Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2643-6760
Review Article(ISSN: 2643-6760) 
Sternal Cleft: Appropriate Approach to Diagnosis and Treatment Volume 1 - Issue 3
Yasser Ali Kamal*
- Department of Cardiothoracic Surgery, Minia University Hospital, Egypt
Received: June 05, 2018; Published: June 11, 2018
Corresponding author: Yasser Ali Kamal, Department of Surgery, Minia University Hospital, El-Minia, 61519, Egypt
DOI: 10.32474/SCSOAJ.2018.01.000112
Abstract
Sternal cleft (SC) is a rare congenital anomaly of the chest wall, isolated or combined with other malformations. We try to provide an appropriate stepwise approach for the diagnosis and treatment of SC. Prenatal ultrasound diagnosis can be obtained after the 18th week of gestation. At birth, diagnosis is based on the findings of clinical examination, chest x-ray, computed tomography and additional investigations for the associated anomalies. Primary approximation of the sternal bars is preferred before the age of 3 months. For older cases and rigid chest wall, the surgical correction can be achieved using prosthetic materials, autologous grafts, or biologic implants. The surgical decision depends on the age of patient, rigidity of the chest wall, and presence of other abnormalities in the chest wall.
Keywords: Chest wall malformations; Sternum; Autologous graft; Biologic matrix
Abbreviations: CPB: Cardiopulmonary Bypass; TEE: Trans-esophageal Echocardiography; Sternal Cleft (SC); MRI: Magnetic Resonance Imaging
Introduction
Normally, the sternum is developed by fusion of two mesenchymal bars, between the 7th and 10th week of gestation, extending from the manubrium to the xiphoid process [1]. Sternal cleft (SC) is a rare chest wall anomaly occurs after failure of the sternal bars to fuse. It accounts for 0.15% of chest wall malformations, and occurs in less than 1:100,000 of live births, with a female predominance [2]. The SC may be partial or total depending on its extension. In regard to its location, the defect may be superior, inferior or complete. Other malformations may occur with SC, like hemangiomas, omphalocele, pentalogy of Cantrell (Ectopia cordis, intracardiac defects, sternal cleft, omphalocele, pericardial defect), PHACES syndrome (posterior fossa brain malformations, facial hemangiomas, arterial anomalies, cardiac defects, eye abnormalities, sternal cleft and supra-umbilical raphe), gastroschisis, pectus excavatum, and hamartoma [1,3].
Main Text
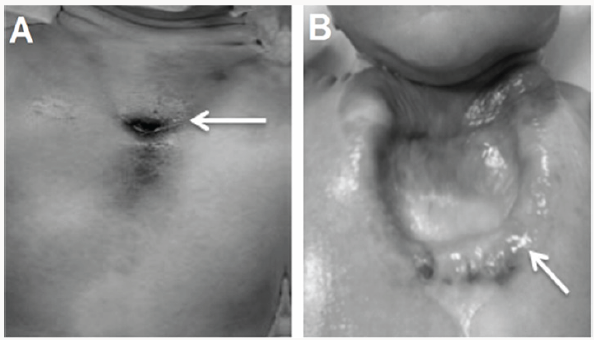
Prenatal diagnosis of SC by fetal ultrasound is well done between the 18th and 26th week of gestation when the number and size of ossification centres are small or absent [4]. Also, prenatal identification of SC is easier when cardiac anomalies exist as these anomalies indicate heedful research of other malformations [1]. At birth, the clinical inspection and palpation of the chest wall can help diagnosis of SC, when cardiac pulsations under the skin are inspected, with V-shaped cleft to xiphoid or U-shaped space between the costal ridges up to 4th costal cartilage (Figure 1) [5].
Figure 3: a) Chest X-ray showing displaced clavicles. b) Axial view of computed tomography showing sternal defect (arrow).
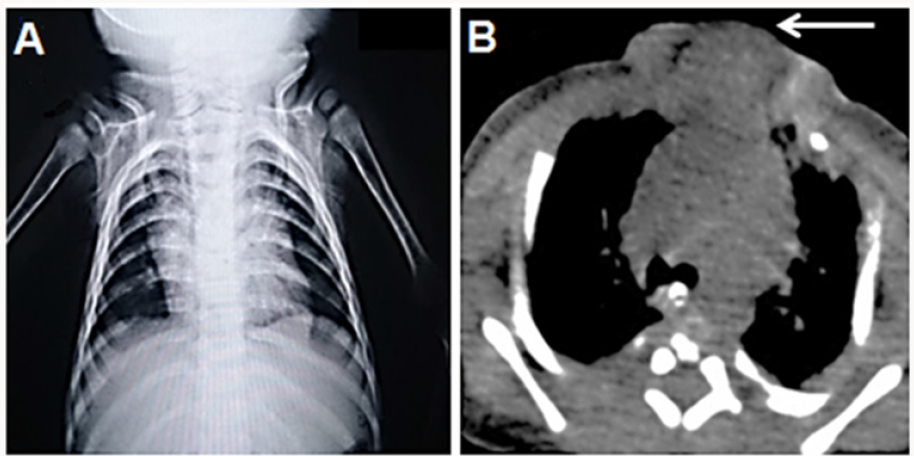
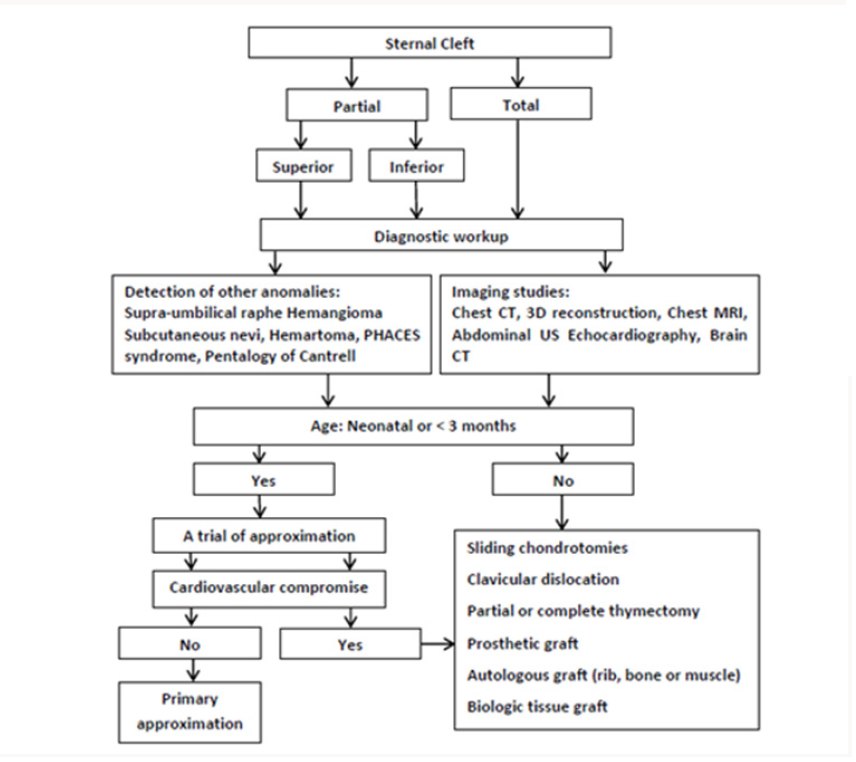
To obtain better results during the management of SC, the physicians should follow a stepwise approach for its diagnosis and treatment (Figure 2). The imaging studies for SC involves lateral view chest X-ray to identify sternal segments and ossification centers. Also, a widened interclavicular distance on posterioranterior view of chest X-ray (Figure 3a), with other clinical findings on examination may confirm the diagnosis [4,6]. The computed tomography (CT) is the gold standard to detect sternal anatomy (Figure 3b). Other imaging modalities to confirm CT findings include 3D reconstruction views and Magnetic Resonance Imaging (MRI) of the chest. It is important to screen other associated congenital anomalies with SC using abdominal ultrasonography and echocardiography [7].
The surgical correction of SC is indicated to avoid the potential injury of unprotected intrathoracic organs, while in absence of vital indications surgery is performed for cosmetic considerations. In the newborn, the sternum is more flexible with minimal compression of the underlying structures, thus surgery is preferred [8]. Early primary closure is the appropriate decision of management in the neonatal period to obtain better surgical and cosmetic outcome. The prognosis after surgery is good when the SC is isolated [3]. In addition to adequate protection of the thoracic organs, surgery should maintain the growth of the chest wall, avoid the prosthetic material when possible, and attain a satisfactory cosmetic outcome. Thus, it is necessary to illustrate the relationship between thoracic organs and chest wall preoperatively using Multi-Detector CT (MDCT). Also, after the surgical correction, MDCT has an important role to detect the adoption of thoracic organs to the reconstructed chest wall [2].
The surgery for SC has anesthetic challenges as multiple organ systems may be involved with a risk of direct injury to the vital organs. The possible perioperative complications include rapid blood loss, arrhythmias, cardiac dysfunction and pneumothorax. Thus, the main requirements during anesthesia for the repair of SC include standby Cardiopulmonary Bypass (CPB), invasive hemodynamic monitoring, use of intraoperative Trans-esophageal Echocardiography (TEE), close observation of the ventilator parameters, elective postoperative mechanical ventilation with adequate analgesia [9]. The sustained physiological changes after operation warrant postoperative intensive care monitoring [10].
Autologous repair and definitive skeletal reconstruction with rib or bone grafts is preferable over prostheses to avoid the possible complications of prosthetic materials, with advantages of less reaction, less infection risk, minimal necrosis of the graft, and neovascularization from the neighboring tissues after the surgery [7,12]. The described techniques using autogenous tissues for reconstruction of a new sternum in older children and adults include: posterior periosteal flap from the sternal bars with chondral grafts; autogenous iliac bone and V-Y myoplasty of pectoralis major muscles; and autogenous rib grafts buttressed with Teflon for bridging wide defects [3,12]. The use of biologic tissue substitutes in reconstruction of SC has promising outcomes. The biologic extracellular tissue matrix derived from porcine dermis forms a strong bio¬logic that can be infiltrated by body’s cells with subsequent integration with the surrounding tissues, good resistance and durability [13].
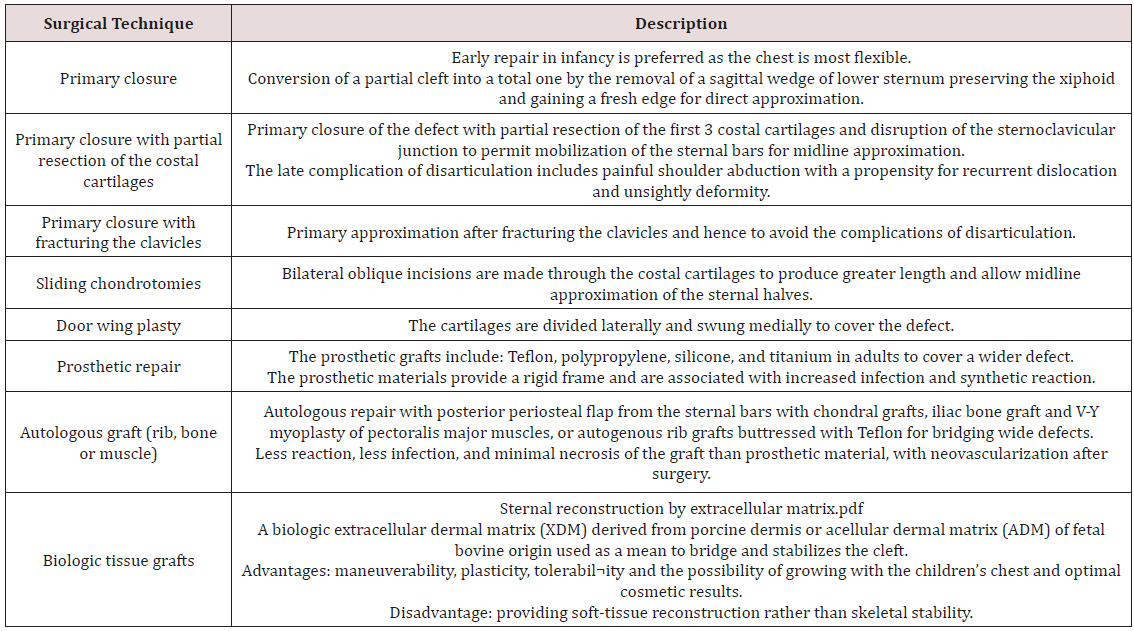
Beyond the neonatal period, particularly after the age of three months, the chest wall becomes rigid and more complicated surgical techniques rather than primary approximation are indicated. These techniques include sliding chondrotomies, fracturing the clavicle, autologous bone or cartilage graft interposition, muscle flap interposition, and prosthetic or biologic mesh grafts (Table 1) [7,11]. In adult patients with SC, prosthetic grafts may be used to cover a wide defect without reduction of the intrathoracic volume. The available prosthetics include methylmethacrylate, polypropylene mesh, silicone prosthesis and titanium plates. The aim of the prosthetic repair is to fix the thorax at its anterior fulcrum in the region of the sternochondral and costochondral junctions [3]. However, the prosthetic material may be complicated by tissue reaction, bradycardia, hypotension and infection, thus alternatives to these prostheses are preferred in management of SC [11]
The reconstruction of SC using biologic extracellular matrix has advantages of maneuverability, plasticity, tolerabil¬ity and the possibility of growing with the children’s chest and optimal cosmetic results. However, a careful approach is recommended when cellular biologic matrices are used considering the location of the SC and the associated chest wall dynamics. The use of a biologic tissue for repair of SC provides soft-tissue reconstruction rather than skeletal stability, which may indicate additional reconstruction with muscle flap or bone graft. Moreover, there is a need for further research to constitute clear criteria for the choice of biologic implants [14]. Other challenges in the surgical correction of SC arise from the association of SC with other chest wall abnormalities, particularly pectus excavatum (PE). Complete SC with PE is an extremely rare entity [15]. Surgery is indicated for significant compression of the heart chambers by the depressed sternum or only for the cosmetic disfigurement [16].
In literature, the described techniques for repair of SC associated with PE in adults rely on bilateral excision of fragments derived from the deformed cartilages, followed by direct approximation of the sternal bars without tension [16,17]. Tocchioni and his colleagues described a combined approach for the correction of complex anterior chest wall anomalies in a teenager with SC and PE. After creation of bilateral periosteal flaps, detachment of the pectoralis major muscles and collection of the portions from the 3rd to 5th costal cartilages, PE was corrected by the introduction of a substernal bar according to the Nuss technique. The steps of sternal reconstruction were: suturing of the periosteal flaps, reinforcement by Prolene mesh, placing the autologous cartilaginous grafts over the reinforced periosteal flaps, reinforcement by another Prolene mesh, and finally suturing the pectoralis major muscles above onto the midline [15].
Conclusion
The proper diagnosis of SC depends on combination of the clinical examination with the findings of imaging studies in addition to screening for other anomalies. The choice of an appropriate surgical technique depends mainly to the age of patient, rigidity of the chest wall, and presence of other abnormalities in the chest wall. The use of biologic grafts has encouraging results but further evaluation is required. When PE exists with SC, the surgical correction of SC may be achieved by approximation of the sternal bars or require more complex procedure.
References
- Zamfir C, Zamfirescu A, Tanase C, Basca I (2014) Sternal cleft - A rare congenital malformation. J Ped Surg Case Reports 2(3): 97-100.
- Yamanaka K, Higuma T, Watanabe K, Okada Y, Ichida F, et al. (2012) Congenital sternal cleft. J Pediatr Surg 47(11): 2143-2145.
- Luthra S, Dhaliwal RS, Singh H (2007) Sternal cleft: a natural absurdity or a surgical opportunity. J Pediatr Surg 42(3): 582-584.
- Ashok Raja J, Mathevan G, Mathiarasan K, Ramasubramaniam P (2014) Closing the cleft over a throbbing heart. BMJ Case Rep 2014: bcr2014204529.
- Yavuzer S, Kara M (2003) Primary repair of a sternal cleft in an infant with autogenous tissues. Interact Cardiovasc Thorac Surg 2(4): 541-543.
- Olusoji OO, Sanni SB, Omodara OO, Akerele OF (2017) Isolated sternal cleft in a patient with atrial septal defect: A rare sole association. Niger Postgrad Med J 24(1): 60-63.
- Alshomer F, Aldaghri F, Alohaideb N, Aljehani R, Murad MA, et al. (2017) Reconstruction of Congenital Sternal Clefts : Surgical Experience and Literature Review. Plast Reconstr Surg Glob Open 5(11): e1567.
- Yuksel M, Kuru P, Ermerak NO, Kiyan G (2016) Intrauterine diagnosed sternal cleft patient and her management. J Vis Surg 2: 48.
- Kumar R, Mohammed S, Biyani G, Karnawat R (2015) Sternal cleft: Anaesthetic management of a rare congenital anomaly. Indian J Anaesth 59(8): 523-525.
- Ramdial S, Pillay D, Madaree A (2016) Primary closure of a sternal cleft in a neonate. World J Plast Surg 5(3): 308-312.
- Jadhav V, Rao S, D’Cruz A (2009) Autologous repair of isolated complete sternal cleft in an adolescent. J Pediatr Surg 44(12): 2414-2416.
- Kuru P, Ermerak NO, Bostanci K, Yuksel M (2015) Reconstruction of sternal cleft with autologous cartilage graft in an adult. Asian Cardiovasc Thorac Ann 23(5): 591-592.
- Badylak SF (2002) The extracellular matrix as a scaffold for tissue reconstruction. Seminars in Cell & Developmental Biology 13(5): 377- 383.
- Molinaro F, Garzi A, Cerchia E, Di Crescenzo VG, Luzzi L, et al. (2016) Sternal reconstruction by extracellular matrix. Open Med (Wars) 11(1):196-199.
- Tocchioni F, Ghionzoli M, Lo Piccolo R, Deaconu DE, Facchini F, et al. (2015) Sternal Cleft and Pectus Excavatum: A Combined Approach for the Correction of a Complex Anterior Chest Wall Malformation in a Teenager. Ann Thorac Surg 99(6): e131-e135.
- Kabiri el H, Traibi A, Boulahya A (2011) Complete sternal cleft in an adult: case report. Gen Thorac Cardiovasc Surg 59(8): 587-589.
- Sarper A, Oz N, Arslan G, Demircan A (2002) Complete congenital sternal cleft associated with pectus excavatum. Tex Heart Inst J 29(3): 206-209.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...