valley; For this reason, the evaluated
species can be used as a protein supplement in ruminant diets. The
fluctuations found in the minerals (Ca, Si) in both climatic seasons
of this species in the present study, in calcium are consistent with
those reported for numerous [21]forage trees Garcia et al. [19].
On the other hand, the silica values are similar to those obtained
by Parra et al. [22] in tropical legumes. This response pattern
could be attributed according to Chavez et al. [23] and Verdecia
et al. [24] to the variability of the soil-climatic conditions (solar
radiation, temperature, rainfall and soil) aspects that influence the
assimilation and distribution in the different parts of the plants,
and changes in the concentration of these elements are encouraged.
The increase in Si, ADF, ADL and cell wall with age could be related
to the physiological and anatomical changes that occur as the plant
ages, which causes a decrease in the proportion of cytoplasmic
content, cell lumen is reduced with its components soluble and
increase fibrous compounds Nogueira-Filho et al. [25]. This is
accentuated as the DM increases, due to the water balance of the
plant and the amount of nitrogen available in the soil, among other
factors.
On the other hand, Ramirez et al. [26] stated that depending
on the type of tissue, as the cell of the plant matures, the cell wall
widens and, commonly, produces a secondary wall of different
composition, with notable deposition of aromatic constituents,
so chemical and anatomical changes that affect digestibility. This
was related to the fact that in the younger ages there is greater
succulence of the leaves and a high amount of tender stems Bayoli
et al. [27]. Hence, at an early age these species presented high
percentages of digestibility. Degradability differences Tables 3 &
4 could be associated with the characteristics of each species and
genus. The relationship between chemical indicators and ruminal
degradability must be kept in mind, which has been described by
numerous authors for various legumes Pedraza et al. [28]. The
low percentages of IVDMD and ISDMD could be attributed to the
concentration of NDF, ADL and Si sufficient to modify the attack of
ruminal microorganisms on plant cells, as was demonstrated by
Valenciaga [29] in C. purpureus and that could be valid for legumes.
Subsequently Verdecia et al. [20] reaffirmed this principle in Lotus
uliginosus, Tithonia diversifolia, Erythrina variegata, Gliricidia
sepium and Leucaena leucocephala. In this sense, Garcia [30]states
that digestibility is due to the characteristics of the material of
origin, age, phenology and period of the year, since these factors
have a decisive influence on the tenors of the fibrous components.
These results corroborate what was stated by Harborne [31],
Labrada et al. [32] and Garcia [30], where the appearance of
secondary compounds is related to the age of the material Tables
5&6, the defense mechanisms of the plant and the effects of soil
and climate. Santacoloma-Varon and Granados [33] in this species
found low amount of tannins, which could be related to the fact
that the samples were taken during the dry season and this can
affect the photosynthetic rate and consequently the synthesis of
secondary metabolites. A It is necessary to take into account that,
although the volume and speed of biochemical reactions may
increase with temperature, most chemical reactions in plants have
a characteristic thermal optimum, which decreases at both higher
and lower temperatures. This is due, in the first instance, to the
fact that the enzymatic activity and integrity of cell membranes are
affected by extreme temperatures Sierra [34].
These results coincide with those reported by Sosa et al.
[35] in L. leucocephala. However, the concentration of TT (21.49
g / kg) found in this group can be a positive element when fully
evaluating the antinutritional characteristics of the polyphenolic
fraction of this species, since they are in the range where, possibly,
the ruminal ecosystem is not affected and increases the probability
of overpassing protein formation and would facilitate post-renal
nitrogen digestibility Aerts et al. [36], Estevez et al. [37]and Olivares
et al. [38] found low levels of TT in this plant (3.01 g / kg). This
behavior at an early age describes that the species with the highest
proportion of fiber have a high concentration of hydroxylated
metabolites, aspects that coincide with the statements made by
numerous authors about the negative effect of cell wall compounds
and tannins on animal nutrition Kumar [39], Makkar [7]. Chew et
al. [40], found lower concentrations of TF (17 g / kg) in the leaves
than in the flowers in L. leucocephala, which could be related to
the process of photosynthesis and its possible accumulation
Bhattacharya et al. [41].
Conclusion
The results of the present investigation evidenced the marked effect of climatic factors and age on the quality of the Jatropha curcas since, as the maturity of the forage progressed, its nutritional contribution decreased with a decrease in the CP, the nutrient content, digestibility and energy intake, as well as an increase in the fibrous fraction and the content of polyphenolic compounds.
References
- Boufennara S, Lopez S, Bousseboua H, Bodas R, Bouazza L (2012) Chemical composition and digestibility of some browse plant species collected from Algerian arid rangelands. Spanish J Agric Res 10(1): 88-98.
- Murgueitio E, RosalesM, Gómez ME (2001) Agroforestería para la producción animal sostenible. Fundación CIPAV. Cali Colombia p.67.
- ToralO, IglesiasGómez JM, MachadoCastro RL (2016) Colecta de Jatropha curcas y su comportamiento durante la primera fase de desarrollo. En GJ Martín (Presidente), Memorias IV Convención Internacional Agrodesarrollo. EEPF Indio Hatuey, Matanzas, Cuba, pp.164-167.
- Soil Survey Staff (2003) Keys to Soil Taxonomy. USDA, Ninth Edition, Washington, USA, pp. 332.
- Goering MK, Van Soest PJ (1970) Forage Fiber Analysis (apparatus, reagents, procedures and some applications). Agricultural USDA Washington DC, USA, pp 379.
- AOAC (2016) Official methods of analysis of AOAC International. (20th Edn), Rockville, MD: AOAC International, ISBN: 978-0-935584-87-5.
- Makkar HPS (2003) Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Ruminant Research 49: 241-256.
- Robinson T (1979) The evolutionary ecology of alkaloids. En: Herbivores:Their interactions with Secondary plant Metabolites. In: Rosenthal GAy JanzenDH (Ed.), Academic Press, USA pp. 413-448.
- Menke KH, Steingass H (1988) Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev 28: 7-55.
- Ørskov ER (2000) The in-situ technique for the estimation of forage degradability in ruminants.In:Givens DI and Owen E (Eds.), Forage Evaluation in Ruminant Nutrition, CAB International, Norwich, United Kingdom,pp. 175-198.
- AumontG, Caudron I, Saminadin G, Xandé A (1995) Sources of variation in nutritive values of tropical forages from the Caribbean. Animal Feed Sci Tech 51(1-2): 1-13.
- Cáceres O, González E (2000) Metodología para la determinación del valor nutritivo de los forrajes tropicales.Pastos y Forrajes 23(1): 87-103.
- Massey FJ (1951) The Kolmogorov-Smirnov test for goodness of fit. Journal of the American Statistical Association 4(543): 68-78.
- Bartlett M (1937) Properties of sufficiency and statistical tests. Proceedings of the Royal Society of London. Ser A 160(2): 268-282.
- Verdecia D, Ramírez JL, Bodas R, González JSY, LópezS(2011) Influencia de los factores climáticos sobre la composición química y la digestibilidad del pasto de Pennisetum purpureum vc. Mott en la región oriental de Cuba: Libro de Actas del I Congreso en Investigación en Agricultura para el Desarrollo (Moreno A. In: Gómez H&Hernández C (Eds.), Agrícola Española SA, Madrid, Spain, 76-77.
- Verdecia D, Herrera RS, Ramírez JL, Leonard I, Álvarez Y, et al. (2012) Valor nutritivo de Leucaena leucocephala con énfasis en el contenido de metabolitos secundarios. Redvet13(11): 1-10.
- Pedraza RM, La OO Estévez, J, Guevara G, Martínez S (2003) Nota técnica: Degradabilidad ruminal efectiva y digestibilidad intestinal in vitro del nitrógeno del follaje de leguminosas arbóreas tropicales. Pastos y Forrajes 26: 237-241.
- Garcia DE, Medina MG, Clavero T, Cova LJ, Domínguez, et al. (2008) Caracterización nutritiva del follaje de seis especies forrajeras con énfasis en sus perfiles polifenól Revista Científica FCV-LUZ 18(2):188-196.
- Garcia DE, Medina MG, Cova LJ, Clavero T, Torres A, et al. (2009) Evaluación integral de recursos forrajeros para rumiantes en el estado Trujillo, Venezuela. Rev Fac Agron 26: 555-582.
- Verdecia DM, Herrera RS, Ramírez JL, Leonard I, Bodas R, et al. (2014) Effect of re-growth age in the secondary metabolites from Neonotonia wightii in the Cauto valley, Cuba.Cuban J Agric Sci 48 (2): 149-154.
- Diaz T (2016) Food security and livestock: The case of Latin America and the Caribbean. Cuban J Agric Sci 43(1).
- Parra R, Combellas J, González E (1972) Composición y valor nutritivo de forrajes producidos en el tró 2. Fracciones químicas que afectan la disponibilidad de los componentes fibrosos. Agron Trop 22: 219-230.
- Chavez L, Álvarez A, Ramírez R (2012) Apuntes sobre reguladores del crecimiento vegetal que participan en la respuesta de las plantas frente al estrés abió Cultivos Tropicales. 33(3): 47-56.
- Verdecia DM, Herrera RS, Ramírez JL, Leonar I, Bodas R, et al. (2013) Effect of the re-growth age on the nutritive quality of Neonotonia wightii in the Cauto valley, Cuba. Cuban J Agric Sci 47(1): 89-95.
- Nogueira Filho JCM, Fondevila M, Barrios Urdaneta A, González Ronquillo M (2000) In vitro microbial fermentation of tropical grasses at an advaced maturity stages. Anim Feed Sci Tech 83:145-147.
- Ramirez R, Ramírez RGy, López F (2002) Factores estructurales de la pared celular del forraje que afectan su digestibilidad. Ciencia UANL 5: 180-189.
- Bayoli JJ, Ngongoni NT, Hamudikuwanda H(2008) Chemical composition and ruminal degradability of cowpea and silverleaf desmodium legumes harvested at different stage of maturity. Tropical and Subtropical Agroecosystems 8: 1-11.
- Sosa EE, Pérez D, Ortega L, Zapata G (2004) Evaluación del potencial forrajero de árboles y arbustos tropicales para la alimentación de ovinos. Técnica Pecuaria México 42(2): 129-144.
- Valenciaga D (2007) Caracterización química y estructural de las paredes celulares de Pennisetum purpureum vc. CUBA CT-115 y su digestibilidad ruminal en búfalos de río (Bubalis bubalus). Tesis DrC. La Habana, Cubapp.110.
- Garcia DE (2004) Los metabolitos secundarios de las especies vegetales. Pastos y Forrajes. 27(1): 1-12.
- Harborne JB (1993) Introduction to ecological biochemestry. 4th Academic Press, Harcomt Brace & Co. Publishers, New York, USA, pp.320.
- Labrada J, Guevara G, Estévez J, Martínez S, Pedraza R (2001) Evaluaciónde algunos indicadores de la composición química del follaje de Erythrina variegata. Rev Prod Anim 13: 35-39.
- Santacoloma Varon LE, Granados J (2010) Evaluación del contenido de metabolitos secundarios en dos especies de plantas forrajeras encontradas en dos pisos térmicos de Colombia. Revista de Investigación Agraria y Ambiental 1(1):31-35.
- Sierra OJ (2005) Fundamentos para el establecimiento de pasturas y cultivos forrajeros. Editorial Universidad de Antioquia pp.120.
- Aerts RJ, Barry TN, McNabb WC (2000) Polyphenols and agriculture: beneficial effect of proanthocyanidins in forages. Agr. Ecosyst. Environ 75: 1-12.
- Estevez JA, Pedraza RM, Guevara G (2001) Algunos indicadores de la composición química del follaje de siete leguminosas arbóreas y arbustivas. Rev Prod Anim 13: 95-97.
- Olivares PJ, Jiménez GR, Rojas HS, Martínez HP (2005) Uso de las leguminosas arbustivas en los sistemas de producción animal en el tró Revista Electrónica de Veterinaria. REDVET 4: 19.
- Kumar R (1992) Antinutritional factors. The potential risks of toxicity and the methods to alleviate them. En: Legumes trees and other fooder trees as protein source for livestock. Speedy, A.W. y Pugliese, P.L. Eds. FAO Animal Production and Health Paper no102: pp.145.
- Chew Y, Chan E, Tan P, Lim Y, Stanslas (2011) Assessment of phytochemical content, polyphenolic composition, antioxidant and antibacterial activities of Leguminosae medicinal plants in Peninsular Malaysia. BMC Complementary and Alternative Medicine.
- Bhattacharya S, Kamat JP, Bandyopadhyay SK, Chattopadhyay S (2009) Comparative inhibitory properties of some Indian medicinal plant extracts against photosensitization-induced lipid damage. Food Chem 113: 975-979.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...