Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6679
Research Article(ISSN: 2637-6679) 
Spondylolisthesis Volume 5 - Issue 4
Mohamed Mostafa Mossaad*
- Professor of Orthopaedic Spine Surgery Zagazig University Egypt
Received: August 18, 2020; Published: September 10, 2020
Corresponding author: Dr. Mohamed Mostafa Mossaad, Professor of Orthopaedic Spine Surgery Zagazig University Egypt
DOI: 10.32474/RRHOAJ.2020.05.000219
Introduction, Natural History, and Pathogenesis of Spondylolisthesis
Spondylolysis refers to a posterior defect in the vertebral body at the pars interarticularis [1]. Usually, this defect is due to trauma or from a chronic repetitive loading and hyperextension. If this instability results in translation of the vertebral body, spondylolisthesis has occurred [1,2]. This process requires a fracture or deformation of the posterior spine elements creating an elongation of the pars. This condition occurs in all ages with the underlying cause varying based on age group. If the slip progresses to the point of neurologic compromise, then surgical intervention may be required to decompress and stabilize the affected segments [3]. In the absence of motor deficits, a nonoperative course of analgesia, activity modification, and injections should be tried for several months [4]. The rates of spondylosis and spondylolisthesis vary widely by age groups. In the pediatric population, spondylosis is present in about 5% of the population, most commonly (90%) at the L5 to S1 motion segment, although pathology at L4 is more likely to be symptomatic [1-9]. Long-term studies estimate that about 15% of those with a defect (spondylosis) will develop a slip (spondylolisthesis) [10,11]. In regard to adults, lumbar spondylolisthesis without a defect in the pars is noted in 5% of men, 10% women [12]. It is not always symptomatic. This degenerative type is usually noted at the L4 to L5 levels (versus isthmic noted at L5 to S1) [6]. Degenerative spondylolisthesis is an acquired type of spondylolisthesis occurring much more frequently and gradually in the adult population [6]. Cohorts with degenerative spondylolisthesis will rarely develop a high-grade spondylolisthesis [13]. Furthermore, the chronic natural history of this disease process is such that with further degenerative changes, the vertebral segments may eventually stabilize, and the patients can have subsequent clinical improvements [13].
Repetitive micro-traumas from hyperextension lead to elongated or absent pars interarticularis. This applies additional stress to the facet joints and subsequent hypermobility leading to advanced degeneration of the disc space [14]. The reduced disc and facet stability results in translation of the vertebral body, creating a stenotic effect on the exiting nerve roots and/or the spinal canal. In the traumatic setting, a flexion distraction energy may cause a localized vertebral body failure at this segment, predisposing the patient to chronic issues if instability develops. Initial evaluation of lower back pain is first directed by obtaining a history from the patient. This history should pertain to the timeline of pain, radiation of pain, and inciting events. Careful attention to prior episodes of trauma should be noted. Low-grade slips and stenotic spinal canals may decompress and relieve pain with leaning forward or sitting. It is important to note patient comments such as decreased pain with pushing a grocery cart or walking upstairs as both common actions have the spinal column in forwarding flexion [15]. It is also important in any evaluation of extremity issues to inspect circulation as vascular claudication may mirror or mimic the neurogenic issues. Classically patients may complain of pain radiating down both buttocks and lower extremities. An evaluation of the patient walking is also critical to better assess the daily impact this pain or deficits is causing. All physical examinations will include evaluation of the neurologic function of the arms, legs, bladder, and bowels. The keys to a thorough exam are organization and patience. One should evaluate not only strength but also sensation and reflexes. It is also important to inspect the skin along the back and document the presence of tenderness to compression or palpable step-off. Performing a straight leg test in a patient positioned supine may also reveal a stenotic canal [16]. This may also cause local sites of pain as hamstring contractures are often associated with spondylolisthesis [17]. As mentioned above, a thorough neurologic examination is required. Commonly a patient will have an L5 radiculopathy resulting in weakness of ankle dorsiflexion and extension of the great toe. This deficit may also diminish the Achilles tendon reflex. An L4 radiculopathy may present with weakness at the quadriceps and a decreased patellar tendon reflex.
Documentation is paramount as these initial findings will likely be used as a baseline for all future evaluations.
Evaluation of patients with low-back pain typically includes anterior-posterior (AP) and lateral radiographs of the impacted area. Some physicians will obtain radiographs of the entire spine. A key component to radiographic evaluation is obtaining flexion/ extension films as this helps illustrate vertebral segment stability [18]. Serial standing lateral radiographs obtained in the clinic will help track any potential progression of the slip. One of the crucial measurements to note in regard to slips is the amount of translation between vertebral bodies. The amount of “uncovering” of one endplate from the other determines the grade. There are four grades of spondylolisthesis: Grade 1: 0% to 25%, Grade 2: 26% to 50%, Grade 3: 51% to 75% and Grade 4: 76% to 99% [12-19]. At 100% displacement, patients have developed spondyloptosis [20]. Grades 1 and 2 are considered low-grade slips [21]. Grades 3 and 4 are considered high-grade spondylolisthesis [19]. An MRI is critical when evaluating patients with suspected spondylosis and spondylolisthesis. Frequently these patients will initially trail a six-week course of physical therapy and upon follow-up, if the symptomology is still present an MRI can be obtained at that time [1]. The focus should be directed to the T2 weighted sagittal and axial images as these will illustrate any compression of neurologic elements.
Classification
various classifications have been described for spondylolisthesis. The Wiltse-Newman classification is one of the most widely used classification systems for spondylolisthesis. This classification system was originally based on a radiographic review of a large series of patients in which anterior translation of the vertebral body was found to be related to a defect in the pars interarticularis [1]. Subsequent modifications and classifications have been proposed to include the Marchetti and Bartolozzi classification system [2,3]. The Wiltse-Newman classification categorizes spondylolisthesis into five different types: 1) dysplastic 2) isthmic 3) degenerative 4) traumatic and 5) pathologic. 2 As the name suggests, dysplastic spondylolisthesis is a result of a failure of formation or abnormal formation of the posterior elements resulting in subsequent instability. The incidence of this type of spondylolisthesis is reported to be anywhere from 14-21% in the literature [1]. Isthmic spondylolisthesis resulting from a defect within the pars interarticularis is the most common type and accounts for the vast majority of cases in children and adolescents. It has been subdivided into three types depending on the etiology of the pars defect.
Degenerative spondylolisthesis, on the other hand, is the most common form of spondylolisthesis seen in adults. In contrast to the dysplastic and isthmic varieties, degenerative spondylolisthesis is characterized by an intact posterior neural arch. The pathogenesis is thought to be related to chronic degenerative changes within the spine and posterior elements resulting in the incompetence of the surrounding ligamentous structures. As a result, degenerative spondylolisthesis occurs most commonly in individuals older than fifty years of age and typically at the lumbar 4-5 level. It is also seen with greater frequency in the African American population and is disproportionately higher in females [4]. Traumatic spondylolisthesis results from a high-energy injury and is considered a fracture- dislocation involving the posterior elements. Pathologic spondylolisthesis can be divided into 1) local – resulting from the local destruction of bone or surrounding support structures as occurs in tumors or malignancy and 2) systemic – such as that occurs from metabolic bone disease [3]. The Marchetti-Bartolozzi classification is etiology-based, and aids in conveying the underlying mechanism and pathogenesis.
Developmental spondylolisthesis can be further divided into high dysplastic and low dysplastic types. In the high dysplastic form, the L5-S1 segment is affected and is characterized by wedging of the L5 segment and there is the presence of a dome-shaped, vertical sacrum.5 The low dysplastic form often presents much later than the high dysplastic type, with individuals symptomatic in early adulthood as opposed to childhood/adolescence. There are four types of acquired spondylolisthesis: 1) degenerative 2) traumatic 3) pathologic and 4) post-surgical. The degenerative, traumatic and pathologic types are similar to that described in the Wiltse-Newman classification. The post-surgical type occurs when iatrogenic destabilization occurs from aggressive resection of the posterior elements. Traumatic spondylolisthesis can occur secondary to high-energy injury or secondary to stress or fatigue through the pars interarticularis. A fracture that occurs through a normal pars interarticularis is referred to as spondylolytic spondylolisthesis [5]. While both classification systems are used readily in the literature, many studies fail to differentiate between the dysplastic and isthmic types of spondylolisthesis as it relates to both diagnosis and treatment. It is for this reason that the Marchetti-Bartolozzi classification has recently been favored [6].
Clinical Presentation
The most common reason for children and adults with spondylolisthesis to seek medical attention is pain or radicular symptoms [7]. The individual may have a history of a previous traumatic event, but insidious onset is also quite common upon presentation to a medical care provider. As with most evaluations, a thorough history and examination assists in elucidating the etiology of symptoms. Patients with spondylolysis or spondylolisthesis may have reproducible pain localized to the lower lumbar spine, which suggesting a traumatic or spondylolytic etiology. Frequently, an individual will have pain and difficulty with attempted hyperextension of the lumbar spine with associated limited flexion and extension. Hamstring contracture is often associated with spondylolisthesis, and in severe cases, can result in a crouched gait pattern [8]. In severe spondylolisthesis or spondyloptosis, neurologic changes can occur to include motor or sensory deficit but even in these circumstances major neurologic complaints are rare. Tension signs may be present with straight-leg testing (SLR) and in severe cases, bowel and bladder symptoms can occur. Although often overlooked and not attempted, a rectal examination should be performed if concern exists about potential compression of the sacral nerve roots.
Patients with degenerative spondylolisthesis present with a variety of symptoms depending on the pathologic processes involved. Patients can have neurogenic claudication resulting from spinal stenosis occurring secondary to listhesis, as well as hypertrophy of the ligamentum flavum and facet degeneration. Typically, patients will present with pain radiating down both buttocks and lower extremities with associated numbness and weakness. This may be alleviated by resting or leaning forward – this is in stark contrast to vascular claudication which is associated with pain even at rest. If pulses are not palpable or pain continues even at rest, a vascular work-up should be initiated to rule out underlying vascular etiology [21-33].
Treatment Spondylolysis
Frederickson et al., have shown in their study that no patients with a unilateral pars defect went onto develop spondylolisthesis, and of those patients with a bilateral pars defect, only a small percentage of patients went onto develop a slip [11]. Non-surgical intervention includes modification of the activity that may have exacerbated the pain, non-steroidal anti-inflammatory medications (NSAIDs), physical therapy, stretching, and the use of a lumbosacral orthosis. Non-operative management has shown a great degree of success in many studies. Debnath [34] in his series of 42 patients with symptomatic unilateral stress injuries of the pars or spondylolysis for a minimum of 2-year follow-up. All but eight of the patients (81%) went onto return to their previous level of activity without further symptoms after a six-month period of activity restriction and bracing. It was not clear [35] whether those patients who improved also had radiographic evidence of union. Sys et al. subsequently followed [36] competitive athletes with either a unilateral or bilateral spondylolysis. All patients were initially treated with a period of bracing and activity restriction. All 11 patients with unilateral pars defects went onto develop osseous union as determined by subsequent CT scan. Only five out of nine patients with bilateral defects went onto develop osseous union, but seven of nine patients reported a good to excellent final outcome. In a recent meta-analysis of patients with either spondylolysis or grade I spondylolisthesis, 83.9% treated non-operatively had a successful clinical outcome after at least one year [36].
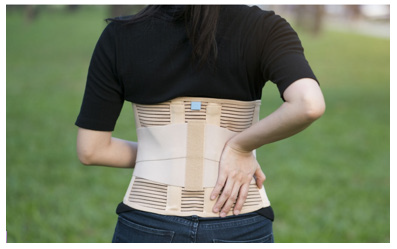
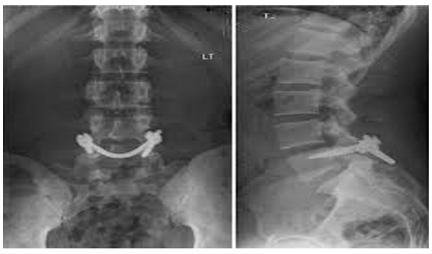
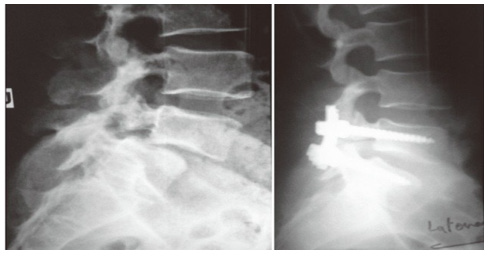
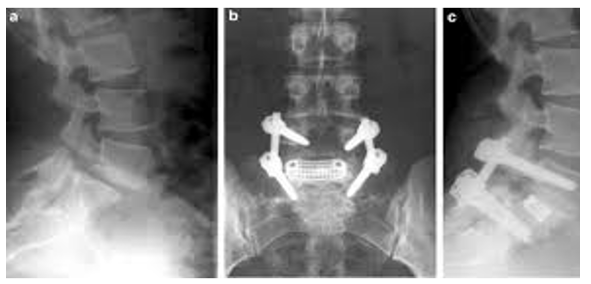
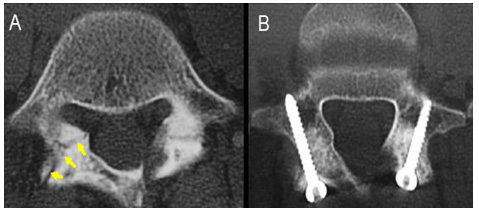
In the same meta-analysis, no significant difference was found when comparing patients treated with or without bracing, implying that bracing may not be necessary for a favorable outcome [36]. Furthermore, lumbosacral orthoses and corsets (Figure 1), have not been shown to be effective below the L4-L5 level [35-37] .The authors suggest that it may be the activity restriction and not bracing that may be the more important role in the overall outcome. There has also been evidence to suggest that the acuity of the fracture may affect overall union but that union is not necessary to have relief in symptoms [38]. In patients who continue to have pain, and in those who have not demonstrated improvement despite a course of extended non-operative treatment, surgical intervention may be indicated. A direct surgical repair of the pars interarticularis is an option to address the defect. The ideal candidate is a child or adolescent with a spondylolytic defect cephalad to L5 with an intact intervertebral disc, absence of radicular symptoms and minimal listhesis; or in a patient with multiple spondylolytic defects at several levels [39]. A direct repair of the pars offers the advantage of avoiding a fusion thereby preserving motion segments – especially in an individual with multi-level involvement (Figure 2). Several techniques for direct repair have been described. The Buck technique involves the use of screw fixation directly across the pars defect with concomitant bone grafting. In his original series, Buck used this technique on 16 patients with only one patient having persistent symptoms ultimately requiring a posterior spinal fusion. One patient required a screw exchange for symptoms resulting from an excessively long screw and one ultimately required screw removal and laminectomy for radicular symptoms. All patients except for the one requiring spinal fusion achieved complete osseous union of their original defect. Debnath [35] reported on a small series of patients (eight) treated using Buck’s technique with seven of eight patients having improvement in their symptoms and return to athletic activity by one year (Figure 3). The one remaining patient ultimately went on to receive a posterior spinal fusion [39,40]. Similar results were reported by Bradford using a segmental wire fixation technique [41]. Several other techniques have been described with variable results in the literature to include the hook screw [41-44]. More recently, Kakiuchi [45] reported his results using a pedicle screw-laminar hook construct.
Figure 3: General Psychopathology, Positive and Negative Symptoms Brain Activity Areas Associated with Addiction.
Low-Grade Spondylolisthesis
Patients with low-grade spondylolisthesis should also be subjected to a course of non-operative management given the relatively benign natural history of the disease process as discussed previously. In those patients with persistent pain, radicular symptoms or those demonstrating progression of their spondylolisthesis, operative intervention is a viable option. Much of the literature regarding the optimal surgical procedure, surgical approach as well as the role for decompression and instrumentation remains controversial. In situ posterolateral fusion has been well-described in the literature with generally favorable clinical outcomes. In a series of 129 adult patients in a randomized study comparing patients undergoing posterolateral fusion for grade I and II spondylolisthesis with and without instrumentation for a minimum of 5-year follow-up. At one year follow-up, both groups achieved radiographic evidence of fusion (77% for the instrumented and 86% for the non-instrumented group). In another prospective, randomized study by Moller [44,45], [46-77] adult patients with grade I or II spondylolisthesis underwent posterolateral fusion with or without instrumentation. Radiographic fusion was achieved in 65% and 78% respectively with both groups having significant improvement in their post-operative pain scores. Similar fusion rates are reported in the majority of the literature irrespective of whether instrumentation was used or not [47-49]. The theoretical advantages of in situ posterolateral fusion alone include achieving posterior stability at the site of instability (at the pars interarticularis) with the added benefit of performing a decompression, if necessary, and the ability to place instrumentation. Also, when compared to anterior approaches or circumferential fusion, there is decreased intra-operative time with decreased blood loss and other morbidities associated with an anterior approach.
There still exists some controversy regarding the need for posterior decompression for patients who have radicular symptoms. While it would intuitively seem that nerve root decompression would serve to improve the overall clinical outcome in patients with radiculopathy, there is literature to suggest otherwise [50]. In a prospective randomized study, Carragee [51] followed 42 patients who had failed non-operative treatment with grade I or II spondylolisthesis. They were randomized to receive in situ posterolateral fusion with or without decompression. Twenty-two percent of patients who had undergone a posterior decompression developed a pseudoarthrosis. Interestingly, all 24 patients who had fusion without decompression went on to achieve radiographic union. One-third of the patients treated with decompression reported an unsatisfactory result in terms of overall pain and functional scores, whereas only one of the 24 patients treated without decompression had an unsatisfactory result. The results from this study suggest that added decompression in the setting of posterolateral fusion for low-grade spondylolisthesis may actually increase the rate of pseudoarthrosis and worsen clinical outcome. This may be due to the fact that decompression further de-stabilizes the posterior elements – these results occurred even in the patients who had received decompression supplemented by instrumentation. Similar results were reported by Garreau de Loubresse [52], reporting a 30% pseudoarthrosis rate in his series of patient who received decompression compared to 8% who had received posterolateral fusion alone.
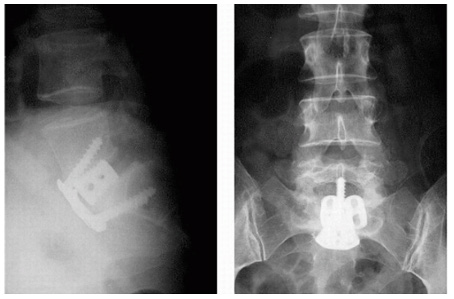
Similarly, much controversy exists regarding the need for instrumentation in the setting of in situ posterolateral fusion. The theoretical benefits of adding instrumentation to the fusion construct include added rigidity and stability thereby enhancing fusion rates and clinical outcomes. Both Moller [46] and Christensen [46] reported a lower radiographic union rate in those patients who had fusion with instrumentation whereas others were unable to demonstrate any clear benefit [47-51]. On the contrary, Carragee [51] reported only one out of 20 patients developed a pseudoarthrosis when managed with supplementary instrumentation as opposed to three out of 22 when managed without. Furthermore, this suggested that instrumentation may play a role in enhancing fusion rates as the patients who did not receive instrumentation were exclusively patients who were found to be non-smokers. All smoking patients had been selected to receive instrumentation. In further support of instrumentation, Zdeblick [50-53] conducted a prospective, randomized trial where patients received either no instrumentation, semi-rigid instrumentation, or rigid instrumentation with pedicle screws (Figure4), The respective rates of fusion were 81%, 89% and 100%. Similar to Carragee’s study, fusion rates were enhanced in the sub-population of smokers. Smoking patients without instrumentation had a fusion rate of 53% compared to 87% of those who had rigid instrumentation. Despite the variation in reported clinical and radiographic outcomes after instrumentation, given the relatively recent literature supporting its use in enhancing fusion, the clear benefit in the subset of patients who smoke, as well as its added benefit in those undergoing a decompression, rigid pedicle screw instrumentation is recommended in conjunction with in situ posterolateral fusion.
Anterior lumbar interbody fusion (ALIF) (Figure 5), has also been reported as an option for the surgical management of lowgrade spondylolisthesis. The ALIF allows for the placement of an interbody graft which can help restore disc height, kyphosis and allows for indirect decompression of the nerve roots. Any bone graft placed from an anterior approach allows for compression of the graft which is biomechanically favorable. However, potential risks with this approach include injury to the nerve plexus resulting in retrograde ejaculation as well as the obvious neurovascular injury from a transperitoneal approach. Furthermore, in those patients with clear signs of radiculopathy, a direct decompression cannot be performed as this requires a posterior approach for direct access and visualization. Most of the literature regarding ALIF has been in conjunction with a posterior fusion to achieve circumferential fusion. In a retrospective review, Ishihara [54] followed 23 patients who underwent ALIF for low-grade spondylolisthesis. The overall fusion rate was 83% with satisfactory low-back pain scores. These low-back pain scores were found to worsen after the 5-year follow- up. In addition, a significant amount of adjacent segment degeneration was noted both radiographically and on MRI; however, there was no comparison to pre-operative imaging to see if these changes had been pre-existing. Cheng [55], has reported in his series 15 of 20 patients (75%) had radiographic evidence of fusion with a minimum of 10-year follow-up with 95% of patients reporting satisfactory to excellent outcomes. One patient required subsequent posterior spinal fusion; however, it was unclear whether the remaining four patients who had failed to achieve union went onto revision surgery.
Surprisingly, there have also been very few direct comparison studies of anterior versus posterior surgery for low-grade spondylolisthesis. As far as we are aware, there only exists one study comparing ALIF with posterolateral fusion and instrumentation. Kim [56] compared 40 patients who received either ALIF (20 patients) or posterolateral fusion with instrumentation (20 patients) with all patients having either a Meyerding grade I or II spondylolisthesis. He noted no statistically significant difference between fusion rates or clinical outcomes in either group with both treatment groups responding favorably. The fusion rates were noted to be 90% in the ALIF group and 95% in the posterolateral fusion group. Similarly, 85% of patients in the ALIF group noted satisfactory results with 90% satisfactory results in the posterolateral fusion group. The results of this study suggest similar clinical and radiographic results regardless of surgical approach; however, this would be better elucidated in a randomized, prospective study. The idea of performing a reduction in high-grade spondylolisthesis remains controversial; however, there is evidence to suggest it may not be necessary in low-grade spondylolisthesis. Patients with lowgrade spondylolisthesis obviously have a lower degree of deformity with less translation and less kyphosis. Furthermore, as already discussed, the natural history of this disease is relatively benign. Naderi [57] reported 30 patients with low-grade spondylolisthesis who all underwent posterolateral fusion with instrumentation. He purposely did not attempt reduction intra-operatively and evaluated the degree of reduction post-operatively. Ninety-three percent of patients had evidence of partial reduction and the degree of reduction averaged 20% when compared to pre-operative imaging. Interestingly, they also noted that in patients with spondylolisthesis occurring at L4-L5 and those with a more sagittal orientation of the facet joints to have a greater degree of reduction. It is also noteworthy that 29 of their 30 patients had a grade I spondylolisthesis.
Circumferential fusion encompasses the benefits provided by both anterior and posterior approaches [55-57]. This can be accomplished by either an ALIF with posterolateral fusion, transforaminal lumbar interbody fusion (TLIF), or with posterior lumbar interbody fusion. There have been significantly higher rates of radiographic union reported in the literature with circumferential fusion. Lauber [58] conducted a prospective study on 39 patients with low grade spondylolisthesis who received TLIF. 19 of the patients had degenerative spondylolisthesis. The overall fusion rate was 94.8% (a separate fusion rate was not given for the non-degenerative spondylolisthesis group) and the mean Oswestry Disability Index scores decreased by 10 points. In a separate study by Rosenberg [59], 22 patients with low-grade spondylolisthesis underwent TLIF. Clinical results were reported with resolution of radiculopathy in all patients who had radicular pain with 16 patients reporting complete resolution of their back pain. The only complications include one patient with a dural tear, one with mild neurologic weakness which had completely resolved, and two superficial wound complications. Radiographic fusion was not reported in this study. Other studies have reported on the safety and high fusion rates with TLIF; however, these have been in conjunction with patients with other pathology and not isolated to spondylolisthesis (i.e. degenerative disc disease, disc herniation). Transforaminal lumbar interbody fusion (TLIF) is a relatively recent technique and there is very little literature directly comparing it to other techniques such as posterolateral fusion [58]. It has been compared to PLIF retrospectively with lower complication rates, but not in patients exclusively with spondylolisthesis [59,60].
Posterior lumbar interbody fusion (PLIF), (Figure 6) has been compared to posterolateral fusion retrospectively in a few series. In a study by Madan [61], 21 patients received posterolateral fusion in situ with instrumentation and 23 patients received PLIF. The patients who underwent PLIF had higher fusion rates (no pseudoarthrosis) but the patients who had received posterolateral fusion had better subjective outcome scores. In another study by La Rosa [62], 18 patients had undergone posterolateral fusion with 17 patients receiving PLIF. At 2-year follow-up, the PLIF group had no evidence of pseudoarthrosis with better maintenance of correction. Clinical outcome measures for both groups were not found to be significantly different [60,61]. In a recent review by Kwon [63], a meta-analysis was performed combining the clinical and radiographic results of multiple studies. They reported fusion rates of 98.2%, 83.3%, and 75.3% for patients who received circumferential, posterior, and anterior procedures respectively. With respect to clinical outcomes, 86.4%, 79.3%, and 74.8% reported a successful outcome with circumferential, anterior, and posterior procedures respectively. In a recent prospective, controlled study by Swan [64], each group of 50 patients was treated with either posterior spinal fusion with instrumentation versus circumferential fusion with anterior and posterior fusion. One pseudoarthrosis was noted in the circumferential fusion group compared to three in the posterior- only group. Clinical outcomes were also superior in the group with a combined procedure. Interestingly, radiographic reduction in the slip angle was only maintained in the group with the group with circumferential fusion. Consistent with the retrospective review by Kwon, this study suggests superior results with circumferential fusion.
High-Grade Spondylolisthesis
Compared with low-grade spondylolisthesis, more patients undergo operative intervention. This is consistent with the known natural history and is to be expected given the fact that the greatest risk factors for progression include the original degree of translation on presentation as well as age. Higher degrees of dysplasia and translation correlate with increasing symptomatology to include pain and radiculopathy. Harris reported on a small series of patients treated non-operatively without serious neurologic complications and mild pain [9]. As with low-grade spondylolisthesis, the decision for operative management is likely to be individualized; however, in young, pre-pubescent patients with high-grade slip and evidence of dysplasia, surgical intervention is recommended to manage the increasing likelihood for progression. In situ posterior spinal fusion without instrumentation has been well-described in the literature for the management of high-grade spondylolisthesis. Johnson [65] followed 17 patients who had undergone in situ arthrodesis with an average follow-up of 14 years. No patients developed a pseudoarthrosis and 16 of 17 patients reported excellent results long-term up to 20 years after the procedure. In the previously cited by Harris9, he also managed a cohort of 21 patients treated with in situ posterolateral fusion. Only one patient had evidence of a pseudoarthrosis, three patients had evidence of bending of the fusion mass, and 95% of patients reported mild or absence of pain symptoms. Similar results of pseudoarthrosis and clinical outcome are reported throughout the literature [64-67].
The concerns with posterolateral fusion without instrumentation and decompression are similar to the literature reported for those patients with low-grade spondylolisthesis. There is a higher reported rate of pseudoarthrosis as well as lower rates of successful clinical outcome as compared to ALIF alone or circumferential fusion. Higher rates of slip progression have also been reported for uninstrumented fusion. In a study by Molinari [68] ,37 patients with high-grade spondylolisthesis were evaluated after receiving one of three different surgical options. One subset underwent in situ posterolateral fusion without instrumentation; the second group underwent instrumented posterolateral fusion, and the third set underwent reduction, decompression and circumferential fusion. Almost 50% of the patients in the uninstrumented group had a pseudoarthrosis, 2 patients (28%) had pseudoarthrosis in the instrumented group while all patients in the combined procedure had evidence of fusion. Similar to the literature on low-grade spondylolisthesis, several studies demonstrate increased rates of arthrodesis as well as improved clinical outcomes with circumferential fusion. Muschik68 directly compared the results of patients undergoing either uninstrumented anterior in situ fusion (29 patients) or anterior fusion with posterior instrumented fusion (30 patients). The group that had undergone circumferential fusion was superior to the anterior fusion group in all parameters to include rate of pseudoarthrosis (7% versus 24%), lumbosacral kyphosis, measurable degree of translation, as well time to fusion. There was no significant difference in overall clinical outcome [69]. In another study by Lamberg [70], anterior, circumferential, and posterolateral uninstrumented fusion was compared over an average of 17 years in 83 patients. The circumferential and anterior fusion groups demonstrated the highest rate of fusion (96% and 100% respectively) whereas the posterolateral fusion population had a rate of 86%. The circumferential group also had the lowest Oswestry Index scores. In a very recent study, Goyal70 reported the first series of patients with highgrade spondylolisthesis to be treated with reduction and TLIF. He had a small series of 13 patients in whom all but one had developed radiographic union. All but one patient reported good to excellent subjective outcome scores. They also averaged a 75% correction of their pre-operative spondylolisthesis.
Perhaps the most controversial area in the management of high-grade spondylolisthesis involves the question of whether reduction is necessary. Proponents of reduction note that reduction results in increased likelihood of fusion, improved biomechanical positioning for graft placement, preservation of motion segments, as well as improvement in lumbosacral kyphosis. One of the biggest concerns in performing a reduction is the resulting neurologic injury. Much of the literature is difficult to critically evaluate regarding this issue as many of the studies performed are retrospective and often times, the control groups have additional variables that are not well controlled. In the previously cited study by Muschik [69], patients who did and did not receive a reduction were compared (reduction group had pseudoarthrosis rate of 7% versus 24%); however, this comparison may be skewed due to the fact that the second cohort also received additional posterior instrumentation which in and of itself may have altered the fusion rates and clinical outcome. Molinari’s [68] study (reduction group had no pseudoarthrosis compared to 7 of 37 total patients without reduction) also compared patients with and without reduction, but again the overall results may be altered as patients were not standardized to receive instrumentation and one specific type of fusion technique. In a more recent study from Finland, a cohort of 11 patients was managed with circumferential fusion with reduction and instrumentation posteriorly while the second cohort was managed with circumferential fusion without instrumentation [69-72]. They had no pseudoarthrosis in either cohort but reported improved clinical outcomes in the group that had received fusion in situ. Again, this scenario is difficult to interpret as the second cohort was not managed with instrumentation; however, one could argue that added instrumentation in this cohort would only serve to improve both radiographic and clinical outcomes (given the trend in favor of instrumentation in the literature).
Several authors recommend partial reduction of the spondylolytic segment due to concern for potential injury to the L5 nerve root. In a novel study by Petraco [73], a cadaveric study was performed to evaluate the path of the L5 nerve root with attempted reduction of a high-grade spondylolisthesis. He found that the strain on the L5 nerve root was essentially non-linear. The total strain on the L5 nerve root with complete reduction of a 100% slip resulted in a strain of 14%. When the first 50% of the slipped segment was reduced, it resulted in a strain of 4%. The second half of the reduction resulted in a 10% strain; therefore, 71% of the strain occurred during the second half of reduction. Furthermore, reduction of the last 12.5% of slip was 6-7 times higher in strain than reduction of the first 12.5% of slip. This study suggests that partial reduction may be beneficial in restoring disc height and other lumbosacral parameters while minimizing injury to the L5 nerve root. Furthermore, this also implies that reduction should probably be avoided in patients with low-grade spondylolisthesis. In patients with 100% translation, or spondyloptosis, surgical intervention is often required as patients tend to have neurologic deficit as well as poor sagittal balance as a result of extreme lumbosacral kyphosis. Traditional reduction maneuvers can be difficult from a posterior only approach as the vertebral segment tends to be in significant kyphosis and sitting beneath S1. Gaines [74] has published his series as well as technique for management of patients with spondyloptosis. It involves a staged procedure where a vertebrectomy of L5 is performed through an anterior approach and the patient is subsequently returned for and L4-S1 posterior spinal fusion . In his recent review of his series of 30 patients, he reported a total of 23 patients with immediate post-operative L5 neuropraxia. At most recent follow-up, he reports that all but 2 have had total motor recovery with some lingering sensory deficits. An independent review was conducted on a series of his first 16 patients [74], and all reported very positive subjective outcomes.
Degenerative Spondylolisthesis
As in other types of spondylolisthesis, a prolonged course of non-operative treatment should be considered. Patients with degenerative spondylolisthesis very rarely develop high-grade spondylolisthesis. The natural history of the disease process is such that with further degenerative changes, the affected vertebral segment will stabilize and patients can have subsequent clinical improvement. Non-operative management can include the use of selective nerve root injections as well as epidural injections. Operative management can be considered in those patients who do not improve despite a prolonged non-operative course or in those patients who continue to deteriorate neurologically or have evidence of bowel/ bladder involvement [75]. Not surprisingly, the treatment options for patients with degenerative spondylolisthesis are also very controversial. Much of the decision making process needs to be guided by symptomatology as well as pre-operative imaging. Patients can have several locations of stenosis or degenerative change that may be responsible for their neurologic or pain symptoms. Controversies exist regarding the need for arthrodesis as well as the need for instrumentation.
Several authors have provided good results treating patients with isolated decompression with fusion. In a large retrospective review of 290 patients by Epstein [76], 82% of patients reported a good to excellent result in those treated with isolated decompression. However, of the patients that were selected, none had evidence of instability on flexion/extension radiographs pre-operatively. Only 2% of patients required a repeat surgery for instability or recurrence of symptoms. In a prospective study, Kleeman [77] reported favorable outcomes using a modified technique of decompression where complete excision of the ligamentum flavum was undertaken through a modified laminotomy. Of the 51 patients, only 15 had true degenerative spondylolisthesis. Eighty-percent of patients reported a good to excellent outcome and only 13% of patients had progression of their slip (the remaining 87% had no change). Arthrodesis should be considered in patients who have pre-operative evidence of instability on dynamic radiographs. Other authors have reported much less favorable results and advocate concomitant fusion with decompression. In a large meta-analysis by Mardjetko [78] of 216 patients, patients who received four different procedures were evaluated – those who received isolated decompression, decompression with uninstrumented fusion, decompression with fusion and instrumentation (not pedicle screws), and decompression and fusion with pedicle screw instrumentation. He found a significant difference with 90% of patients undergoing fusion reporting satisfactory clinical outcomes compared to 69% without fusion. In another frequently cited study, Herkowitz [79] prospectively evaluated 50 patients with degenerative spondylolisthesis treated with decompression with or without posterolateral fusion. Twenty-four of 25 patients in the fusion group reported good to excellent clinical results even with nine pseudoarthroses. Only 28% of the patients who had undergone fusion had increased post-operative slip, whereas 96% of the patient with isolated decompression had increased translation.
Similarly, much controversy exists regarding the need for instrumentation concomitantly with arthrodesis. While the literature has demonstrated in the cases of non-degenerative spondylolisthesis that increased fusion tends to correlate with improved clinical outcome (hence the argument for instrumentation as it has been shown to enhance fusion rates), there has been conflicting data with respective to degenerative spondylolisthesis. In an important prospective, randomized study by Fischgrund [80], 67 patients were randomized to receive decompressive laminectomy and arthrodesis with or without instrumentation. He reported a fusion rate of 82% in the instrumented group compared to 45%; however, there was no significant difference found in the overall outcome. Therefore, while the fusion rate was significantly improved with instrumentation, the authors concluded that instrumentation may be unnecessary as it does not improve the overall clinical outcome. Conversely, Kornblum [81] followed 47 patients prospectively (originally from the Herkowitz and Fischgrund’s studies followed to long-term) who all received decompression with uninstrumented fusion to determine the overall fusion rate and their associated clinical outcomes. 86% of patients who achieved radiographic evidence of fusion had a good to excellent clinical outcome compared to 56% of the patients with a pseudoarthrosis. Contrary to the previous study, Kornblum demonstrated that the final clinical outcome is correlated with the presence of a successful fusion. Furthermore, in a recent meta-analysis , a clear benefit was demonstrated with concomitant fusion as opposed to isolated decompression; however, they could not conclude that instrumentation resulted in an improvement in clinical outcome (despite the fact that instrumentation clearly demonstrated an increase in successful fusion).
ALIF and circumferential fusion have been very sparely reported in the literature. A few authors [82-84] have reported satisfactory results in their small retrospective series of patients with ALIF; however, they all concede that it may have a limited role in advanced stages of disease or in older patients given the extensive involvement of disease posteriorly that would only be addressed indirectly through an anterior approach. Similarly, very little has been published on PLIF and TLIF. Previously cited, Lauber [58] evaluated a small series of 39 patients who had undergone TLIF for isthmic and degenerative spondylolisthesis (19 patients). He reported a total fusion rate of 94.8% but no individual fusion rate was given for the patient with degenerative spondylolisthesis. Interestingly, patients in the degenerative group continued to have improvement in their clinical outcome scores throughout the 2-year follow-up. Given the paucity of literature on interbody fusion, further literature to include randomized, clinical trials will be necessary to elucidate the role of circumferential fusion in this population. As is evidenced by the many studies that were evaluated, there is still quite a bit of controversy regarding the optimal treatment for spondylolisthesis. We attempted to evaluate many of the studies that are commonly presented in the literature and critically evaluate them. Further prospective, randomized studies are needed to best ascertain the optimal approach in terms of fusion and patient outcomes.
References
- Cavalier R, Herman MJ, Cheung EV, Pizzutillo PD (2006) Spondylolysis and spondylolisthesis in children and adolescents: I. Diagnosis, natural history, and nonsurgical management. J Am Acad Orthop Surg 14(7): 417-424.
- Foreman P, Griessenauer CJ, Watanabe K, Conklin M, Shoja MM, et al. (2013) L5 spondylolysis/spondylolisthesis: a comprehensive review with an anatomic focus. Childs Nerv Syst 29(2): 209-216.
- Cheung EV, Herman MJ, Cavalier R, Pizzutillo PD (2006) Spondylolysis and spondylolisthesis in children and adolescents: II. Surgical management. J Am Acad Orthop Surg 14(8): 488-498.
- Herman MJ, Pizzutillo PD, Cavalier R (2003) Spondylolysis and spondylolisthesis in the child and adolescent athlete. Orthop Clin North Am 34(3): 461-467, vii.
- Jones TR, Rao RD (2009) Adult isthmic spondylolisthesis. J Am Acad Orthop Surg 17(10): 609-167.
- Eismont FJ, Norton RP, Hirsch BP (2014) Surgical management of lumbar degenerative spondylolisthesis. J Am Acad Orthop Surg 22(4):203-213.
- Ibebuike K, Roussot M, Watt J, Dunn R (2018) Management challenges of traumatic spondylolisthesis of the Axis with an unusual C2-C3 posterior subluxation in a paediatric patient: case report and literature review. Afr Health Sci 18(2): 458-467.
- Boxall D, Bradford DS, Winter RB, Moe JH (1979) Management of severe spondylolisthesis in children and adolescents. J Bone Joint Surg Am 61(4): 479-495.
- Saraste H (1987) Long-term clinical and radiological follow-up of spondylolysis and spondylolisthesis. J Pediatr Orthop 7(6): 631-638.
- Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, et al. (2009) Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 34(2): 199-205.
- Omidi Kashani F, Ebrahimzadeh MH, Salari S (2014) Lumbar spondylolysis and spondylolytic spondylolisthesis: who should be have surgery? An algorithmic approach. Asian Spine J 8(6): 856-863.
- Bouras T, Korovessis P (2015) Management of spondylolysis and low-grade spondylolisthesis in fine athletes. A comprehensive review. Eur J Orthop Surg Traumatol 25 Suppl 1: S167-75.
- Schulte TL, Ringel F, Quante M, Eicker SO, Muche Borowski C, et al. (2016) Surgery for adult spondylolisthesis: a systematic review of the evidence. Eur Spine J 25(8): 2359-2367.
- Shah SA, Saller J (2016) Evaluation and Diagnosis of Back Pain in Children and Adolescents. J Am Acad Orthop Surg 24(1):37-45.
- Syrmou E, Tsitsopoulos PP, Marinopoulos D, Tsonidis C, Anagnostopoulos I, et al. (2010) Spondylolysis: a review and reappraisal. Hippokratia 14(1): 17-21.
- Scaia V, Baxter D, Cook C (2012) The pain provocation-based straight leg raise test for diagnosis of lumbar disc herniation, lumbar radiculopathy, and/or sciatica: a systematic review of clinical utility. J Back Musculoskelet Rehabil 25(4): 215-223.
- Miyamoto N, Hirata K, Kimura N, Miyamoto Mikami E (2018) Contributions of Hamstring Stiffness to Straight-Leg-Raise and Sit-and-Reach Test Scores. Int J Sports Med 39(2): 110-114.
- Chen X, Zhou QS, Xu L, Chen ZH, Zhu ZZ, et al. (2018) Does kyphotic configuration on upright lateral radiograph correlate with instability in patients with degenerative lumbar spondylolisthesis? Clin Neurol Neurosurg 173: 96-100.
- Harris IE, Weinstein SL (1987) Long-term follow-up of patients with grade-III and IV spondylolisthesis. Treatment with and without posterior fusion. J Bone Joint Surg Am 69(7): 960-969.
- Mishra A, Agrawal D, Gupta D, Sinha S, Satyarthee GD (2015) Traumatic spondyloptosis: a series of 20 patients. J Neurosurg Spine 22(6): 647-652.
- Evans N, McCarthy M (2018) Management of symptomatic degenerative low-grade lumbar spondylolisthesis. EFORT Open Rev 3(12): 620-631.
- Kirkaldy Willis WH, Farfan HF (1982) Instability of the lumbar spine. Clin Orthop 165:110-123.
- Yue WM, Brodner W, Gaines, RW (2005) Abnormal spinal anatomy in 27 cases of surgically corrected spondyloptosis. Spine 30(6S): S22-S26.
- Sakamaki T, Sairyo K, Katoh S, Endo H, Komatsubara S (2003) The pathogenesis of slippage and deformity in the pediatric lumbar spine: a radiographic and histologic study using a new rat in vivo model. Spine 28: 645-651.
- Kajiura K, Katoh S, Sairyo K, Ikata T, Goel V (2001) Slippage mechanism of pediatric spondylolysis: biomechanical study using immature calf spines. Spine 26: 2208-2213.
- Konz RJ, Goel VK, Grobler LJ, Grosland NM, Spratt KF (2001) The pathomechanism of spondylolytic spondylolisthesis in immature primate lumbar spines. Spine 26: E38-E49.
- Cyron BM, Hutton WC (1978) The fatigue strength of the lumbar neural arch in spondylolysis. J Bone Joint Surg Br 60: 234-238.
- Rosenberg NJ, Bargar WL, Friedman B (1981) The incidence of spondylolysis and spondylolisthesis in non-ambulatory patients. Spine 6: 35-38.
- Jackson DW, Wiltse LL, Cirincoine RJ (1976) Spondylolysis in the female gymnast. Clin Orthop Relat Res 117: 68-73.
- Ferguson RJ, McMaster JH, Stanitski CL (1974) Low back pain in college football linemen. J Sports Med 2: 63-69.
- Boden SD, Riew KD, Yamaguchi K, Branch TP, Schellinger D (1996) Orientation of the lumbar facet joints: association with degenerative disc disease. J Bone Joint Surg Am 78: 403-411.
- Meyerding HW (1932) Spondylolisthesis. Surg Gynecol Obstet 54: 371-377.
- Sairyo K, Katoh S, Takata Y, Terai T, Yasui N, et al. (2006) MRI signal changes of the pedicle as an indicator for early diagnosis of spondylolysis in children and adolescents. Spine 31: 206-211.
- Labelle H, Roussouly P, Berthonnaud E, Dimnet J, O’Brien M (2005) The importance of spino-pelvic balance in L5-S1 developmental spondylolisthesis. Spine 30(6S): S27-S34.
- Debnath UK, Freeman BJC, Grevitt MP, Sithole J, Scammell BE, et al. (2007) Clinical outcome of symptomatic unilateral stress injuries of the lumbar pars interarticularis. Spine 32: 995-1000.
- Sys J, Michielsen J, Bracke P, Martens M, Verstreken J (2001) Nonoperative treatment of active spondylolysis in elite athletes with normal x-ray findings: literature review and results of conservative treatment. Eur Spine J 10: 498-504.
- Klein G, Mehlman CT, McCarty M (2009) Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults. J Pediatr Orthop 29: 146-156.
- Miller RA, Hardcastle P, Renwick SE (1992) Lower spinal mobility and external immobilization in the normal and pathologic condition. Orthop Rev 21: 753-757.
- Fujii K, Katoh S, Sairyo K, Ikata T, Yasui N (2004) Union of defects of the pars interarticularis of the lumbar spine in children and adolescents: the radiological outcome after conservative treatment. J Bone Joint Surg Br 86: 225.
- Cheung EV, Herman MJ, Cavalier R, Pizzutillo PD (2006) Spondylolysis and spondylolisthesis in children and adolescents: II. Surgical management. J Am Acad Orthop Surg 14: 488-498.
- Buck JE (1970) Direct repair of the defect in spondylolisthesis. J Bone Joint Surg Br 52B:432-437.
- Nicol RO, Scott JH (1986) Lytic spondylolysis: repair by wiring. Spine 11: 1027-1030.
- Bradford DS, Iza J (1985) Repair of the defect in spondylolysis or minimal degrees of spondylolisthesis by segmental wire fixation and bone grafting. Spine 10: 673-679.
- Morscher E, Gerber B, Fasel J (1984) Surgical treatment of spondylolisthesis by bone grafting and direct stabilization of spondylolysis by means of a hook screw. Arch Orthop Trauma Surg 103:175-178.
- Kakiuchi M (1997) Repair of the defect in spondylolysis. Durable fixation with pedicle screws and laminar hooks. J Bone Joint Surg Am 79: 818-825.
- Christensen FB, Hansen ES, Laursen M, Thomsen K, Bunger CE (2002) Long-term functional outcome of pedicle screw instrumentation as a support for posterolateral spinal fusion. Spine 27: 1269-1277.
- Moller H, Hedlund R (2002) Instrumented and noninstrumented posterolateral fusion in adult spondylolisthesis. Spine 25: 1716-1721.
- Boos N, Marchesi D, Aebi M (1991) Treatment of spondylolysis and spondylolisthesis with Cotrel-Dubousset instrumentation: a preliminary report. J Spinal Disord 4: 472-479.
- Schnee CL, Freese A, Ansell LV (1997) Outcome analysis for adults with spondylolisthesis treated with posterolateral fusion and transpedicular screw fixation. J Neurosurg 86: 56-63.
- McGuire RA, Amundson GM (1993) The use of primary internal fixation in spondylolisthesis. Spine 18: 1662-1672.
- Carragee E (1997) Single-level posterolateral arthrodesis, with and without posterior decompression, for the treatment of isthmic spondylolisthesis in adults. A prospective, randomized study. J Bone Joint Surg Am 79: 1175-1180.
- Garreau de Loubresse C, Bon T, Deburge A (1996) Posterolateral fusion for radicular pain in isthmic spondylolisthesis. Clin Orthop 323: 194-201.
- Zdeblick TA (1993) A prospective, randomized study of lumbar fusion. Preliminary results. Spine 18: 983-991.
- Ishihara H, Osada R, Kanamori M (2001) Minimum 10-year follow-up study of anterior lumbar interbody fusion for isthmic spondylolisthesis. J Spinal Disord 14: 91-99.
- Cheng CL, Fang D, Lee PC (1989) Anterior spinal fusion for spondylolysis and isthmic spondylolisthesis: long term results in adults. J Bone Joint Surg Br 71: 264-267.
- Kim NH, Lee JW (1999) Anterior interbody fusion versus posterolateral fusion with transpedicular fixation for isthmic spondylolisthesis in adults. A comparison of clinical results. Spine 24: 812-817.
- Naderi S, Manisali M, Acar F, Ozaksoy D, Mertol T, et al. (2003) Factors affecting reduction in low-grade lumbosacral spondylolisthesis. J Neurosurg 99: 151-156.
- Lauber S, Schulte TL, Liljenqvist U, Halm H, Hackenberg L (2006) Clinical and radiologic 2-4 year results of transforaminal lumber interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and2. Spine 31: 1693-1698.
- Rosenberg WS, Praveen VM (2001) Transforaminal lumbar interbody fusion: technique, complications, and early results. Neurosurgery 48: 569-574.
- Humphreys SC, Hodges SD, Patwardhan AG, Eck JC, Murphy RB, et al. (2001) Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine 26: 567-571.
- Madan S, Boeree NR (2002) Outcome of posterior lumbar interbody fusion versus posterolateral fusion for spondylolytic spondylolisthesis. Spine 27: 1536-1542.
- La Rosa G, Conti A, Cacciola F, Cardali S, La Torre D, et al. (2003) Pedicle screw fixation for isthmic spondylolisthesis: does posterior lumbar interbody fusion improve outcome over posterolateral fusion? J Neurosurg 99: 143-150.
- Kwon BK, Albert TJ (2005) Adult low-grade spondylolytic spondylolisthesis evaluation and management. Spine 30(6S): S35-S41.
- Swan J, Hurtwitz E, Malek F, van den Haak E, et al. (2006) Surgical treatment for unstable low-grade isthmic spondylolisthesis in adults: a prospective controlled study of posterior instrumented fusion compared with combined anterior-posterior fusion. Spine J 6: 606-614.
- Johnson JR, Kirwarn EO (1983) The long-term results of fusion in situ for severe spondylolisthesis. J Bone Joint Surg Br 65: 43-46.
- Seitsalo S, Osterman K, Hyvarinen J, Schlenzka D, Poussa M (1990) Severe spondylolisthesis in children and adolescents. A long-term review of fusion in situ. J Bone Joint Surg Br 72: 259-265.
- Newton PO, Johnston CE (1997) Analysis and treatment of poor outcomes following in situ arthrodesis in adolescent spondylolisthesis. J Pediatr Orthop 17: 754-761.
- Molinari RW, Bridwell KH, Lenke LG, Ungacta FF, Riew KD (1999) Complications in the surgical treatment of pediatric high-grade isthmic dysplastic spondylolisthesis. A comparsion of three surgical techniques. Spine 24: 1701-1711.
- Muschik M, Zippel H, Perka C (1997) Surgical management of severe spondylolisthesis in children and adolescents: anterior fusion in situ versus anterior spondylodesis with posterior transpedicular instrumentation and reduction. Spine 22: 2036-2042.
- Lamberg T, Remes V, Helenius I, Schlenzka D, Seitsalo S, et al. (2007) Uninstrumented in situ fusion for high-grade childhood and adolescent isthmic spondylolisthesis: long-term outcome. J Bone Joint Surg Am. 89: 512-518.
- Goyal N, Wimberley DW, Hyatt A, Zeiller S, Vacarro AR, et al. (2009) Radiographic and clinical outcomes after instrumented reduction and transforaminal lumbar interbody fusion of mid and high-grade isthmic spondylolisthesis. J Spinal Disord Tech 22: 321-327.
- Poussa M, Remes V, Lamberg T, Tervahartiala P, Schlenzka D, et al. (2006) Treatment of severe spondylolisthesis in adolescence with reduction or fusion in situ: long-term clinical, radiologic, and functional outcome. Spine 31: 583-590.
- Petraco DM, Spivak JM, Cappadona, JG, Kummer FJ, Neuwirth MG (1996) An anatomic evaluation of L5 nerve stretch in spondylolisthesis reduction. Spine 21: 1133-1138.
- Gaines RW (2005) L5 vertebrectomy for the surgical treatment of spondyloptosis. Spine 30(6S): S66-S70.
- Lehmer SM, Steffee AD, Gaines RW (1994) Treatment of L5-S1 spondyloptosis by staged L5 resection with reduction and fusion of L4 onto S1 (Gaines procedure). Spine 19: 1916-25.
- Epstein NE (1998) Decompression in the surgical management of degenerative spondylolisthesis: advantages of a conservative approach in 290 patients. J Spinal Disord 11: 116-23.
- Kleeman TJ, Hiscoe AC, Berg EE (2000) Patient outcomes after minimally destabilizing lumbar stenosis decompression. Spine 25: 865-870.
- Marjedtko SM, Connolly PJ, Shott S (1994) Degenerative lumbar spondylolisthesis: a meta-analysis of the literature 1970-1193. Spine 19(suppl): S2256-2265.
- Herkowitz HN, Kurz LT (1991) Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am 73: 802-808.
- Fischgrund JS, Mackay M, Herkowitz HN, Brower R, Montgomery DM, et al. (1997) 1997 volvo award winner in clinical studies: degenerative lumber spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine 22:2807-2812.
- MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, et al. (2004) Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective long-term study comparing fusion and pseudoarthrosis. Spine 29: 726-734.
- Martin CR, Gruszczynski AT, Braunsfurth HA, Fallatah SM, O’neil J, et al (2007). The surgical management of degenerative lumbar spondylolisthesis. A systematic review. Spine 32: 1791-1798.
- Takahashi K, Kitahara H, Yamagata M (1990) Long-term results of anterior interbody fusion for treatment of degenerative spondylolisthesis. Spine 15: 1211-1215.
- Inoue S, Watanabe T, Goto S (1988) Degenerative spondylolisthesis: pathophysiology and results of anterior interbody fusion. Clin Ortop 227: 90-98.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...

.png)