Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6679
Research Article(ISSN: 2637-6679) 
Polymorphic Estimation of Clopidogrel hydrogen sulfate Form-II in Clopidogrel hydrogen sulfate Form-I by X-ray diffractometer Volume 7 - Issue 2
Amit Gosar, Shivaji Jadhav, Mithun Gharat*, Mahesh Patil and Dyandev Kadu
- Amit Gosar, Shivaji Jadhav, Mithun Gharat*, Mahesh Patil and Dyandev Kadu
Received: December 07, 2021 Published: December 15, 2021
Corresponding author: Mithun Gharat, Department of Chemistry, University of Mumbai, India
DOI: 10.32474/RRHOAJ.2021.07.000257
Abstract
Clopidogrel hydrogen sulfate Form-I possibly may convert into Clopidogrel hydrogen sulfate Form-II and determination of Clopidogrel hydrogen sulfate form-II content with accurate quantitation is difficult and Clopidogrel hydrogen sulfate Form-II determination method is not reported in literature. Hence, novel specific and accurate method has been developed for the Polymorph estimation of Clopidogrel hydrogen sulfate Form-II by X-ray crystallography. The method has been developed and validated as per International conference on Harmonization guidelines (Q2R1) and found out specific Linear, Precise and accurate in validation. The described method is accurate and validated and successfully applied for the Polymorph estimation of Clopidogrel hydrogen sulfate Form-II in Clopidogrel hydrogen sulfate Form-I.
Keywords: Breast cancer; Softmax function; Adam algorithm; Biological activity
Introduction
Clopidogrel, sold as the brand name Plavix among others. is an antiplatelet medication that is used to reduce the risk of heart disease and stroke in those at high risk [1]. It is also used together with aspirin in heart attacks and following the placement of a coronary arterystent. It is taken by mouth. Onset of effects is about 2 hours and lasts for 5 days [2]. Clopidogrel is in thethienopyridine-class of antiplatelets [3]. It works by irreversibly inhibiting a receptor called P2Y12 on platelets [4,5]. Clopidogrel hydrogen sulfate is a pharmaceutical Active Ingredient with a novel mechanism of action for the reduction of atherosclerotic events. There are only two crystalline forms Clopidogrel hydrogen sulfate I and Clopidogrel hydrogen sulfate II among the six known polymorphs of Clopidogrel hydrogen sulfate have therapeutic activity. The structure of the Clopidogrel hydrogen sulfate I polymorph is unknown and the structure of the Clopidogrel hydrogen sulfate II polymorph is known only partially. In past, two techniques of X-ray diffraction quantitative phase analysis have been developed in this work for the quantification of Clopidogrel hydrogen sulfate I and Clopidogrel hydrogen sulfate II in their mixtures. The first technique is based on use of the whole powder pattern decomposition method. The second technique is based on the classical direct method. Both methods show lower sensitivity towards detection [6,7]. X-ray powder diffraction is a powerful tool to detect crystalline phases, the sensitivity of the laboratory instruments can be a serious limitation with respect to Detection of multiple physical forms of the Active Pharmaceutical Ingredient in the drug substance and drug products during processing and storage [8]. The analysis data available is of by DSC, TGA with Hot Stage Microscopy, FTIR and XRD. In X-ray diffraction analysis, Uvarov and Popov have developed the Clopidogrel phase content using Xray powder pattern decomposition and classical direct methods. The limit of detection was found to be 1.0-1.5 wt.% in both methods. In another quantitative analysis, Nemet and coworkers combined IR and Raman spectroscopy with chemometrics and quantified 2% and 3% of Form II in Form I, respectively, with more than 1% limit of detection. Recently, a microscopy combined with image analysis was developed [9]. In this study, X-ray microtomography was used for selective and sensitive detection of clopidogrel bisulphate polymorphs. The limit of detection was also found to be 1%. From all above data search, it was found that technique available to detect Clopidogrel Polymorph is about 1% [10,11]. The Quantitative estimation of Clopidogrel hydrogen sulfate form-II by X-ray diffraction is found available with High limit of detection value more than 1%. Hence, the method was not available with limit of detection less than 1%. Accordingly, experimental, and exploratory work has been carried out and successfully delineate in this invention [12].
Experimental Work
Experimental work is carried out for Polymorphic estimation of Clopidogrel hydrogen sulfate Form-II in Clopidogrel hydrogen sulfate Form-I by X-ray powder diffractometer.
Materials and Reagent
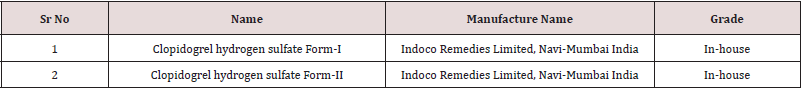
Clopidogrel hydrogen sulfate form-I, Clopidogrel hydrogen sulfate form-II was obtained from Indoco Remedies Ltd, Navi Mumbai, India. The primary goal was to develop method to obtain the most stable, reproducible, consistent method. Various Steps were taken during method development as mentioned below.
Preparation of Samples
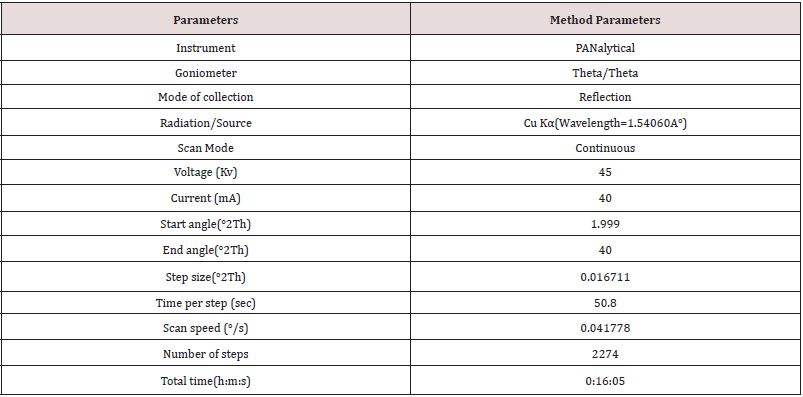
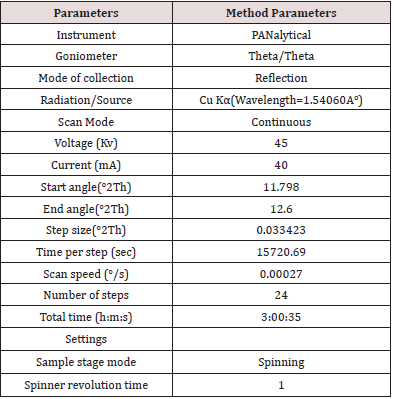
Prepared the sample holder of Clopidogrel hydrogen sulfate Form-II and Clopidogrel hydrogen sulfate Form-I in a PAN alytical sample holder with back loading technique, using sample holder tool kit and carried out the analysis as per instrumental conditions mentioned in (Tables 1 & 2).
Observation
From the overlay diffractogram of Clopidogrel hydrogen sulfate Form-II and Clopidogrel hydrogen sulfate Form-I, it was observed that the Characteristic peak at 2θ 12.3° of Clopidogrel hydrogen sulfate Form-II was not having any interference from Clopidogrel hydrogen sulfate Form-I and shall be selected as characteristic peak for determination of Clopidogrel hydrogen sulfate Form- II content in Clopidogrel hydrogen sulfate Form-I. For better response Trial-2 to be taken with change in Time per step from 50.800(s) to 299.625(s) and scan range from 2°-40° to 11.798° to12.6° and Proceeded for next trial of 1 Hr. slow scan method with concentration of 5% level of Clopidogrel hydrogen sulfate Form II spiked in Clopidogrel hydrogen sulfate Form I to check the effect of peak intensity of marker peak of Clopidogrel hydrogen sulfate Form II standard at 2θ 12.3°.
a) Preparation of 5% spiked Clopidogrel hydrogen sulfate form-II in Clopidogrel hydrogen sulfate form-I
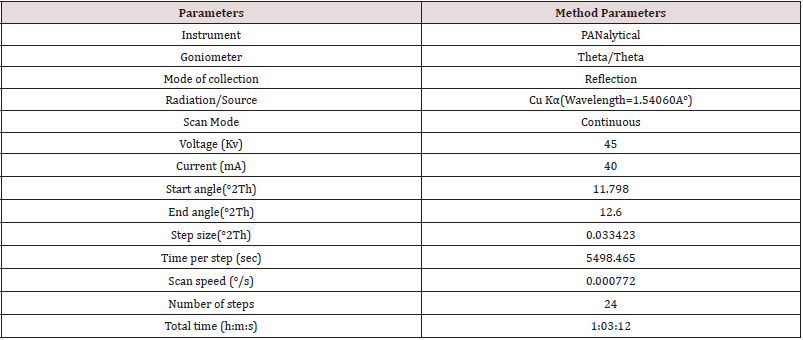
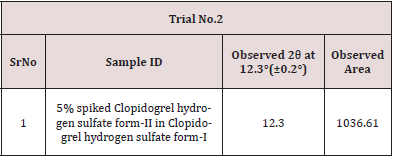
Mixed 25.17 mg of Clopidogrel hydrogen sulfate Form-II (B.No: CLOPIDOGREL HYDROGEN SULFATE -II/17001) in 475.34 mg of Clopidogrel hydrogen sulfate form-II (B.No: CLOPIDOGREL HYDROGEN SULFATE -I/18001) by geometric mixing. Then filled the mixture in a PANalytical sample holder with back loading technique, using sample holder tool kit and carried out the analysis as per 1-hour slow scan method as described in Table 2 by changing in time per step from 50.8 sec to 5498.465 sec and run time from 00:16:05(h:m:s) to 01:03:12(h:m:s) and recorded data in Table 3.
Result
As from above observation, 1-hour slow scan method as described in method of Table 2 is able to detect of Clopidogrel hydrogen sulfate form-II. Further, to increase the sensitivity of the method next Trial No.3 with 3- hour slow scan shall be performed.
a) Preparation of 5% spiked Clopidogrel hydrogen sulfate form-II in Clopidogrel hydrogen sulfate form-I
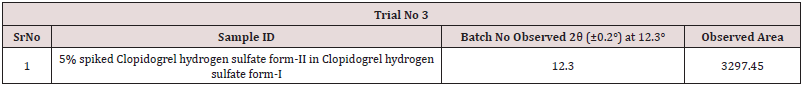
Mixed 25.24 mg of Clopidogrel hydrogen sulfate Form-II (B.No: CLOPIDOGREL HYDROGEN SULFATE -II/17001) in 475.26 mg of Clopidogrel hydrogen sulfate form-II (B.No: CLOPIDOGREL HYDROGEN SULFATE -I/18001) by geometric mixing. Thenfilled the mixture in a PANalytical sample holder with back loading technique, using sample holder tool kit and carried out the analysis as per 3-hour slow scan method as described in Table 4 by changing in time per step from 5498.465 sec to 15720.690 sec and run time from 01:03:12 (h:m:s) to 03:00:35 (h:m:s) and recorded data in Table 5. From the above observation table, slow scan method of Trial No.3 with run time 03:00:35(h:m:s) is found suitable for determination of Clopidogrel hydrogen sulfate form-II in Clopidogrel hydrogen sulfate form-I. The suitability of the method can be confirmed by performing Validation includes parameters such as Specificity, Linearity & LOD LOQ determination, Precision, LOQ Precision and Accuracy.
Exploratory Work
The validation work was conducted according to the ICH (International Conference on Harmonization) guidelines Q2R1.
Specificity
The Specificity of the method is confirmed by compiling data from finalized Step 3 are tabulated as follows, From the overlay diffractogram, There is no significance interference observed in Clopidogrel hydrogen sulfate Form-I from Clopidogrel hydrogen sulfate form-II at 12.3 (±0.2°). Hence, method is found Specific (Tables 6-8).
Linearity, Lod and Loq Determination
a) Preparation of Linearity level
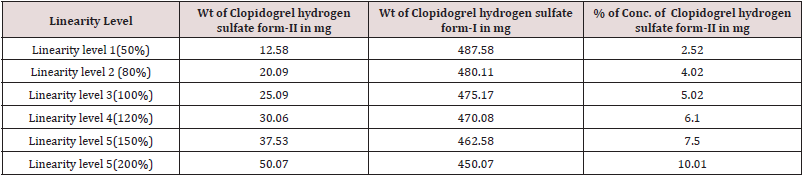
For preparation of Linearity levels, Clopidogrel hydrogen sulfate form-II was mixed in Clopidogrel hydrogen sulfate form-I using geometric mixing technique in different proportion to obtain linearity levels as mentioned in Table 9. The same linearity data was used to determine LOD, LOQ using Standard deviation method.
b) Procedure
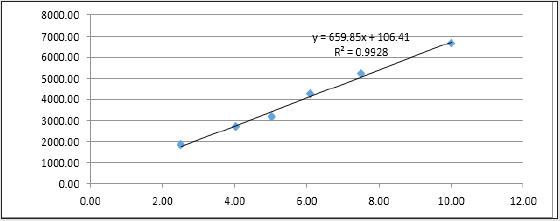
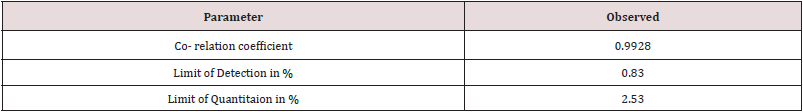
The sample was analysed as per Trial No-3. Peak area observed at 12.3° (2-Theta) was then used to calculate Co-relation coefficient(R) and the data is recorded in Table 10. The Linearity graph was plotted for peak area at 12.3° (2-theta) against %Conc. of Clopidogrel hydrogen sulfate form-II (Figure 1). From the above data calculated limit of detection and limit of quantitation for Clopidogrel hydrogen sulfate form-II in Clopidogrel hydrogen sulfate Form-I by formula given below and recorded the data in Table 9.
c) Calculation
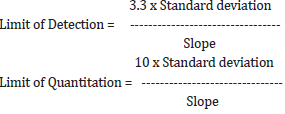
Conclusion
From the above data, it is observed that the method is linear in range 0.5% to 2.0% of Clopidogrel hydrogen sulfate form-II in Clopidogrel hydrogen sulfate form-I with regression coefficient 0.9928 and the LOD, LOQ is established as 0.83 % & 2.53 % respectively.
Precision
Preparation of 100% Level Preparation of 5% spiked Clopidogrel hydrogen sulfate form-II in Clopidogrel hydrogen sulfate Form-I
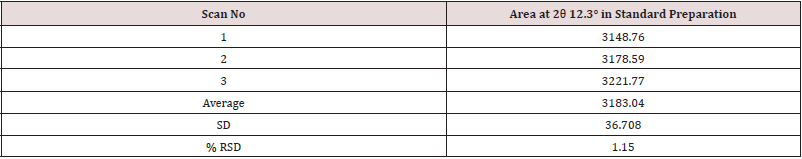
Mixed 25.09 mg of Clopidogrel hydrogen sulfate Form-II in 475.17 mg of Clopidogrel hydrogen sulfate form-I using mixer mill machine by geometric mixing. Then filled the mixture in a PANalytical sample holder with back loading technique. Then loaded the sample holder between the X-ray optics path and scan using the instrumental conditions mentioned in (Tables 11 & 12). The %RSD for the area of significant peak of Clopidogrel hydrogen sulfate form-II at about 12.3° 2θ in 3 Standard preparations (5%) is found 1.15%.
Precision at Limit of Quantitation (Loq)
Preparation of 100% Level Preparation of 5% spiked Clopidogrel hydrogen sulfate form-II in Clopidogrel hydrogen sulfate Form-I
Mixed 25.09 mg of Clopidogrel hydrogen sulfate Form-II in 475.17 mg of Clopidogrel hydrogen sulfate form-I using mixer mill machine by geometric mixing. Then filled the mixture in a PANalytical sample holder with back loading technique. Then loaded the sample holder between the X-ray optics path and scan using the instrumental conditions mentioned in Table 4.
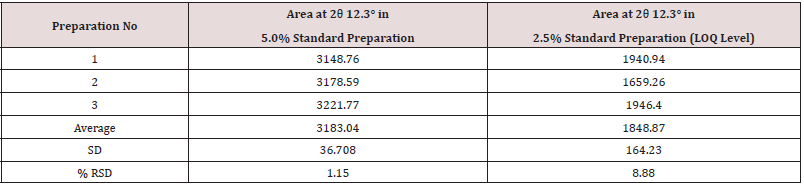
Preparation of 50% Level (LOQ Level=2.53%)
Mixed 12.58 mg of Clopidogrel hydrogen sulfate Form-II in 487.58 mg of Clopidogrel hydrogen sulfate form-I using mixer mill machine by geometric mixing. Then filled the mixture in a PANalytical sample holder with back loading technique. Then loaded the sample holder between the X-ray optics path and scan using the instrumental conditions mentioned in Table 13.
Observations
Result
The %RSD for the area of Clopidogrel hydrogen sulfate form-II at 12.3° in LOQ level is found 8.88 %. Hence, the method is validated for precision at LOQ level.
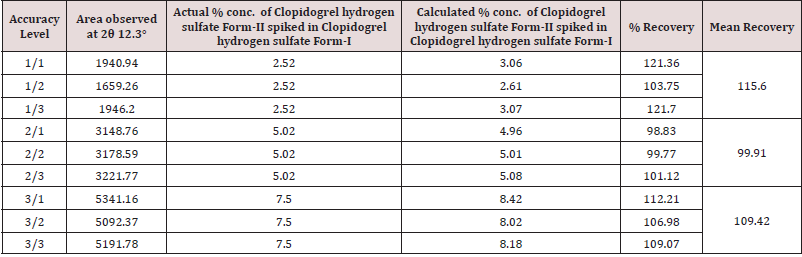
Accuracy at Different Levels
Procedure Preparation of Accuracy level
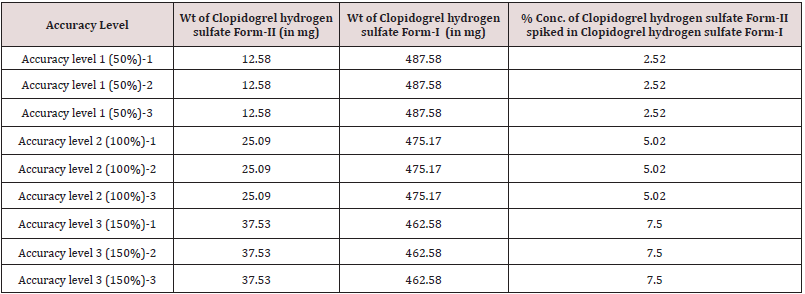
For preparation of Accuracy levels, Clopidogrel hydrogen sulfate form-II was mixed in Clopidogrel hydrogen sulfate form-I using geometric mixing technique in different proportion to obtain Accuracy levels as mentioned in Table 14 and carried out the analysis for each level in triplicate preparation as per instrument parameter mentioned in Step-3. Calculated % Recovery for Clopidogrel hydrogen sulfate form-II in Clopidogrel hydrogen sulfate form-I and recorded the data in Table 15.
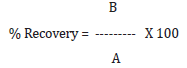
Result
The %RSD for the area of significant peak of Clopidogrel hydrogen sulfate form-II at about 12.3° 2θ in 3 Standard preparations (5%) is found 1.15%. Mean % recovery value at 50% (LOQ), 100% and 150% level concentration is found 115.60%, 99.91% and 109.42% respectively. Hence, Method is validated for accuracy at 50% (LOQ), 100% and 150% concentration levels.
Results and Conclusion
Method as defined in Step-3 with run time 03:00:35mins is found Specific, Precise, Linear with Co-relation coefficient =0.9964, LOQ= 2.53% and LOD= 0.83% respectively, LOQ Precision with % RSD of 8.88 and Mean % recovery value at 50% (LOQ), 100% and 150% level concentration is found 115.60%, 99.91% and 109.42% respectively. The method as defined in Step 3 is validated for Specificity, Precision, Linearity, LOD and LOQ Determination, LOQ Precision and Accuracy. Hence, the method is found suitable for its intended use to determine polymorphic content of Clopidogrel Hydrogen Sulfate Form II in Clopidogrel Hydrogen Sulfate Form I by X-Ray Diffractometer.
References
- Lestari, Maria LAD, Gunawan Indrayanto, and Harry G Brittain (2010) Clopidogrel bisulfate. Profiles of drug substances, excipients and related methodology. Academic Press 35: 71-115.
- Mannacio VA, Di Tommaso L, Antignan A (2012) Aspirin plus clopidogrel for optimal platelet inhibition following off-pump coronary artery bypass surgery: results from the CRYSSA (prevention of Coronary arteRY bypaSS occlusion After off-pump procedures) randomised study. Randomized Controlled Trial 98(23):1710-1715.
- Farfan, Silvia (2012) Comparative dissolution and polymorphism study of clopidogrel bisulfate tablets available in Argentine. Journal of Applied Pharmaceutical Science 10.10 (2020): 62-71.
- Damman, Peter (2012) P2Y12 platelet inhibition in clinical practice. Journal of thrombosis and thrombolysis 33(2): 143-153.
- Jeong, Young-Hoon (2010) Carriage of cytochrome 2C19 polymorphism is associated with risk of high post-treatment platelet reactivity on high maintenance-dose clopidogrel of 150 mg/day: results of the ACCEL-DOUBLE (Accelerated Platelet Inhibition by a Double Dose of Clopidogrel According to Gene Polymorphism) study. JACC: Cardiovascular Interventions 3(7): 731-741.
- Uvarov V, Popov I (2008) Development and metrological characterization of quantitative X-ray diffraction phase analysis for the mixtures of clopidogrel bisulphate polymorphs. Journal of Pharmaceutical and Biomedical Analysis 46(4): 676-682.
- Azhad Chowdhury (2016) Understanding and Evaluating Crystal Polymorphism By Second Harmonic Generation Microscopy.
- Chowdhury Purdue University Department of Pharmaceutical Technology (Formulations), National Institute of Pharmaceutical Education and Research (NIPER).
- Lee, Alfred Y, Deniz Erdemir, and Allan S Myerson (2011) Crystal polymorphism in chemical process development. Annual review of chemical and biomolecular engineering (2011): 259-280.
- (2021) Applications of synchrotron powder X-ray diffractometry in drug substance and drug product characterization Citation DataTrAC Trends in Analytical Chemistry 136 : 116-181.
- Brittain, Harry G (2012) Polymorphism and solvatomorphism 2010. Journal of pharmaceutical sciences 101(2): 464-484.
- Liu Y, Huang HW, Wu JM, Shi YQ, Yang LH (2013) Polymorphs of clopidogrel bisulfate. Yao Xue Xue Bao. 48(8): 1358-1360.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...

.png)