
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4676
Review Article(ISSN: 2637-4676) 
Diversification of Glomermycota form Arbuscular Mycorrhizal Fungi Associated with Vegetable Crops Cultivated Underneath Natural Ecosystems in Arid Region of Rajasthan, India Volume 9 - Issue 2
Kamal Prasad*
- Warkem Biotech Pvt. Limited, Agro Division, Swastik Disha Business Parks, via. Vadhani Industrial Estate, LBS Marg, Mumbai- 400086, Maharashtra, India
Received: January 5, 2021; Published: March 18, 2021
Corresponding author: Dr. Kamal Prasad, Warkem Biotech Pvt. Limited, Agro Division, Swastik Disha Business Parks, via. Vadhani Industrial Estate, LBS Marg, Mumbai- 400086, Maharashtra, India
DOI: 10.32474/CIACR.2021.09.000310
Abstract
An investigation was carried out for twenty-two plant species, cultivated widely as vegetable crops in arid region of Rajasthan, state of India belonged to eight different families to measure their affinity in harbouring symbiotic association with arbuscular mycorrhizal fungi (AM fungi) and nutrient status in rhizospheric soil. Twenty out of the twenty two species were having developed AM fungal colonization in their root tissues with a range of 16.33% to 91.33%. All the soil samples tested were slightly acidic to alkaline and housed AM fungal spore with a density ranges from 80.33-199 spores/ 50g air dried soil. The AM fungal spore density in the soil was not found to have any effect on symbiotic colonization in root tissues of studied vegetable crop plants by Mycorrhizae. The soil chemical analysis was also noticed to have no correlation with both colonization of root tissues by AM fungi and spore densities in the rhizospheric soil. Variations in AM fungal root colonization’s and spore densities were found statistically significant. Plant species had a significant role in root tissue colonization by AM fungi.
Keywords: Soil; AM fungi; Vegetables Crop plant; Arid region; Rajasthan
Introduction
Mycorrhizal fungi are important members of the plant microbiome, forming interdependency with the roots of most vascular plants on Earth. Maximum plant species partner with either endomycorrhizal or ectomycorrhizal fungi or these symbioses are thought to represent plant variations to quick and slow soil nutrient cycling rates. Members of the Glomeromycota are responsible for forming mutualistic associations called endomycorrhizae with the roots of more than 90% of the world’s vascular plants. These endomycorrhizae are also known as arbuscular mycorrhizal fungi (AM fungi). AM fungi are grouped in a monophyletic group, the phylum Glomermycota. The Glomeromycota were belong to the order Glomerales (Glomales) of the Zygomycota. Glomeromycota form relatively large asexual spores in the soil. Endo and ecto mycorrhizal fungi are reported to beneficial for several host plants in macro and micro nutrients mobilization and uptake, inducing tolerance to drought, soil salinity or alkalinity, and resistance to pathogens [1-5]. Vegetables crops are also reported to have mycorrhizal association [6]. Application of a large amount of inorganic and organic fertilizers to the vegetable field soils also affects the microbial population and their role in nutrient cycling. Soil microbes influence the mutability and uptake of inorganic nutrients by plants. Symbiotic entophytes and mycorrhizae are involved in the uptake of nutrient elements of vital plants. Management of soil microbe is necessary to optimize nitrogen and phosphate nutrition of plants. Before attaining the true management of soil microbes, it is essential to understand better the interactions between plants and microbes in the soil around the roots [7]. There are extensive microbial activities in rhizosphere soil which is colonized by a wide range of microbes having important effects on plant nutrition, overall growth, productivity and soil health. Vesicular arbuscular mycorrhizal fungi (VAMF) and arbuscular mycorrhizas fungi (AM fungi) are mutualistic symbioses formed between the roots of most plants and fungi [8-12]. Mycorrhizal fungi assist the plants to absorb mineral nutrients from the soil, particularly low available elements such as phosphorus (P), nitrogen (N), potassium (K), iron (Fe), molybdenum (Mo) and cobalt (Co) [13-14]. These fungi are also reported to consistently stimulate plant absorption of zinc and copper and also increase plant resistance to various stresses like water, drought, salt and heavy metal toxicity [13-15]. AM fungi infection enables the most of Cu, Zn and Cd to be retained by roots, allowing less heavy metal translocation to leaves. There is evidence that mycorrhizal plants contain higher concentration of growth hormones than their non-mycorrhizal equivalents [16]. Effective nutrient acquisition by AM fungi is generally attributed to the extensive hyphal growth beyond the nutrient depletion zone surrounding the root [9] and principal avoidance strategies of plants for adaptation to adverse soil conditions is increase in root surface area via mycorrhizae [17].
Mycorrhizae are associated with the roots of most species of angiosperms, gymnosperms, pteridophytes and bryophytes and are important in agriculture, horticulture and forestry. A better understanding of the mycorrhizae of agronomic crops is necessary because of their potential involvement in the sustainable agriculture systems [18]. The abundance and distribution of AM fungi in various plants have been studied in various parts of the world. A number of studies have also been performed on the occurrence and abundance of AM fungi in various plants and the effect of AM fungi on crop plants [19-25]. The present investigation was undertaken to study the AM fungal association in some vegetable crops cultivated in arid zone of Rajasthan state of India on the light of distribution and abundance.
Materials and Methods
Root material and rhizosphere soil sampling
The standard methodology for root and rhizosphere soil sampling was adopted. Soil and root samples of twenty-two vegetable crop plants were collected from various crop fields of different sites of Rajasthan state. Four replications were made for each collection site in case of every sample. Soil samples with roots of respective plant species were collected and placed in plastic bags and tagged for analysis.
Quantification of AM fungal biomass and glomalin stock and pertinent soil quality parameters
Assessment of AM fungal colonization percentage (AMFCP)
Approximately 2.0 g (fresh weight) of fine roots were used for staining and the assessment of AM fungal colonization. Freshly collected roots were thoroughly washed with water several times and cut in to uniform pieces approximately 1cm long. Then the roots were cleared with 10% KOH, acidified with 1 N HCl, and stained in 0.05% Trypan blue [26]. Mycorrhizae infection in roots was expressed as percentage of segments containing fungal structures like mycelia, vesicles and arbuscules.
Assessment of AM fungal spore population (AMFSP)
Air dried soil was taken in four replicates for the determination of spore population. The AM fungal spores were extracted using the modified wet sieving and decanting method of Daniels and Skipper [27] and Gerdemann and Nicolson [28] from rhizosphere soil samples collected from different vegetable species. The contents from all the sieves were taken on filter paper and AM fungal spore density was counted under stereo binocular microscope.
Establishment of monoculture of AM fungal spore
It is a well-known fact that, at a particular time naturally occurring spores may not reflect the actual symbiotic fungal structure and AM fungal species determination often requires more than few healthy spores. For detail understanding of AM fungal composition and their structural adaptations, separate trap cultures were established for identification.
Identification of AM Fungal Species
AM fungal spores were separated under stereo binocular microscope and mounted in PVLG and PVLG Meltzer’s reagent. Gentle pressure was applied on coverslip to rupture the spores for details of wall layers. Characterization of individual AM fungal spores was carried out after being subject to morphogenetic and micrometric analysis based on their colour, diameter, shape, wall layers, surface content, hyphal colour, hyphal width, and hyphal attachment with the wall. On this basis, dominant genera of AM fungi were categorized (Table 3). The AM fungi were identified and named made at species level with the help of relevant literature [29-33]. The identity of the AM fungi was further confirmed with the species description of INVAM (http://invam.wvu.edu/).
Physico-chemical analysis of rhizospheric soil samples
Air dried soil samples were used for different physico-chemical analysis. pH was determined in 1.25 (w/v) solutions of samples in water and the same was used for determination of electrical conductivity (EC). Air dried samples was processed (addition of 40% NaoH and distillation) using a Kel Plus Nitrogen estimation system (Class DX, Pelican Equipment’s) followed by determination of available nitrogen by titration with 0.02N H2SO4 [34]. Available phosphorus was determined by Olsen’s method [35] and available potassium was determined in a 1 N ammonium acetate extract using flame photometer [36].
Statistical Analysis
Observations of AM fungal colonization, spore density and physico- chemical properties of vegetable species were analyzed using SPSS (SPSS Inc. version 17.0). Results were subjected to one way analysis of variance and the significant difference was determined according to Duncan’s Multiple Range Test at significant level of P<0.05.
Results and Findings
Physico-chemical properties of rhizospheric soil samples
pH values of rhizospheric soils were found ranging from slightly acidic to near alkaline (6.50 to 7.53) in a total of 22 angiosperm plant species belonging to eight family, cultivated widely as vegetable crops in arid region of Rajasthan, India (Tables 1,2). Variations in pH, EC, OC and available NKP values among the plant species of all samples was statistically significant at P< 0.05 level (Tables 1,2).
Table 1: Chemical analysis of rhizospheric soils (pH, EC and OC) of different species of vegetables species growing in the arid region of Rajasthan.
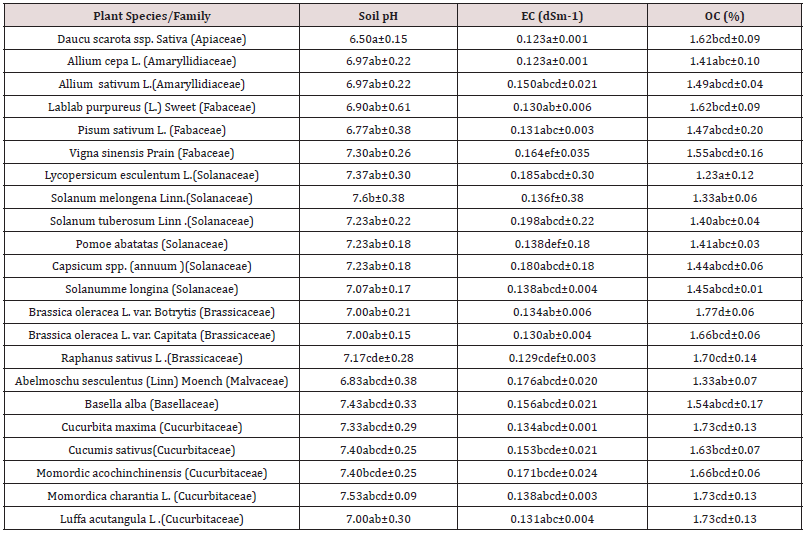
Values are mean of four replicates. SE-Std error; Values in a column followed by the same letter are not significantly different at P< 0.05 according to DMRT
Table 2: Chemical analysis of rhizospheric soils (Available NPK) of different species of vegetables species growing in the arid region of Rajasthan.
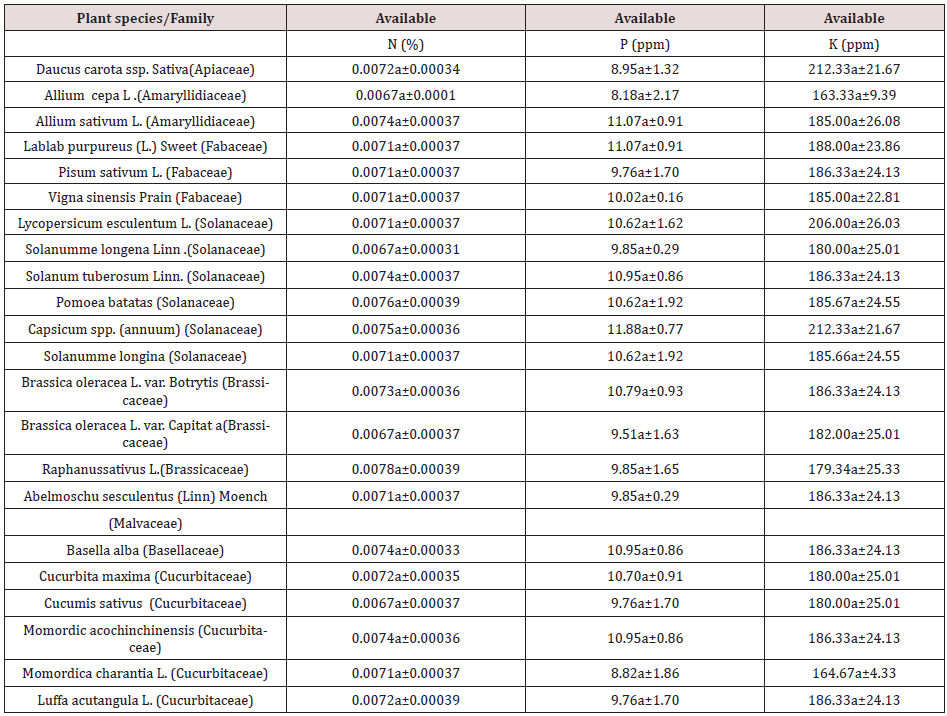
Values are mean of four replicates. SE-Std error; Values in a column followed by the same letter are not significantly different at P< 0.05 according to DMRT
AM fungal root colonization percentage
Root colonization of AM fungi largely depends on the quantity of AM fungal inoculum potential present within the soil. Though pre-existing hyphae and infected root fragments are shown to successfully colonize the roots of host, germinating spores are considered to be the key players in new host establishment. The distribution and abundance of AM fungal colonization in the root tissues of studied crop plants and AM fungal spore density in the rhizospheric soils of vegetable crops are presented in Table 3. Twenty species of this present investigation out of twenty-two showed their capacity in harbouring mycorrhizal root colonization. The intensity of mycorrhizal fungal colonization in root tissues ranged from 16.33 ± 0.88 to 91.33 ± 2.85 in terms root length examined. The highest root length AM fungal colonization was recorded in Allium sativum L. of Amaryllidiaceae family (91.33a±2.85) and the lowest root colonization in Abelmoschus esculentus (Linn.) Moench of Malvaceae family (16.33a±0.88). The species Brassica oleracea L var. capitata of family Brassicaceae and Basella alba of family Basellaceae appeared to have no mycorrhizal association (Table 3). The AM fungal colonization spectrum of studied families recorded were 41.33%, 85.00-91.33%’, 58.67-72.66%, 34.00-56.66%, 0-19.67%, 16.33% and 19.67-77.33% respectively for Apiaceae, Amaryllidaceae, Fabaceae, Solanaceae, Brassicaceae, Malvaceae and Cucurbitaceae. The descending order of eight families in terms of their capacity to harbouring mycorrhizal colonization in average is Amaryllidaceous (81.17%) Cucurbitaceae (61.47%) >Fabaceae (64.55%) >Solanaceae (49.00%) > Apiaceae (41.00%)> Brassicaceae (19.67%) Malvaceae (16.33%)> Basellaceae (0.0%). The AM fungal colonization consisted of fungal hyphae, vesicles, and arbuscules (Plate1-2). The percentage of colonization varied among the plant species. A similar study with colonization quantum of 30-68% in case of 21 weed species under 15 families from India was reported by Prasad [21].
Table 3: AM fungi association in roots and spore propagules and species in soil for different vegetable species growing in the arid region of Rajasthan
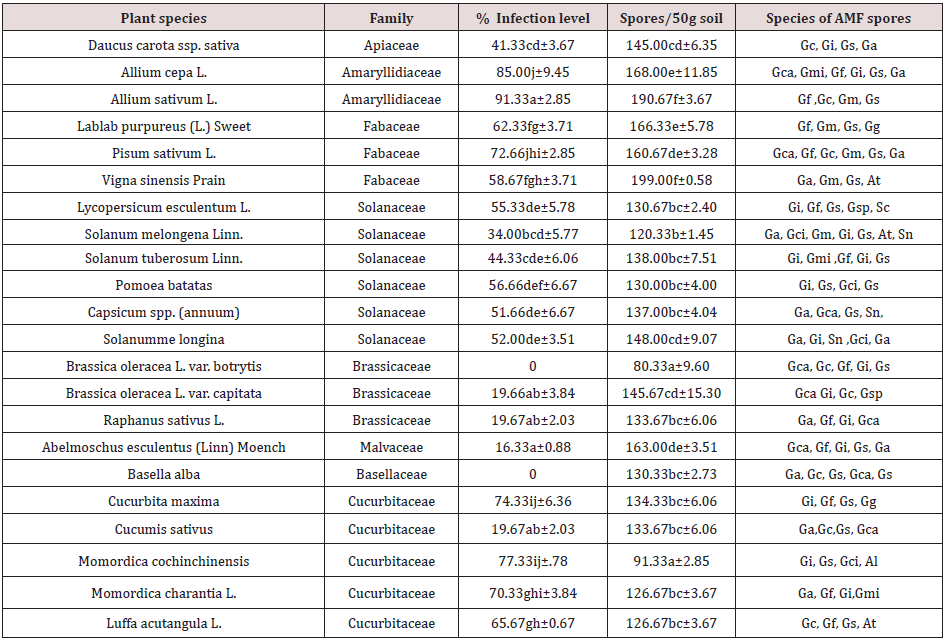
Values are mean of four replicates. SE-Std error; Values in a column followed by the same letter are not significantly different
at P< 0.05 according to DMRT
Al- Acaulospora laecunosa; At- Acaulospora tuberculata; Ga-Gigaspora albida; Gc -Glomus constrictum; Gca-Glomus caledonium;
Gf -Glomus fasciculatum; Gi -Glomus intraradices; Gm -Glomus mosseae; Gmi - Glomus macrocarpum; Gs - Glomus
species; Gci-Gigaspora candida; Gg - Gigaspora gigantea; Gsp - Gigaspora spp.; Sn - Sclerocystis nigra; Sc -Sclerocystis spp.
AM fungal spore population and identification
The Glomeromycota have generally coenocytic mycelia and
reproduce asexually through blastic development of the hyphal
tip to produce Glomerospores known as AM fungal spores with
diameters of 50–500 μm.The collected soil samples for estimation
of AM fungal density were of different types and had varying pH
values. The mycorrhizal spore population in soil was higher in this
pH range, and dominant genera of AM fungi were categorized as
Glomus. The concentration of mycorrhizal fungal population in soil
ranged from 80.33 ± 9.60 to 199.00 ±0.58. The maximum spore
density was recorded in Vigna sinensis of Fabaceae family and
the minimum in Brassica oleracea L. Var. botrytis of Brassicaceae
family. A variety of spores were recovered from soils and root
washings, mainly belonging to the genus Glomus (Plate 3). However,
azygospore of Acaulospora and Gigaspora, and sporocarps of
Sclerocystis were also recovered, though these were rare.
To facilitate identification, the rhizosphere soil samples of all
the vegetable species were processed for isolating different AM
fungal propagules. Based on the AM fungal spore characters, the
following fungi were identified: two species of Acaulospora, namely,
A. Laecunosa and A. tuberculata, six species of Glomus, namely,
Glomus constrictum, Glomus caledonium, Glomus fasciculatum,
Glomus intraradices, Glomus mosseae, and Glomus macrocarpum;
three species of Gigaspora, namely Gigaspora albida, Gigaspora
candida and Gigaspora gigantean; one species of Sclerocystis
nigra; one unidentified spore each of Gigaspora, Sclerocystis and
17 unidentified spore of Glomus. G. intraradices seems to be the
most predominant species, noticed in most of the rhizosphere
soil samples of different vegetable species (14 vegetable species)
followed by G. fasciculatum (12 vegetable species), G. caledonium
(9 vegetable species), G. constrictum (7 vegetable species), Glomus
mosseae (5 vegetable species), G. macrocarpum (3 vegetable
species) and unidentified Glomus spp. (17 vegetable species).
The spore of A. tuberculata was found in three vegetable species
namely Vigna sinensis , Solanum melongena and Luffa acutangula
while that of A. laecunosa was found only in one vegetable species
(Momordica cochinchinensis) in the rhizosphere soil samples. The
spore of Gigaspora (G. albida) was noticed in five vegetable species,
namely Daucus carota, Allium cepa, Pisum sativum, Solanumme
longina and Abelmoschus esculentus followed by G. calaspora
was recorded in three vegetable species, namely Pomoea batatas,
Solanum melongina and Momordica cochinchinensis while G.
gigantea was noticed in two vegetable species of rhizosphere soil
samples, namely Lablab purpureus and Cucurbita maxima. The
spore of Sclerocystis nigra was recorded only in the rhizosphere
soil samples of two vegetable species, namely, Capsicum spp.
(annuum) and Solanum melongina and one unidentified species
in Lycopersicum esculentum soil sample (Table 3). No definite
correlation could be established between AM fungal spore
and mycorrhizal root colonization. As multiplication of an
endomycorrhiza depends on its association with plant roots, the
number of its spores in soils is likely to differ, as shown in the
present investigation. The prevalence of AM fungi suggests that
the mycorrhizae may be of great importance for plant growth
and development in tropical soils, especially in arid and semi-arid
zones, which have extremely low soil moisture and are relatively
poor in macro and micro nutrients. The activity of mycorrhizae fungal population, in terms of root colonization and the number
of AM fungal spores has been shown to be greatly affected by soil
conditions [37-39].
Statistical analyses showed that variations in the amount of
mycorrhizal root colonization/infection in various plant species and
AM fungal spore density of their rhizospheric soils were significant
in most of the cases even at P< 0.005 level. ANOVA showed that
plant species played a significant role in the colonization of root
tissues by mycorrhizal fungi. Present study is in agreement with
the findings of Mosse and Bowen [40], Read et al. [41], Prasad [21],
Prasad and Gautam [42] and Pringle et al., [43] Prasad [21] reported
a range of 5-370 spores/100g dried soil in India. Comparatively a
low spore density like this present study might have been caused by
a warmer climate inserting less pressure for sporulation [44] which
supports the findings of Nadarajah and Nawawi [45-47] and Prasad
[21]. The present study reveals that AM fungi are quite common
in most of the vegetable species examined from various places in
the arid zones of Rajasthan, India with species of Glomus found to
be widely spread among the vegetable of this region. The Glomus
species of AM fungi help the various crop plants of the arid region
in yield productivity in sustainable manner.
Conflict of Interest Statements
There is no conflict of interest.
References
- Bryla DR, Duniway JM (1998) the Influence of the Mycorrhiza Glomus Etunicatum on Drought Acclimation in Safflower and Wheat. Physiologia Plantarum Volume 104(1): 87-96.
- Prasad K (2021) Effect of Dual Inoculation of Arbuscular Mycorrhiza Fungus and Cultivar Specific Bradyrhizobium Japonnicum on the Growth, Yield, chlorophyll, Nitrogen and Phosphorus Contents of Soybean Grown on Alluvial Soil. Journal of Innovation in Applied Research Volume 4(1): 1-12.
- Prasad K (2021) Impact of Biological Fertilizer Arbuscular Mycorrhizal Fungi and Conventional Fertilizers Mobilization on Growth, Yield, Nutrients Uptake, Quercet in and Allin Contents in Allium Crops Cultivation under Field Conditions in Semi-Arid Region6 of India. South Asia Journal of Experimental Biology Volume 11(1): 15-26.
- Prasad K, Warke RV, Khadke K (2019) Management of Soilborne Pathogens to Improve Productivity of Pulses using Organic Technologies for Sustainable Agriculture. International Journal of Research and Analytical Reviews Volume 6(2): 82-101.
- Prasad K (2020) Positive Importance of Arbuscular Mycorrhizal Fungi for Global Sustainable Agriculture and Environment Management for Green Technology. Current Investigations in Agriculture and Current Research Volume 9(2): 1182-1185.
- Prasad K (2000) Occurrence of Vesicular Arbuscular Mycorrhizal Fungi in some Cultivated Crop PLANTS. In: Maheshwari DK, Dubey RC, Prasad G, Navneet (Eds.), Microbes: Agriculture, Industry and Environment. Dehra Dun, 65-69.
- Tacon F, Le RD, Fraga-Beddiar A, Diagne O (1971) Interactions among Rhizospheric Microorganisms, VAM Fungi and Symbiotic Nitrogen Fixing Bacteria. In: David A, Kenneth G, MacDicken (Eds.), Research on Multipurpose Tree Species in Asia. 450-561.
- Menge JA (1983) Utilization of Vesicular-Arbuscular Mycorrhizal Fungi in Agriculture. Canadian Journal of Botany Volume 61(3): 1015-1024.
- Tisdale SL, Nelson WL, Baton JD (1985) Soil Fertility and Fertilizers. Macmillan Publishing Collier: pp. 754.
- Shi Z, Zhang J, Lu S, Li Y, Wang F (2020) Arbuscular Mycorrhizal Fungi Improve the Performance of Sweet Sorghum Grown in a Mo-contaminated Soil. Journal of Fungi Volume 6(2): 44-46.
- Prasad K (2015) Biofertilizers: a New Dimension for Agriculture and Environmental Development to Improve Production in Sustainable Manner. Journal of Basic and Applied Mycology Volume 11(1& II): 5-13.
- Prasad K (2017) Biology, Diversity and Promising Role of Mycorrhizal Entophytes for Green technology. In: Maheshwari DK (Ed.), Endophytes: Biology and Biotechnology. Switzerland, 267- 301.
- Mosse B (1973) Advances in the Study of Vesicular-Arbuscular Mycorrhiza. Annual Review of Phytopathology Volume 11(1): 171-196.
- Prasad K (2021) Influence of Arbuscular Mycorrhizal Fungal Biostimulants and Conventional Fertilizers on some Solanaceous Crops for Growth, Productivity and Nutrient Toichiometry under Field Conditions in Semi- Arid Region of Maharashtra, India. Journal of Experimental Biology and Agricultural Sciences Volume 9(1): 75-86.
- Prasad K (2013) Arbuscular Mycorrhizal Fungus Plays a Major Role in Agriculture and Natural Ecosystems to Improve Production in Sustainable Manner. In: Jamaluddin, Singh AK (Eds.), Microbes and Sustainable Plant Productivity. Jodhpur, pp.189.
- Skimmer FA (1985) Agriculture and Biotechnology In: Higgins IJ, Best D, Jones J (Eds.), Biotechnology: Principles and Applications. United States.
- Turk MA, Assaf TA, Hameed KM, Al-Tawaha AM (2006) Significance of Mycorrhizae. World Journal of Agriculture Science Volume 2(1): 16-20.
- Khalil S, Loynachan TE, McNabb HS (1992) Colonization of Soybean by Mycorrhizal Fungi and Spore Populations in Oowa soils. Agronomy Journal Volume 84(5): 832-836.
- Atithi D, Saha AJ, Das P (2014) Arbuscular Mycorrhizal Colonization in Aquilaria Malaccensis Lamk and in its Surrounding Herbaceous Community. Mycorrhiza News Volume 25(4): 2-7.
- Muthukumar T, Senthilkumar M, Rajangam M, Udaiyan K (2007) Arbuscular Mycorrhiza Morphology and dark septate fungal Association in Medicinl and Aromatic Plants of Western Ghats, Southern India. Mycorrhiza Volume 17(1): 11-24.
- Prasad K (1998) Surveys of Vesicular-Arbuscular Mycorrhizal Fungal Colonization in some Weed Specie. Indian Journal of Applied and Pure Biology Volume 13(2): 87-90.
- Prasad K, Deploey JJ (1999) Incidence of Arbuscular Mycorrhizae and their Effect on Certain Species of Trees. Journal of the Pennsylvania Academy of Science Volume 73(3): 117- 122.
- Prasad K (2003) Arbuscular Mycorrhizal Fungal Association and Root Colonization in some Important Tree Species. In: A Sadanandam (Ed.), Plant Biotechnology. Hyderabad, 222-226.
- Prasad K, Meghavanshi MK, Harwani D, Mahna SK, Werner D (2006) Distribution of Arbuscular Mycorrhizal Fungi in Soybean. Rhizosphere Mycorrhiza News Volume 17(4): 14-17.
- Prasad K, Meghavanshi MK, Khan AA (2011) Incidence of Arbuscular Mycorrhizal Fungi in Tree Species in Arid Zones of Ajmer region of Rajasthan. Mycorrhiza News Volume 22(4): 12-15.
- Phillips JM, Hayman DS (1970) Improved Procedure for Clearing Roots and Staining Parasitic and VAM Fungi For Rapid Assessment of Infection. Transactions of the British Mycological Society Volume 55(1): 158-161.
- Daniels BA, Skipper HA (1982) Methods for the Recovery and Quantitative Estimation of Propagules from Soil. In: Schenck NC (Ed.), Methods and Principles of Mycorrhizal Research. St. Paul, Minnesota, USA, 29–35.
- Gerdemann JW, Nicolson TH (1963) Spores of Mycorrhizal Endogone Species Extracted from Soil by Wet Sieving and Decanting. Transactions of the British Mycological Society Volume 46(2): 235-244.
- Trappe JM (1982) Synoptic Key to the Genera and Species of Zygomycetes Mycorrhizal Fungi. Phytopathology Volume 72(8): 1102–1108.
- Morton JB, Benny GL (1990) Revised Classification of Arbuscular Mycorrhizal Fungi (Zygomycetes): a New Order, Glomales, Two New Suborders, Glomineae and Gigasporineae and Two New Families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomaceae. Mycotaxon Volume 37: 471–491.
- Schenck NC, Perez Y (1990) a Manual for Identification of Vesicular Arbuscular Mycorrhizal Fungi. Gainesville: Florida: University of Florida.
- Hao W, Lin X, Wu T, Shi Y (2002) Screening of Arbuscular Mycorrhizal Fungi for the Revegetation of Eroded Red Soils in Subtropical China. Plant and Soil 239: 225–235.
- Prasad K, Rajak RC (1999) Recent Advances in Mycorrhizal Taxonomy: Morphological and Molecular Criteria. In: Rajak RC (Ed.), Microbial Biotechnology for Sustainable Development and Productivity. Jodhpur, 62-72.
- Subbiah BV, Asija GL (1956) a Rapid Procedure for the Determination of Available Nitrogen in Soils. Current Science Volume 25: 259-260.
- Olsen SR, Cole CV, Wantabe FS, Dean LA (1954) Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate. Cric. US. Agric: CRC Press pp. 939.
- Singh D, Chhonkar PK, Dwivedi BS (2007) Manual on Soil, Plant and Water Analysis. Westville Publishing House: 1-200.
- Mukerji KG, Sabharwal A, Kochar B (1984) Vesicular Arbuscular Mycorrhizae: Concept and Advances. In: Mukerji KG, Agnihotri VP, Singh RP (Eds.), Progress in Microbial Ecology. Lucknow, India, 489–525.
- Prasad K (1999) Vesicular Arbuscular Mycorrhizal Fungi Associated with Prosopis Juliflora. Journal of Tropical Forestry Volume 15: 233-236.
- Mishra Rani, Kehri HK, Akhtar O (2016) Diversity and Status of Arbuscular Mycorrhizal Fungi in the Ornamental Plants Growing under Natural Conditions at Different Sites of Allahabad, Uttar Pradesh. Journal of Basic and Applied Mycology Volume 12(2): 64-76.
- Mosse B, Bowen GD (1968) the Distribution of Endogone Spores in some Australian and New Zealand Soils and in an Experimental Field Soil at Roth Amsted. Transactions of the British Mycological Society Volume 51: 485-492.
- Read DJ, Koucheki HK, Hodgson J (1976) Vesicular-Arbuscular Mycorrhiza in Natural Vegetation Systems I. the Occurrence of Infection. New Phytologist Volume 77(3): 641-653.
- Prasad K, Gautam SP (2000) Arbuscular Mycorrhizal Spore Types Present in the Root Zone of Dalbergia sissoo L. Vislesana Research Journal (Science) Volume 7(2): 13- 18.
- Pringle A, Adams RI, Cross HB, Bruns TD (2009) the Ectomycorrhizal Fungus Amanita Phalloides was Introduced and is Expanding its Range on the West Coast of North America. Molecular Ecology Volume 18(5): 817-833.
- Hetrick BAD, Bloom J (1983) Vesicular- Arbuscular Mycorrhizal Fungi Associated with Native Tall Grass Prairie and Cultivated Winter Wheat. Canadian Journal of Botany Volume 61(8): 2140-2146.
- Nadarajah P, Nawawi A (1990) Zygomycetous Endomycorrhizal Fungi in some Malaysian Soils under Rubber (Hevea brasiliensis). In: David A, Taylor G, Kenneth G, MacDicken (Eds.), Research on Multipurpose Tree Species in Asia.
- Liu M, Zhao Z, Chen L, Wang L, Ji L, et al. (2020) Influences of Arbuscular Mycorrhizae, Phosphorus Fertiliser and Biochar on Alfalfa Growth, Nutrient Status and Cadmium Uptake. Ecotoxicology and Environmental Safety Volume 196: 110537
- Shi Z, Zhang J, Lu S, Li Y,Wang F (2020) Arbuscular Mycorrhizal Fungi Improve the Performance of Sweet Sorghum Grown in a Mo-contaminated Soil. Journal of Fungi (Basel) Volume 6(2): 44-46.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...




