Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6679
Review Article(ISSN: 2637-6679) 
Molecular detection of aflatoxin producing Aspergillus species isolates in some chicken meat cuts in Gharbiya governorate, Egypt Volume 7 - Issue 1
Shaltout FA1, Heikal GI2 and Ghanem AM3*
- 1Food Hygiene and Control Department, (Meat Hygiene), Faculty of Veterinary Medicine, Benha University, Egypt
- 2Food Hygiene Department, Animal Health Research Institute (Tanta branch), Egypt
- 3Food Security Sector, Egyptian Armed Forces, Egypt
Received: November 22, 2021 Published: December 06, 2021
Corresponding author: Ayman Ghanem, Department of Food Hygiene and Control (Meat Hygiene), Faculty of Veterinary Medicine, Benha University, Egypt
DOI: 10.32474/RRHOAJ.2021.07.000255
Abstract
Contamination with fungi and their toxins is considered one of the most dangerous hidden pollutants that threaten the health of the consumer. The presence of mycotoxins in various foods has been recorded, despite their apparent safety for human consumption. Therefore, the current study was conducted to evaluate the prevalence of Aspergillus species by culture method; and aflatoxinproducing genes molecularly in total of 75 random samples of chicken cuts represented by wing, breast and thigh (25 of each) that were collected from various groceries and poultries shops located at Gharbiya governorate, Egypt. Results of culture and isolation techniques revealed detection of Aspergillus sp. in 36, 48 and 44% of the examined wing, breast and thigh samples, respectively. Moreover, microbiological identification of the isolated strains showed presence of A. niger, A. flavus, A. fumigatus, A. terreus and A. parasiticus in 16, 13.3, 10.6, 1.3 and 1.3% of the total population of the examined samples. Molecular detection of some aflatoxin production regulating genes (OmtA, Nor1 and Ver1) in ten Aspergillus sp. isolates revealed their detection in 8/10 (80%), 8/10 (80%) and 7/10 (70%) represented by positive bands at molecular weight of 1024, 400 and 537 bp, respectively. Referring to the recorded results, chicken cuts may possess a great silent hazard to the human being under improper good manufacturing practices and inadequate hygienic conditions during handling and storage.
Keywords: Aspergillus Species; Chicken Meat Cuts; Cpcr; Egypt
Introduction
Chicken meat and meat products production in developing countries plays an essential role in supporting food security and poultry meat demands Wong et al. [1]. Contamination of meat products with molds can be occur during different preparation stages during slaughtering under bad hygienic conditions using contaminated water or by adding contaminated spices with mold spores or during packing, handling, transportation and storage Khalalfalla et al. [2]. Contamination of meat with Aspergillus species, especially Flavi section, is one of the most hazardous microbial contamination as the majority of Aspergillus species are able to aflatoxins production that can cause diseases associated with aflatoxin poisoning and carcinogenic effects Leggieri et al. [3]. Acute aflatoxin poisoning may lead to death as was recorded in Kenya in 2004 Probst et al. [4], while chronic poisoning may lead to various recorded mutagens and cancers Benkerroum [5]. Aspergillus sp. was classified into two groups depending on their toxigenic impacts on food and human health; 1st group includes the aflatoxigenic species such as A. flavus and A. parasiticus, while the 2nd group contains the non-aflatoxin-producing species such as A. tamarii and A. oryzae Frisvad et al. [6]. Molecular analyses have been used to confirm aflatoxin productivity of Aspergillus species isolates. omtA, nor1 and ver1 genes are from the commonly used genes encoded aflP, aflD and aflM toxins detection in food items Sohrabi and Taghizadeh [7] yield an accurate, rapid and reliable records of toxigenic aspergillus species especially in food chain Sadhasivam et al. [8]. Therefore, the main target of the current study was to investigate the presence of toxigenic aspergillus species in some chicken meat cuts collected from Gharbiya Governorate markets, Egypt.
Material and Methods
Collection of samples : A total of seventy-five random samples of
raw chilled chicken wing, chicken thigh, chicken breast (25 of each)
was collected from different local poultries shops and different
supermarkets at Gharbia governorate, Egypt. Samples were taken
aseptically in polyethylene bags and were transferred to the
laboratory in ice box for mycological examination.
Preparation of samples (ISO [9]): Twenty-five grams from each
sample were carefully and aseptically homogenized in blinder after
mixing with 225 ml of sterile peptone water 0.1% to form a dilution
of 1:10, from which tenth fold serial dilutions were prepared.
Determination of Aspergillus species
Culture of the prepared samples was performed according to
ISO [10], where 0.1ml of the previously prepared serial dilutions
was spreaded by mean of sterile L-shape glass rod over two Petridishes
contained solidified Dichloran Rose Bengal agar with
chloramphenicol (DRBC) then were incubated at upright position
at 25Oc for 5 -7 days.
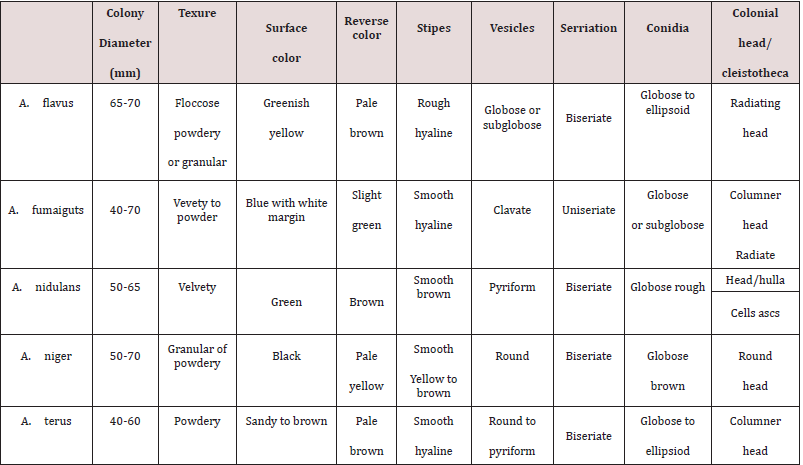
Identification of isolated strains was performed according
to Pitt and Hocking [11] macroscopically and microscopically as
recorded in Table 1.
Molecular detection of some aflatoxin producing genes of some
isolated Aspergillus strains by cPCR
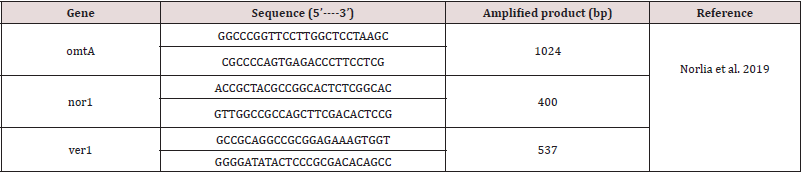
Oligonucleotide primers used in cPCR
Three pairs of omtA, nor1 and ver1 primers were prepared and collected from Metabion (Germany). Their special sequence and amplify certain products as were be displayed in Table 2.
Mycological DNA was extracted following QIAamp DNeasy
Plant Mini kit Catalogue no. 69104.
Preparation of master mix and thermal profile was adapted
according to the manufacturer instructions (Emerald Amp GT PCR
mastermix (Takara) Code No. RR310A).
Results
As recorded in Table 3, Aspergillus sp. was detected in 32(42.6%) of the total examined samples. In detail, breast samples recorded the highest contamination level (48%); followed by thigh and wing samples, respectively. Regarding with the genus identification, A. niger had the highest detection levels (16%) in the examined samples (Table 4). Referring to the obtained results of molecular detection of some aflatoxin producing genes as recorded in (Table 5) and (Figures 1-3); omtA, nor1 and ver1 genes were detected in 8/10 (80%), 8/10 (80%) and 7/10 (70%) of the examined A. flavus isolates, respectively. presence of these genes indicated the producibility of the examined strain for aflatoxins P, D and M, respectively.
Table 3: Prevalence of Aspergillus species in the examined chicken meat cut samples (n= 25 of each).
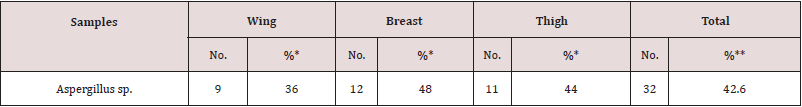
%* prevalence in relation to the number of each sample (25).
%** prevalence in relation to the total number of samples population (75).
Table 4: Prevalence of identified aspergillus sp. in the examined chicken meat cuts (n= 25 of each).
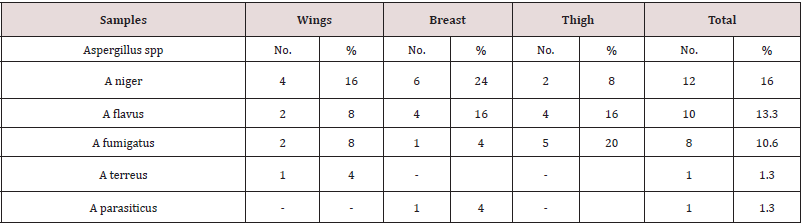
Table 5: Prevalence of aflatoxin producing genes in A. flavus isolates from the examined samples (n= 10).
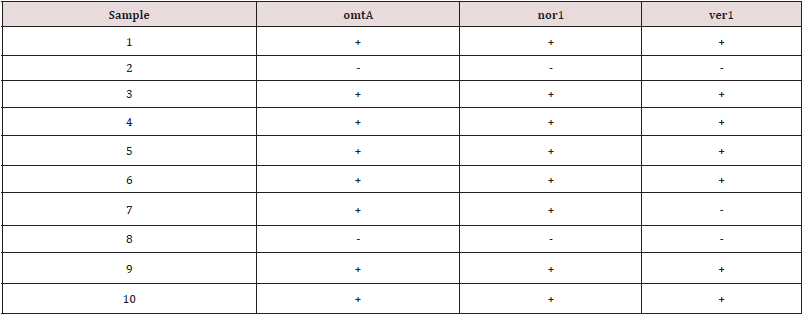
Figure 1: Agarose gel electrophoresis of cPCR of omtA (1024 bp) gene of A. flavus.
Lane L: 100 bp ladder as molecular size DNA marker.
Lane P: Control positive A. flavus for omtA gene.
Lane N: Control negative.
Lanes 1, 3, 4, 5, 6, 7, 9 and 10: Positive A. flavus for omtA gene.
Lanes 2 and 8: Negative A. flavus for omtA gene.
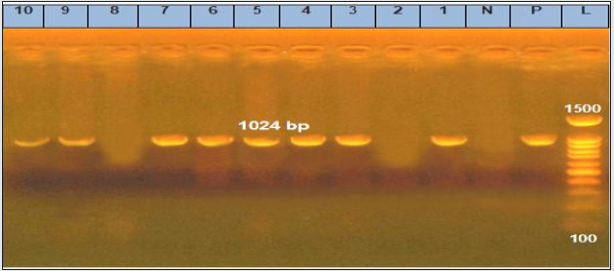
Figure 2: Agarose gel electrophoresis of cPCR of nor1 (400 bp) gene of A. flavus.
Lane L: 100 bp ladder as molecular size DNA marker.
Lane P: Control positive A. flavus for nor1 gene.
Lane N: Control negative.
Lanes 1, 3, 4, 5, 6, 7, 9 and 10: Positive A. flavus for nor1 gene.
Lanes 2 and 8: Negative A. flavus for nor1 gene.
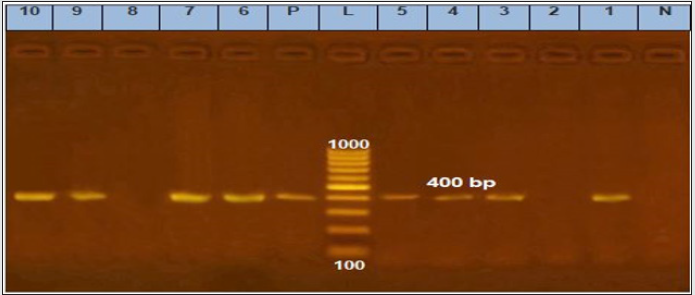
Figure 3: Agarose gel electrophoresis of cPCR of ver1 (400 bp) gene of A. flavus.
Lane L: 100 bp ladder as molecular size DNA marker.
Lane P: Control positive A. flavus for ver1 gene.
Lane N: Control negative.
Lanes 1, 3, 4, 5, 6, 9 and 10: Positive A. flavus for ver1 gene.
Lanes 2, 7 and 8: Negative A. flavus for ver1 gene.
Discussion
Chicken meat and meat products comply an important source of human protein supplement all over the world because they provide good source of digestible protein, low cholesterol fat, essential amino acids, minerals, and different types of vitamins and minerals. In Egypt, as well as human population increasing, demand of animal proteins also is increasing represents a serious challenge in which poultry industry plays an essential role in filling nutrition gap as a rapid and more economic source of proteins (Shaltout et al. [12]). Mold contamination of meat and meat products have been considered a serious source of food spoilage resulting in different organoleptic changes in flavor, color, texture, odor referred mainly to the fungal deterioration especially in poor developing countries due to lack of hygienic measures during processing and handling (Lorenzo et al. [7]). Presence of mold in foods may be referred to the rapid, easy disperse and wide spread of the fungal spores which are abundant in the environment introducing food chain through dust, water, workers and equipment. Their presence in food samples is a serious public health concern as these fungi may be associated with the production of mycotoxins (Benedict et al. [13]). Aspergillus species represents an important mycotic infection in public health concern as a human pathogen and as toxin-producing food contaminant. It releases a lot of spores which found in air, water, soil, plant debris, manure and animal feed. As fungal spore’s growing, it secretes digestive enzyme and mycotoxins leading to food spoilage and human mycotoxicosis (Richardson and Rautemaa-Richardson [14]). Referring to the recorded results in Table 3, Aspergillus sp. was prominently detected in breast samples other than wings and thighs samples, which came in agree with the previously recorded results of Darwish et al. [15] and Shaltout et al. [16] who found that the examined breast samples were more contaminated with fungal infection than wing and thigh samples. While the current prevalence of aspergillus species in the examined samples came lower than those recorded by Hassan [17] who found Aspergillus sp. in all the examined samples (100%) collected from Gharbiya governorate, Egypt. Moreover, Abuzaid et al. [18] also detected A. flavus and A. niger in 40 and 80% of the examined sausage samples of chicken origin, respectively. Referring to the obtained results of the microbiological identification of Aspergillus sp. isolates as recorded in Table 4, they came in agree with the previously reported results by Darwish et al. [15] who found that A. niger was the predominant detected strain, followed by A. flavus and A. parasiticus in the examined samples of chicken cuts collected from Zagazig city, Egypt. Some mold species can cause respiratory infections representing a significant risk for individual with severely weakened immune system (OSHA [19]). Presence of mold in high incidence indicate bad hygienic measures adopted during handling, preparation and processing El Abbasy [20]. Mycotoxins have been defined as naturally occurring secondary fungal metabolites produced in meat and meat products by direct growth of toxigenic molds such as Aspergillus species which produce Aflatoxins and Ochratoxins which threat public health due to their carcinogenic, hepatotoxic, nephrotoxic, teratogenic and mutagenic effects in human and animals Agriopoulou et al. [21]. Aflatoxins are produced by a polyketide pathway that pass through about twenty-seven enzymatic reactions which have been regulated by sets of genes including nor-1, ver-1 and omtA have been shown to be involved in this process. aflD (nor-1) encodes a norsolorinic acid ketoreductase needed for the conversion of the 10-keto group of Norsolorinic Acid (NOR) to the 10-hydroxyl group of Versicolorin A (VERA) Zhou and Linz [22]. aflM (ver-1), predicted to encode a ketoreductase, is involved in the conversion of VERA into Sterigmatocystin (ST) Henry and Townsend [23]; aflP (omtA) codes for O-methyltransferase, which is one of the main genes responsible for transforming ST into O-methylsterigmatocystin (OMST) that is the precursor for aflatoxin production Yabe et al. [24]. Many other previous studies recorded detection of these genes in their Aspergillus isolates of food origin by various PCR techniques; Manonmani et al. [25], Rodrigues et al. [26], and Hassan et al. [27], who conducted several studies investigating the aflatoxigenicity of Aspergillus sp., could detect different genes in their Aspergillus isolates [28,29].
Conclusion
It could be concluded that, breast samples revealed the highest contamination levels with Aspergillus sp.; in addition, A. niger was the prominently detected strain. PCR technique is a unique diagnostic tool for detection and identification of aflatoxigenic Aspergillus strains especially if the field of food safety. So, application of strict hygienic measures, proper use of water supply and food additives from good sources is recommended.
References
- Wong JT, Bruyn JD, Bagnol B, Grieve H, Li M, Pym R and Alders RG (2017) Small-scale poultry and food security in resource-poor settings: A review. Glob Food Secur 15: 43-52.
- Khalalfalla FA, Ali FHM and Saif-Alnasr MM (2017) Microbiological quality of retail meats. J Vet Med Res 24(2): 311-321.
- Leggieri MC, Toscano P and Battilani P (2021) Predicted aflatoxin B1 increase in Europe due to climate change: actions and reactions at global level. Toxins 13(4): 292.
- Probst C, Njapau H and Cotty PJ (2007) Outbreak of an acute aflatoxicosis in Kenya in 2004: identification of the causal agent Appl Environ. Microbiol 73(8): 2762-2764.
- Benkerroum N (2020) Chronic and acute toxicities of aflatoxins: mechanisms of action. Inter Environ. Res Pub Hlth 17(2): pp. 423.
- Frisvad JC, Hubka V, Ezekiel CN, Hong SB, Nováková A, and Chen AJ, et al. (2019) Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Studies in Mycology 93: 1-63.
- Lorenzo JM, Munekata PE, Dominguez R, Pateiro M, Saraiva JA and Franco D (2018) Main groups of microorganisms of relevance for food safety and stability: general aspects and overall description. Innov Technol Food Preserv 53-107.
- Sadhasivam S, Britzi M, Zakin V, Kostyukovsky M, Trostanetsky A, Quinn, E, and Sionov E (2017) Rapid detection and identification of mycotoxigenic fungi and mycotoxins in stored wheat grain. Toxins 9(10): 302-310.
- International Organization for Standardization (2017) Microbiology of the food chain - Preparation of test samples, initial suspension and decimal dilutions for microbiological examination - Part 1: General rules for the preparation of the initial suspension and decimal dilutions.
- International Organization for Standardization (2008) Microbiology of food and animal feeding stuffs-Horizontal method for the enumeration of yeasts and moulds - Part 1: Colony count technique in products with water activity greater than 0,95.
- Pitt JI and Hocking AD (2009) Fungi and Food Spoilage. 2nd Ed. Springer Boston MA Ch 4: p.19.
- Shaltout FA, Zakaria IM, Eltanani J and Elmelegy AS (2015) Microbiological status of meat and chicken received to University student hostel. Benha Vet Med J 29(2): 187-192.
- Benedict K, Chiller TM and Mody RK (2016) Invasive fungal infections acquired from contaminated food or nutritional supplements: A review of the literature. Foodborne Pathogens and Disease 13(7): 343-349.
- Richardson M and Rautemaa Richardson R (2019) Exposure to Aspergillus in home and healthcare facilities' water environments: focus on biofilms. Microorganisms 7(1): 7-12.
- Darwish WS, El Bayomi RM, Abd El-Moaty AM and Gad TM (2016) Mould contamination and aflatoxin residues in frozen chicken meat-cuts and giblets. Jap J Vet Res 64(2): 167-171.
- Shaltout FA, Nasief MZ, Lotfy LM and Gamil BT (2019) Microbiological status of chicken cuts and its products. Benha Vet Med J 37: 57-63.
- Hassan W (2019) Some studies on effect of turmeric and lemon extracts on fungal contamination of chicken meat. Thesis, Master of Vet Med (Meat Hygiene), Benha Univ, Egypt.
- Abuzaid KE, Shaltout FA, Salem R and El Diasty EM (2020) Microbial aspect of some processed meat products with special reference to aflatoxins. Benha Vet Med J 39: 24-28.
- OSHA" Occupational safety and Health Administration" (2010) A brief guide to mold in workplace. OSHA Gov, California, USA.
- El Abbasy TM (2007) Mycological aspects of quail carcasses with a trial to improve their sanitary status. Thesis Master of Vet Med (Meat Hygiene) Zagazig Univ, Egypt.
- Agriopoulou S, Stamatelopoulou E and Varzakas T (2020) Advances in occurrence, importance, and mycotoxin control strategies: prevention and detoxification in Foods. Foods (Basel, Switzerland) 9(2): pp.137.
- Zhou R and Linz JE (1999) Enzymatic function of the Nor-1 protein in aflatoxin biosynthesis in Aspergillus parasiticus. Appl Environ Microbiol 65: 5639-5641.
- Henry KM and Townsend CA (2005) Ordering the reductive and cytochrome P450 oxidative steps in demethylsterigmatocystin formation yields general insights into the biosynthesis of aflatoxin and related fungal metabolites. J Am Chem Soc 127: 3724-3733.
- Yabe K, Ando Y, Hashimoto J, Hamasaki T (1989) Two distinct O-methyltransferases in Aflatoxin biosynthesis. Appl Environ Microbiol 55: 2172-2177.
- Manonmani HK, Anand S, Chandrashekar A and Rati ER (2005) Detection of aflatoxigenic fungi in selected food commodities by PCR. Process Biochemistry 40: 2859-2864.
- Rodrigues P, Venencio A, Kozakiewicz Z, and Lima N (2009) A polyphasic approach to the identification of aflatoxigenic and non-aflatoxigenic strains of Aspergillus section Flavi isolated from Portuguese almonds. Inter J Food Microbiol 129: 187-193.
- Hassan MN, El Sayed, AA and Nada HM (2015) Detection of aflatoxins by HPLC and the expression of biosynthetic nor-1 gene of aflatoxin and ocrA gene of ochratoxin. Benha Vet Med J 29(2): 1-10.
- Norlia M, Jinap S, Nor Khaizura, MAR, Radu S, Samsudin NIP and Azri FA (2019) Aspergillus section Flavi and aflatoxins: occurrence, detection, and identification in raw peanuts and peanut-based products along the supply chain. Front. Microbiol 10: pp.2602.
- Sohrabi N and Taghizadeh M (2018) Molecular identification of aflatoxigenic Aspergillus species in feedstuff samples. Curr Med Mycol 4(2): 1-6.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...

.png)